Abstract
A severe decline in child births has occurred over the past half century, which will lead to considerable population declines, particularly in industrialized regions. A crucial question is whether this decline can be explained by economic and behavioural factors alone, as suggested by demographic reports, or to what degree biological factors are also involved. Here, we discuss data suggesting that human reproductive health is deteriorating in industrialized regions. Widespread infertility and the need for assisted reproduction due to poor semen quality and/or oocyte failure are now major health issues. Other indicators of declining reproductive health include a worldwide increasing incidence in testicular cancer among young men and alterations in twinning frequency. There is also evidence of a parallel decline in rates of legal abortions, revealing a deterioration in total conception rates. Subtle alterations in fertility rates were already visible around 1900, and most industrialized regions now have rates below levels required to sustain their populations. We hypothesize that these reproductive health problems are partially linked to increasing human exposures to chemicals originating directly or indirectly from fossil fuels. If the current infertility epidemic is indeed linked to such exposures, decisive regulatory action underpinned by unconventional, interdisciplinary research collaborations will be needed to reverse the trends.
Key points
-
Industrialized regions have birth rates so low that their populations cannot be sustained; declines in birth rates are generally ascribed to socioeconomic and cultural factors, although human infertility is widespread.
-
Decreasing fertility rates were already recorded around 1900 in Denmark, a few decades after the beginning of utilization of fossil fuels that were, and still are, drivers of modern industrialization and wealth.
-
We hypothesize that declines in fertility rates might be linked to exposures to chemicals originating from fossil fuels causing human reproductive problems and cancer; early gestation might be a sensitive period.
-
The current unsustainable birth rates will eventually result in decreasing populations.
-
A key research challenge remains: how to distinguish biological from socioeconomic and behavioural factors?
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Vollset, S. E. et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet 396, 1285–1306 (2020).
Lee, S. J., Li, L. & Hwang, J. Y. After 20 years of low fertility, where are the obstetrician-gynecologists? Obstet. Gynecol. Sci. 64, 407–418 (2021).
Lutz, W., O’Neill, B. C. & Scherbov, S. Demographics. Europe’s population at a turning point. Science 299, 1991–1992 (2003).
GBD 2017 Population and Fertility Collaborators. Population and fertility by age and sex for 195 countries and territories, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1995–2051 (2018).
Zegers-Hochschild, F. et al. The international glossary on infertility and fertility care, 2017. Hum. Reprod. 32, 1786–1801 (2017).
Priskorn, L., Dahl, C. L., Pihl, A. S., Skakkebaek, N. E. & Juul, A. High maternal age at first and subsequent child births in Denmark in the mid-1800s–Letter to the editor. Eur. J. Obstet. Gynecol. Reprod. Biol. 241, 137–138 (2019).
Fellman, J. & Eriksson, A. W. Temporal differences in the regional twinning rates in Sweden after 1750. Twin Res. 6, 183–191 (2003).
Blomberg, J. M., Priskorn, L., Jensen, T. K., Juul, A. & Skakkebaek, N. E. Temporal trends in fertility rates: a vationwide registry based study from 1901 to 2014. PloS ONE 10, e0143722 (2015).
Lackie, E. & Fairchild, A. The birth control pill, thromboembolic disease, science and the media: a historical review of the relationship. Contraception 94, 295–302 (2016).
Sandström, G., Marklund, E. Fertility differentials in Sweden during the first half of the twentieth century: the changing effect of female labor force participation and occupational field. Presented at the Annual Meeting of the Population Association of America, Chicago, 27–29 April 2017.
Skakkebaek, N. E. et al. Populations, decreasing fertility, and reproductive health. Lancet 393, 1500–1501 (2019).
Oeppen, J. & Vaupel, J. W. Demography. Broken limits to life expectancy. Science 296, 1029–1031 (2002).
Statistics Bureau of Japan. Japan’s Population Estimates Released https://www.stat.go.jp/english/info/news/1910.html (2010).
Tillotson, J. E. America’s obesity: conflicting public policies, industrial economic development, and unintended human consequences. Annu. Rev. Nutr. 24, 617–643 (2004).
Haagen-Smit, A. J. A lesson from the smog capital of the world. Proc. Natl Acad. Sci. USA 67, 887–897 (1970).
Wang, F., Zheng, P., Dai, J., Wang, H. & Wang, R. Fault tree analysis of the causes of urban smog events associated with vehicle exhaust emissions: a case study in Jinan, China. Sci. Total. Environ. 668, 245–253 (2019).
World Health Organization. State of the Science of Endocrine Disputing Chemicals – 2012. https://www.unep.org/resources/publication/state-science-endocrine-disputing-chemicals-ipcp-2012 (2013).
Crinnion, W. J. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physicians. Altern. Med. Rev. 15, 101–109 (2010).
Bergman, A. et al. The impact of endocrine disruption: a consensus statement on the state of the science. Environ. Health Perspect. 121, A104–A106 (2013).
Andersson, A. M. et al. Adverse trends in male reproductive health: we may have reached a crucial ‘tipping point’. Int. J. Androl. 31, 74–80 (2008).
Christin-Maitre, S. History of oral contraceptive drugs and their use worldwide. Best. Pract. Res. Clin. Endocrinol. Metab. 27, 3–12 (2013).
Mears, E. Clinical trials of oral contraceptives. Br. Med. J. 2, 1179–1183 (1961).
Finer, L. B. & Zolna, M. R. Declines in unintended pregnancy in the United States, 2008-2011. N. Engl. J. Med. 374, 843–852 (2016).
Mumford, S. L., Sapra, K. J., King, R. B., Louis, J. F. & Buck Louis, G. M. Pregnancy intentions–a complex construct and call for new measures. Fertil. Steril. 106, 1453–1462 (2016).
Sedgh, G. et al. Abortion incidence between 1990 and 2014: global, regional, and subregional levels and trends. Lancet 388, 258–267 (2016).
Jatlaoui, T. C. et al. Abortion surveillance – United States, 2016. MMWR Surveill. Summ. 68, 1–41 (2019).
Jensen, T. K. et al. Declining trends in conception rates in recent birth cohorts of native Danish women: a possible role of deteriorating male reproductive health. Int. J. Androl. 31, 81–92 (2008).
Lassen, T. H. et al. Trends in rates of natural conceptions among Danish women born during 1960-1984. Hum. Reprod. 27, 2815–2822 (2012).
Hognert, H. et al. High birth rates despite easy access to contraception and abortion: a cross-sectional study. Acta Obstet. Gynecol. Scand. 96, 1414–1422 (2017).
Lidegaard, Ø. et al. Pregnancy loss: A 40-year nationwide assessment. Acta Obstet. Gynecol. Scand. 99, 1492–1496 (2020).
Rossen, L. M., Ahrens, K. A. & Branum, A. M. Trends in risk of pregnancy loss among US women, 1990-2011. Paediatr. Perinat. Epidemiol. 32, 19–29 (2018).
Tong, S. & Short, R. V. Dizygotic twinning as a measure of human fertility. Hum. Reprod. 13, 95–98 (1998).
Asklund, C. et al. Twin pregnancy possibly associated with high semen quality. Hum. Reprod. 22, 751–755 (2007).
Pison, G., Monden, C. & Smits, J. Twinning rates in developed countries: trends and explanations. Popul. Dev. Rev. 41, 629–649 (2015).
Präg, P. & Mills, M. C. in Childlessness in Europe: Contexts, Causes, and Consequences (eds Kreyenfeld, M. & Konietzka, D.) 289–309 (Springer, 2017).
Bracken, M. B. Oral contraception and twinning: an epidemiologic study. Am. J. Obstet. Gynecol. 133, 432–434 (1979).
Rachootin, P. & Olsen, J. Secular changes in the twinning rate in Denmark 1931 to 1977. Scand. J. Soc. Med. 8, 89–94 (1980).
Olsen, J. & Rachootin, P. The end of the decline in twinning rates? Scand. J. Soc. Med. 11, 119 (1983).
Andersen, A. N. & Erb, K. Register data on assisted reproductive technology (ART) in Europe including a detailed description of ART in Denmark. Int. J. Androl. 29, 12–16 (2006).
De Geyter, C. et al. ART in Europe, 2015: results generated from European registries by ESHRE. Hum. Reprod. Open 2020, hoz038 (2020).
Kamphuis, E. I., Bhattacharya, S., van der Veen, F., Mol, B. W. & Templeton, A. Are we overusing IVF? BMJ 348, g252 (2014).
Sundhedsdatastyrelsen. Assisteret Reproduktion 2019. https://docplayer.dk/204335156-Assisteret-reproduktion-2019.html (2019).
Garcia, D., Brazal, S., Rodriguez, A., Prat, A. & Vassena, R. Knowledge of age-related fertility decline in women: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 230, 109–118 (2018).
Habbema, J. D., Eijkemans, M. J., Leridon, H. & te Velde, E. R. Realizing a desired family size: when should couples start? Hum. Reprod. 30, 2215–2221 (2015).
Hassan, M. A. & Killick, S. R. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil. Steril. 79 (Suppl 3), 1520–1527 (2003).
Tsao, C. W. et al. Exploration of the association between obesity and semen quality in a 7630 male population. PLoS ONE 10, e0119458 (2015).
Nieschlag, E., Lammers, U., Freischem, C. W., Langer, K. & Wickings, E. J. Reproductive functions in young fathers and grandfathers. J. Clin. Endocrinol. Metab. 55, 676–681 (1982).
Ge, Z. J., Schatten, H., Zhang, C. L. & Sun, Q. Y. Oocyte ageing and epigenetics. Reproduction 149, R103–R114 (2015).
Gruhn, J. R. et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science 365, 1466–1469 (2019).
Handyside, A. H. Molecular origin of female meiotic aneuploidies. Biochim. Biophys. Acta 1822, 1913–1920 (2012).
Newman, J. E., Fitzgerlad, O., Paul, R. C. & Chambers, G. M. Assisted reproductive technology in Australia and New Zealand 2017. https://npesu.unsw.edu.au/sites/default/files/npesu/data_collection/Assisted%20Reproductive%20Technology%20in%20Australia%20and%20New%20Zealand%202017.pdf (2019).
Neels, K., Murphy, M., Ni Bhrolchain, M. & Beaujouan, E. Rising educational participation and the trend to later childbearing. Popul. Dev. Rev. 43, 667–693 (2017).
Joham, A. E., Palomba, S. & Hart, R. Polycystic ovary syndrome, obesity, and pregnancy. Semin. Reprod. Med. 34, 93–101 (2016).
Koninckx, P. R. et al. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best. Pract. Res. Clin. Obstet. Gynaecol. 71, 14–26 (2021).
Noriega, N. C., Ostby, J., Lambright, C., Wilson, V. S. & Gray, L. E. Jr. Late gestational exposure to the fungicide prochloraz delays the onset of parturition and causes reproductive malformations in male but not female rat offspring. Biol. Reprod. 72, 1324–1335 (2005).
Buck Louis, G. M. et al. Paternal exposures to environmental chemicals and time-to-pregnancy: overview of results from the LIFE study. Andrology 4, 639–647 (2016).
Lum, K. J., Sundaram, R., Barr, D. B., Louis, T. A. & Buck Louis, G. M. Perfluoroalkyl chemicals, menstrual cycle length, and fecundity: findings from a prospective pregnancy study. Epidemiology 28, 90–98 (2017).
Mínguez-Alarcón, L. & Gaskins, A. J. Female exposure to endocrine disrupting chemicals and fecundity: a review. Curr. Opin. Obstet. Gynecol. 29, 202–211 (2017).
Buck Louis, G. M., Kannan, K., Sapra, K. J., Maisog, J. & Sundaram, R. Urinary concentrations of benzophenone-type ultraviolet radiation filters and couples’ fecundity. Am. J. Epidemiol. 180, 1168–1175 (2014).
Smarr, M. M., Sundaram, R., Honda, M., Kannan, K. & Louis, G. M. Urinary concentrations of parabens and other antimicrobial chemicals and their association with couples’ fecundity. Environ. Health Perspect. 125, 730–736 (2017).
Abu-Halima, M. et al. Panel of five microRNAs as potential biomarkers for the diagnosis and assessment of male infertility. Fertil. Steril. 102, 989–997 (2014).
Steinmetz, R., Brown, N. G., Allen, D. L., Bigsby, R. M. & Ben-Jonathan, N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology 138, 1780–1786 (1997).
Steinmetz, R. et al. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology 139, 2741–2747 (1998).
Spearow, J. L., Doemeny, P., Sera, R., Leffler, R. & Barkley, M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science 285, 1259–1261 (1999).
Spearow, J. L. et al. Genetic variation in physiological sensitivity to estrogen in mice. APMIS 109, 356–364 (2001).
Spearow, J. L. & Barkley, M. Reassessment of models used to test xenobiotics for oestrogenic potency is overdue. Hum. Reprod. 16, 1027–1029 (2001).
Amann, R. P. & Howards, S. S. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J. Urol. 124, 211–215 (1980).
Franca, L. R., Russell, L. D. & Cummins, J. M. Is human spermatogenesis uniquely poor? Ann. Rev. Biomed. Sci. 4, 19–40 (2002).
Short, R. V. The testis: the witness of the mating system, the site of mutation and the engine of desire. Acta Paediatr. Suppl. 422, 3–7 (1997).
Hess, R. A. & França, L. R. in Molecular Mechanisms in Spermatogenesis (ed. Cheng, C.) 1–15 (Landes Bioscience, 2007).
França, L. R., Ogawa, T., Avarbock, M. R., Brinster, R. L. & Russell, L. D. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol. Reprod. 59, 1371–1377 (1998).
França, L. R., Avelar, G. F. & Almeida, F. F. Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology 63, 300–318 (2005).
Carlsen, E., Giwercman, A., Keiding, N. & Skakkebæk, N. E. Evidence for decreasing quality of semen during past 50 years. BMJ 305, 609–613 (1992).
Levine, H. et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum. Reprod. Update 23, 646–659 (2017).
Huang, C. et al. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil. Steril. 107, 83–88.e2 (2017).
Yuan, H. F. et al. Decline in semen concentration of healthy Chinese adults: evidence from 9357 participants from 2010 to 2015. Asian J. Androl. 20, 379–384 (2018).
Huang, X. et al. Association of exposure to ambient fine particulate matter constituents with semen quality among men attending a fertility center in China. Environ. Sci. Technol. 53, 5957–5965 (2019).
Priskorn, L. et al. Average sperm count remains unchanged despite reduction in maternal smoking: results from a large cross-sectional study with annual investigations over 21 years. Hum. Reprod. 33, 998–1008 (2018).
Jørgensen, N. et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2, e000990 (2012).
Bonde, J. P. E. et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 352, 1172–1177 (1998).
Guzick, D. S. et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 345, 1388–1393 (2001).
Slama, R. et al. Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum. Reprod. 17, 503–515 (2002).
World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. https://www.who.int/publications/i/item/9789240030787 (2021).
Skakkebaek, N. E. Normal reference ranges for semen quality and their relations to fecundity. Asian J. Androl. 12, 95–98 (2010).
Skakkebæk, N. E., Rajpert-De Meyts, E. & Main, K. M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978 (2001).
Clemmesen, J. A doubling of morbidity from testis carcinoma in Copenhagen, 1943–1962. APMIS 72, 348–349 (1968).
Znaor, A. et al. Testicular cancer incidence predictions in Europe 2010-2035: a rising burden despite population ageing. Int. J. Cancer 147, 820–828 (2020).
Møller, H. Clues to the aetiology of testicular germ cell tumours from descriptive epidemiology. Eur. Urol. 23, 8–15 (1993).
Bergström, R. et al. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. J. Natl Cancer Inst. 88, 727–733 (1996).
Grumet, R. F. & MacMahon, B. Trends in mortality from neoplasms of the testis. Cancer 11, 790–797 (1958).
Case, R. A. Cohort analysis of cancer mortality in England and Wales; 1911–1954 by site and sex. Br. J. Prev. Soc. Med. 10, 172–199 (1956).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Bray, F. et al. Cancer incidence in five continents, Vol. XI (electronic version). Lyon: International Agency for Research on Cancer. https://ci5.iarc.fr/CI5-XI/Default.aspx (2017).
International Agency for Research on Cancer. CI5plus: Cancer Incidence in Five Continents Time Trends. http://ci5.iarc.fr/CI5plus/Default.aspx (2018).
Znaor, A., Lortet-Tieulent, J., Jemal, A. & Bray, F. International variations and trends in testicular cancer incidence and mortality. Eur. Urol. 65, 1095–1106 (2014).
Harbuz, R. et al. A recurrent deletion of DPY19L2 causes infertility in man by blocking sperm head elongation and acrosome formation. Am. J. Hum. Genet. 88, 351–361 (2011).
Dam, A. H. et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am. J. Hum. Genet. 81, 813–820 (2007).
Tüttelmann, F., Ruckert, C. & Röpke, A. Disorders of spermatogenesis: perspectives for novel genetic diagnostics after 20 years of unchanged routine. Med. Genet. 30, 12–20 (2018).
Krausz, C. & Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 15, 369–384 (2018).
Nagirnaja, L. et al. Variant PNLDC1, defective piRNA processing, and azoospermia. N. Engl. J. Med. 385, 707–719 (2021).
Kasak, L. & Laan, M. Monogenic causes of non-obstructive azoospermia: challenges, established knowledge, limitations and perspectives. Hum. Genet. 140, 135–154 (2020).
Barban, N. et al. Genome-wide analysis identifies 12 loci influencing human reproductive behavior. Nat. Genet. 48, 1462–1472 (2016).
Stolk, L. et al. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat. Genet. 41, 645–647 (2009).
Day, F. R. et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 49, 834–841 (2017).
Ruth, K. S. et al. Genome-wide association study of anti-Müllerian hormone levels in pre-menopausal women of late reproductive age and relationship with genetic determinants of reproductive lifespan. Hum. Mol. Genet. 28, 1392–1401 (2019).
Stolk, L. et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat. Genet. 44, 260–268 (2012).
Lutzmann, M. et al. MCM8- and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol. Cell 47, 523–534 (2012).
Cavalli, G. & Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 571, 489–499 (2019).
Almstrup, K. et al. Pubertal development in healthy children is mirrored by DNA methylation patterns in peripheral blood. Sci. Rep. 6, 28657 (2016).
Chen, S. et al. Age at onset of different pubertal signs in boys and girls and differential DNA methylation at age 10 and 18 years: an epigenome-wide follow-up study. Hum. Reprod. Open 2020, hoaa006 (2020).
Kresovich, J. K. et al. Reproduction, DNA methylation and biological age. Hum. Reprod. 34, 1965–1973 (2019).
Meehan, R. R., Thomson, J. P., Lentini, A., Nestor, C. E. & Pennings, S. DNA methylation as a genomic marker of exposure to chemical and environmental agents. Curr. Opin. Chem. Biol. 45, 48–56 (2018).
Almstrup, K., Frederiksen, H., Andersson, A. M. & Juul, A. Levels of endocrine-disrupting chemicals are associated with changes in the peri-pubertal epigenome. Endocr. Connect. 9, 845–857 (2020).
Tobi, E. W. et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat. Commun. 5, 5592 (2014).
Richmond, R. C. et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum. Mol. Genet. 24, 2201–2217 (2015).
Leitão, E. et al. The sperm epigenome does not display recurrent epimutations in patients with severely impaired spermatogenesis. Clin. Epigenet. 12, 61 (2020).
Soubry, A. et al. Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environ. Epigenet. 3, dvx003 (2017).
Wu, H. et al. Preconception urinary phthalate concentrations and sperm DNA methylation profiles among men undergoing IVF treatment: a cross-sectional study. Hum. Reprod. 32, 2159–2169 (2017).
Greeson, K. W. et al. Detrimental effects of flame retardant, PBB153, exposure on sperm and future generations. Sci. Rep. 10, 8567 (2020).
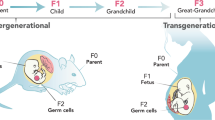
Beck, D., Sadler-Riggleman, I. & Skinner, M. K. Generational comparisons (F1 versus F3) of vinclozolin induced epigenetic transgenerational inheritance of sperm differential DNA methylation regions (epimutations) using MeDIP-Seq. Environ. Epigenet 3, dvx016 (2017).
Nätt, D. & Öst, A. Male reproductive health and intergenerational metabolic responses from a small RNA perspective. J. Intern. Med. 288, 305–320 (2020).
Trigg, N. A., Eamens, A. L. & Nixon, B. The contribution of epididymosomes to the sperm small RNA profile. Reproduction 157, R209–R223 (2019).
Sharma, U. et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351, 391–396 (2016).
Grandjean, V. et al. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep. 5, 18193 (2015).
Xu, H. et al. MicroRNA expression profile analysis in sperm reveals hsa-mir-191 as an auspicious omen of in vitro fertilization. BMC Genomics 21, 165 (2020).
Kong, A. et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 488, 471–475 (2012).
Nybo Andersen, A. M. & Urhoj, S. K. Is advanced paternal age a health risk for the offspring? Fertil. Steril. 107, 312–318 (2017).
Whorton, D., Milby, T. H., Krauss, R. M. & Stubbs, H. A. Testicular function in DBCP exposed pesticide workers. J. Occup. Med. 21, 161–166 (1979).
Goldsmith, J. R., Potashnik, G. & Israeli, R. Reproductive outcomes in families of DBCP-exposed men. Arch. Environ. Health 39, 85–89 (1984).
Potashnik, G., Goldsmith, J. & Insler, V. Dibromochloropropane-induced reduction of the sex-ratio in man. Andrologia 16, 213–218 (1984).
Skakkebaek, N. E. et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol. Rev. 96, 55–97 (2016).
Messiaen, S. et al. Rad54 is required for the normal development of male and female germ cells and contributes to the maintainance of their genome integrity after genotoxic stress. Cell Death Dis. 4, e774 (2013).
Mandl, A. M., Beaumont, H. M. & Hughes, G. C. in Effects of Ionizing Radiation on the Reproductive System (eds Carlson, W. D. & Gassner, F. X.) 165 (Pergamon Press, 1964).
Mandl, A. M. The radiosensitivity of germ cells. Biol. Rev. 39, 288–371 (1964).
Gray, L. E. Jr. et al. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 58, 350–365 (2000).
Fisher, J. S., Macpherson, S., Marchetti, N. & Sharpe, R. M. Human “testicular dysgenesis syndrome”: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum. Reprod. 18, 1383–1394 (2003).
Gray, L. E. Jr., Ostby, J. S. & Kelce, W. R. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxixol. Appl. Pharmacol. 129, 46–52 (1994).
Hass, U. et al. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ. Health Perspect. 115 (Suppl 1), 122–128 (2007).
Welsh, M. et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Invest. 118, 1479–1490 (2008).
Van den Driesche, S. et al. Experimentally induced testicular dysgenesis syndrome originates in the masculinization programming window. JCI Insight 2, e91204 (2017).
Kortenkamp, A. Which chemicals should be grouped together for mixture risk assessments of male reproductive disorders? Mol. Cell Endocrinol. 499, 110581 (2020).
Howdeshell, K. L., Hotchkiss, A. K. & Gray, L. E. Jr Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int. J. Hyg. Environ. Health 220, 179–188 (2017).
Gray, L. E. Jr & Ostby, J. S. In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring. Toxixol. Appl. Pharmacol. 133, 285–294 (1995).
Lovekamp-Swan, T. & Davis, B. J. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 111, 139–145 (2003).
Guerra, M. T., Scarano, W. R., de Toledo, F. C., Franci, J. A. & Kempinas Wde, G. Reproductive development and function of female rats exposed to di-eta-butyl-phthalate (DBP) in utero and during lactation. Reprod. Toxicol. 29, 99–105 (2010).
Johansson, H. K. L. et al. Putative adverse outcome pathways for female reproductive disorders to improve testing and regulation of chemicals. Arch. Toxicol. 94, 3359–3379 (2020).
Mocarelli, P. et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ. Health Perspect. 119, 713–718 (2011).
Hardell, L., van Bavel, B., Lindstrom, G., Eriksson, M. & Carlberg, M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int. J. Androl. 29, 228–234 (2006).
Krysiak-Baltyn, K. et al. Association between chemical pattern in breast milk and congenital cryptorchidism: modelling of complex human exposures. Int. J. Androl. 35, 294–302 (2012).
Main, K. M. et al. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ. Health Perspect. 115, 1519–1526 (2007).
Hemminki, K. & Li, X. Cancer risks in Nordic immigrants and their offspring in Sweden. Eur. J. Cancer 38, 2428–2434 (2002).
Schmiedel, S., Schuz, J., Skakkebæk, N. E. & Johansen, C. Testicular germ cell cancer incidence in an immigration perspective, Denmark, 1978 to 2003. J. Urol. 183, 1378–1382 (2010).
Myrup, C. et al. Testicular cancer risk in first- and second-generation immigrants to Denmark. J. Natl Cancer Inst. 100, 41–47 (2008).
Nielsen, H., Nielsen, M. & Skakkebæk, N. E. The fine structure of a possible carcinoma-in-situ in the seminiferous tubules in the testis of four infertile men. APMIS 82, 235–248 (1974).
Almstrup, K. et al. Genomic and gene expression signature of the pre-invasive testicular carcinoma in situ. Cell Tissue Res. 322, 159–165 (2005).
Almstrup, K. et al. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 64, 4736–4743 (2004).
Moch, H., Cubilla, A. L., Humphrey, P. A., Reuter, V. E. & Ulbright, T. M. The 2016 WHO classification of tumours of the urinary system and male genital organs–part a: renal, penile, and testicular tumours. Eur. Urol. 70, 93–105 (2016).
Sharpe, R. M. & Skakkebæk, N. E. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil. Steril. 89, e33–e38 (2008).
Lottrup, G. et al. Identification of a novel androgen receptor mutation in a family with multiple components compatible with the testicular dysgenesis syndrome. J. Clin. Endocrinol. Metab. 98, 2223–2229 (2013).
Depue, R. H., Pike, M. C. & Henderson, B. E. Cryptorchidism and testicular cancer. J. Natl Cancer Inst. 77, 830–832 (1986).
Moller, H. & Skakkebæk, N. E. Risks of testicular cancer and cryptorchidism in relation to socio-economic status and related factors: case-control studies in Denmark. Int. J. Cancer 66, 287–293 (1996).
Krabbe, S. et al. High incidence of undetected neoplasia in maldescended testes. Lancet 313, 999–1000 (1979).
Serrano, T., Chevrier, C., Multigner, L., Cordier, S. & Jegou, B. International geographic correlation study of the prevalence of disorders of male reproductive health. Hum. Reprod. 28, 1974–1986 (2013).
Jørgensen, N. et al. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum. Reprod. 17, 2199–2208 (2002).
Berthelsen, J. G. & Skakkebæk, N. E. Gonadal function in men with testis cancer. Fertil. Steril. 39, 68–75 (1983).
Berthelsen, J. G. Andrological Aspects of Testicular Cancer 9–44 (Scriptor, 1984).
Petersen, P. M. et al. Impaired testicular function in patients with carcinoma in situ of the testis. J. Clin. Oncol. 17, 173–179 (1999).
Jacobsen, R. et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. Br. Med. J. 321, 789–792 (2000).
Andersson, A. M. et al. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J. Clin. Endocrinol. Metab. 92, 4696–4705 (2007).
Travison, T. G., Araujo, A. B., O’Donnell, A. B., Kupelian, V. & McKinlay, J. B. A population-level decline in serum testosterone levels in American men. J. Clin. Endocrinol. Metab. 92, 196–202 (2007).
Sharma, R., Harlev, A., Agarwal, A. & Esteves, S. C. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 70, 635–645 (2016).
Jensen, T. K. et al. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1,770 young men from the general population in five European countries. Am. J. Epidemiol. 159, 49–58 (2004).
Ramlau-Hansen, C. H. et al. Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am. J. Epidemiol. 165, 1372–1379 (2007).
Jensen, T. K. et al. Adult and prenatal exposures to tobacco smoke as risk indicators of fertility among 430 Danish couples. Am. J. Epidemiol. 148, 992–997 (1998).
Radin, R. G. et al. Active and passive smoking and fecundability in Danish pregnancy planners. Fertil. Steril. 102, 183–191.e2 (2014).
Sapra, K. J., Barr, D. B., Maisog, J. M., Sundaram, R. & Buck Louis, G. M. Time-to-pregnancy associated with couples’ use of tobacco products. Nicotine Tob. Res. 18, 2154–2161 (2016).
Wesselink, A. K. et al. Prospective study of cigarette smoking and fecundability. Hum. Reprod. 34, 558–567 (2019).
Holmboe, S. A. et al. Use of e-cigarettes associated with lower sperm counts in a cross-sectional study of young men from the general population. Hum. Reprod. 35, 1693–1701 (2020).
Gundersen, T. D. et al. Association between use of marijuana and male reproductive hormones and semen quality: a study among 1,215 healthy young men. Am. J. Epidemiol. 182, 473–481 (2015).
Nassan, F. L. et al. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum. Reprod. 34, 715–723 (2019).
Jensen, T. K. et al. Does moderate alcohol consumption affect fertility? Follow up study among couples planning first pregnancy. BMJ 317, 505–510 (1998).
Ricci, E. et al. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod. Biomed. Online 34, 38–47 (2017).
Salas-Huetos, A., James, E. R., Aston, K. I., Jenkins, T. G. & Carrell, D. T. Diet and sperm quality: nutrients, foods and dietary patterns. Reprod. Biol. 19, 219–224 (2019).
Nassan, F. L. et al. Association of dietary patterns with testicular function in young Danish men. JAMA Netw. Open 3, e1921610 (2020).
Grieger, J. A. et al. Pre-pregnancy fast food and fruit intake is associated with time to pregnancy. Hum. Reprod. 33, 1063–1070 (2018).
Lee, S., Min, J. Y., Kim, H. J. & Min, K. B. Association between the frequency of eating non-home-prepared meals and women infertility in the United States. J. Prev. Med. Public Health 53, 73–81 (2020).
Hatch, E. E. et al. Intake of sugar-sweetened beverages and fecundability in a North American preconception cohort. Epidemiology 29, 369–378 (2018).
Supramaniam, P. R., Mittal, M., McVeigh, E. & Lim, L. N. The correlation between raised body mass index and assisted reproductive treatment outcomes: a systematic review and meta-analysis of the evidence. Reprod. Health 15, 34 (2018).
Mushtaq, R. et al. Effect of male body mass index on assisted reproduction treatment outcome: an updated systematic review and meta-analysis. Reprod. Biomed. Online 36, 459–471 (2018).
Sermondade, N. et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum. Reprod. Update 19, 221–231 (2013).
Ma, J. et al. Association between BMI and semen quality: an observational study of 3966 sperm donors. Hum. Reprod. 34, 155–162 (2019).
Vaamonde, D., Da Silva-Grigoletto, M. E., Garcia-Manso, J. M., Barrera, N. & Vaamonde-Lemos, R. Physically active men show better semen parameters and hormone values than sedentary men. Eur. J. Appl. Physiol. 112, 3267–3273 (2012).
Gaskins, A. J. et al. Physical activity and television watching in relation to semen quality in young men. Br. J. Sports Med. 49, 265–270 (2015).
Lalinde-Acevedo, P. C. et al. Physically active men show better semen parameters than their sedentary counterparts. Int. J. Fertil. Steril. 11, 156–165 (2017).
Sun, B. et al. Physical activity and sedentary time in relation to semen quality in healthy men screened as potential sperm donors. Hum. Reprod. 34, 2330–2339 (2019).
Hajizadeh Maleki, B. & Tartibian, B. Moderate aerobic exercise training for improving reproductive function in infertile patients: a randomized controlled trial. Cytokine 92, 55–67 (2017).
Ritchie, H. & Roser, M. Fossil fuels. Our World in Data https://ourworldindata.org/fossil-fuels (2020).
Thompson, R. C., Moore, C. J., vom Saal, F. S. & Swan, S. H. Plastics, the environment and human health: current consensus and future trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2153–2166 (2009).
Francis, M. About 7% of fossil fuels are consumed for non-combustion use in the United States. U.S. Energy Information System https://www.eia.gov/todayinenergy/detail.php?id=35672 (2018).
Festel, G., Evans, D. & Jackson, B. Trade sustainability impact assessment for the negotiations of a partnership and cooperation agreement between the EU and China. https://trade.ec.europa.eu/doclib/docs/2008/september/tradoc_140583.pdf (2008).
Woodruff, T. J., Zota, A. R. & Schwartz, J. M. Environmental chemicals in pregnant women in the US: NHANES 2003-2004. Environ. Health Perspect. 119, 878–885 (2011).
Rogan, W. J. et al. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects of maternal factors and previous lactation. Am. J. Public Health 76, 172–177 (1986).
Fang, J., Nyberg, E., Bignert, A. & Bergman, A. Temporal trends of polychlorinated dibenzo-p-dioxins and dibenzofurans and dioxin-like polychlorinated biphenyls in mothers’ milk from Sweden, 1972-2011. Environ. Int. 60, 224–231 (2013).
Frederiksen, H. et al. UV filters in matched seminal fluid-, urine-, and serum samples from young men. J. Expo. Sci. Environ. Epidemiol. 31, 345–355 (2020).
Apel, P. et al. Time course of phthalate cumulative risks to male developmental health over a 27-year period: biomonitoring samples of the German Environmental Specimen Bank. Environ. Int. 137, 105467 (2020).
Lewtas, J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat. Res. 636, 95–133 (2007).
Vohra, K. et al. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: results from GEOS-Chem. Environ. Res. 195, 110754 (2021).
Bamberger, M. et al. Surface water and groundwater analysis using aryl hydrocarbon and endocrine receptor biological assays and liquid chromatography-high resolution mass spectrometry in Susquehanna County, PA. Environ. Sci. Process. Impacts 21, 988–998 (2019).
Harville, E. W., Shankar, A., Zilversmit, L. & Buekens, P. The Gulf oil spill, miscarriage, and infertility: the GROWH study. Int. Arch. Occup. Environ. Health 91, 47–56 (2018).
Mocarelli, P., Brambilla, P., Gerthoux, P. M., Patterson, D. G. Jr & Needham, L. L. Change in sex ratio with exposure to dioxin. Lancet 348, 409–409 (1996).
Silva, M. J. et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ. Health Perspect. 112, 331–338 (2004).
Colborn, T. & Clement, C. Chemically-Induced Alterations in Sexual and Functional Development: the Wildlife/Human Connection (Princeton Scientific, 1992).
Baskin, L. S., Himes, K. & Colborn, T. Hypospadias and endocrine disruption: is there a connection? Environ. Health Perspect. 109, 1175–1183 (2001).
Hauser, R. et al. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European union. J. Clin. Endocrinol. Metab. 100, 1267–1277 (2015).
Rajpert-De Meyts, E., McGlynn, K. A., Okamoto, K., Jewett, M. A. & Bokemeyer, C. Testicular germ cell tumours. Lancet 387, 1762–1774 (2016).
Sallmén, M., Weinberg, C. R., Baird, D. D., Lindbohm, M. L. & Wilcox, A. J. Has human fertility declined over time? Why we may never know. Epidemiology 16, 494–499 (2005).
Joffe, M. et al. Studying time to pregnancy by use of a retrospective design. Am. J. Epidemiol. 162, 115–124 (2005).
Ahrenfeldt, L. J. et al. Heritability of subfertility among Danish twins. Fertil. Steril. 114, 618–627 (2020).
Buck, G. M. et al. Prospective pregnancy study designs for assessing reproductive and developmental toxicants. Environ. Health Perspect. 112, 79–86 (2004).
Scheike, T. H. & Keiding, N. Design and analysis of time-to-pregnancy. Stat. Methods Med. Res. 15, 127–140 (2006).
Slama, R. et al. Feasibility of the current-duration approach to studying human fecundity. Epidemiology 17, 440–449 (2006).
Hatch, E. E. et al. Evaluation of selection bias in an internet-based study of pregnancy planners. Epidemiology 27, 98–104 (2016).
KOSIS. Vital statistics of Korea. https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B8000F&language=en (2021).
Leal, M. C. & França, L. R. The seminiferous epithelium cycle length in the black tufted-ear marmoset (Callithrix penicillata) is similar to humans. Biol. Reprod. 74, 616–624 (2006).
de Oliveira, C. F. A., Lara, N., Cardoso, B. R. L., de França, L. R. & de Avelar, G. F. Comparative testis structure and function in three representative mice strains. Cell Tissue Res. 382, 391–404 (2020).
Garner, D. L. & Hafez, E. S. E. Spermatozoa and seminal plasma. in Reproduction in Farm Animals (ed. Hafez, E. S. E.) 165–187 (Lea and Febiger, 1993).
Valle Rdel, R. et al. Semen characteristics of captive common marmoset (Callithrix jacchus): a comparison of a German with a Brazilian colony. J. Med. Primatol. 43, 225–230 (2014).
Bezerra, M. J. B. et al. Major seminal plasma proteome of rabbits and associations with sperm quality. Theriogenology 128, 156–166 (2019).
Okamura, A. et al. Broken sperm, cytoplasmic droplets and reduced sperm motility are principal markers of decreased sperm quality due to organophosphorus pesticides in rats. J. Occup. Health 51, 478–487 (2009).
Prins, G. in Encyclopedia of Reproduction Vol. 4 (eds Knobil, E. & Neill, J. D.) 360–367 (Academic, 1998).
Bhattacharjee, R. et al. Targeted disruption of glycogen synthase kinase 3A (GSK3A) in mice affects sperm motility resulting in male infertility. Biol. Reprod. 92, 65 (2015).
Harris, T., Marquez, B., Suarez, S. & Schimenti, J. Sperm motility defects and infertility in male mice with a mutation in Nsun7, a member of the Sun domain-containing family of putative RNA methyltransferases. Biol. Reprod. 77, 376–382 (2007).
Acknowledgements
We thank M. Laversanne from the International Agency for Research on Cancer for assistance with the first draft of Fig. 4. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization. The authors acknowledge financial support from the Innovation Fund Denmark, Danish Ministry of Environment (CEHOS), Danish Ministry of Health, The ReproUnion consortium/EU Interreg ÖKS, Brazilian institutions (CNPq and CAPES) and Minas Gerais State Foundation (FAPEMIG). Finally, the authors thank A. Wahlberg, Department of Anthropology, University of Copenhagen, and K. Kjær, GLOBE Institute, University of Copenhagen, for most valuable discussions prior to writing the paper.
Author information
Authors and Affiliations
Contributions
N.E.S., R.L.-J., A.-M.A., S.A.H., E.V.B., K.A., L.R.F., A.Z., R.J.H. and A.J. researched data for the article, contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission. H.L., N.J., K.M.M., Ø.L. and A.K. contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission. L.P. researched data for the article, contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.J.H. is the Medical Director of Fertility Specialists of Western Australia and has equity interests in Western IVF. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks A.-S. Parent and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Databank, World Development Indicators: https://databank.worldbank.org/reports.aspx?source=world-development-indicators
Family Planning 2020: https://www.familyplanning2020.org/
Statistics Denmark: http://www.statistikbanken.dk/statbank5a/default.asp?w=1600
Supplementary information
Rights and permissions
About this article
Cite this article
Skakkebæk, N.E., Lindahl-Jacobsen, R., Levine, H. et al. Environmental factors in declining human fertility. Nat Rev Endocrinol 18, 139–157 (2022). https://doi.org/10.1038/s41574-021-00598-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-021-00598-8
This article is cited by
-
A bivariate Poisson regression to analyse impact of outlier women on correlation between female schooling and fertility in Malawi
BMC Women's Health (2024)
-
Inter- and trans-generational impacts of real-world PM2.5 exposure on male-specific primary hypogonadism
Cell Discovery (2024)
-
Bisphenol A Negatively Impacts Human Sperm MicroRNA and Protein Profiles
Exposure and Health (2024)
-
Reversing Uteropathies Including Cancer-Like Changes in Mice by Transplanting Mesenchymal Stromal Cells or XAR Treatment
Stem Cell Reviews and Reports (2024)
-
Frequency, morbidity and equity — the case for increased research on male fertility
Nature Reviews Urology (2024)