Abstract
Polycystic ovary syndrome (PCOS) is the main cause of female infertility worldwide and is associated with a substantially increased lifetime risk of comorbidities, including type 2 diabetes mellitus, psychiatric disorders and gynaecological cancers. Despite its high prevalence (~15%) and substantial economic burden, the aetiology of PCOS remains elusive. The genetic loci linked to PCOS so far account for only ~10% of its heritability, which is estimated at 70%. However, growing evidence suggests that altered epigenetic and developmental programming resulting from hormonal dysregulation of the maternal uterine environment contributes to the pathogenesis of PCOS. Male as well as female relatives of women with PCOS are also at an increased risk of developing PCOS-associated reproductive and metabolic disorders. Although PCOS phenotypes are highly heterogenous, hyperandrogenism is thought to be the principal driver of this condition. Current treatments for PCOS are suboptimal as they can only alleviate some of the symptoms; preventative and targeted treatments are sorely needed. This Review presents an overview of the current understanding of the aetiology of PCOS and focuses on the developmental origin and epigenetic inheritance of this syndrome.
Key points
-
Polycystic ovary syndrome (PCOS) is a common heritable disorder strongly linked to hyperandrogenism and hyperinsulinaemia.
-
Disentangling the genetic and non-genetic contributions to the transmission of PCOS will require further investigation.
-
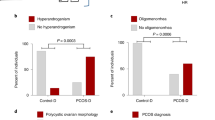
PCOS-like phenotypic traits are transgenerationally inherited in female offspring of androgen-exposed or anti-Müllerian hormone-exposed dams up to the F3 generation, indicating long-lasting effects of an aberrant maternal–fetal environment.
-
Studies in mouse models of PCOS demonstrate that epigenetic modulation connects early-life exposures to subsequent phenotypes and contributes to the development and familial transmission of PCOS.
-
Inheritance through epigenetic mechanisms opens a path towards novel treatment strategies for PCOS-like phenotypic traits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Azziz, R., Marin, C., Hoq, L., Badamgarav, E. & Song, P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J. Clin. Endocrinol. Metab. 90, 4650–4658 (2005).
March, W. A. et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 25, 544–551 (2010).
Yildiz, B. O., Bozdag, G., Yapici, Z., Esinler, I. & Yarali, H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum. Reprod. 27, 3067–3073 (2012).
The Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Fertil. Steril. 81, 19–25 (2004).
Teede, H. J. et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 33, 1602–1618 (2018).
Lizneva, D. et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 106, 6–15 (2016).
Kataoka, J. et al. Prevalence of polycystic ovary syndrome in women with severe obesity — effects of a structured weight loss programme. Clin. Endocrinol. 91, 750–758 (2019).
Ibanez, L. et al. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm. Res. Paediatr. 88, 371–395 (2017).
Mills, G., Badeghiesh, A., Suarthana, E., Baghlaf, H. & Dahan, M. H. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: a population-based study on 9.1 million pregnancies. Hum. Reprod. 35, 1666–1674 (2020).
Maliqueo, M. et al. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 166, 151–155 (2013).
Maliqueo, M. et al. Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum. Reprod. 30, 692–700 (2015).
Dumesic, D. A. et al. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr. Rev. 36, 487–525 (2015).
Legro, R. S., Kunselman, A. R., Dodson, W. C. & Dunaif, A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J. Clin. Endocrinol. Metab. 84, 165–169 (1999).
Rubin, K. H., Glintborg, D., Nybo, M., Abrahamsen, B. & Andersen, M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 102, 3848–3857 (2017).
Glintborg, D., Rubin, K. H., Nybo, M., Abrahamsen, B. & Andersen, M. Cardiovascular disease in a nationwide population of Danish women with polycystic ovary syndrome. Cardiovasc. Diabetol. 17, 37 (2018).
Gunning, M. N. et al. Cardiometabolic health in offspring of women with PCOS compared to healthy controls: a systematic review and individual participant data meta-analysis. Hum. Reprod. Update 26, 103–117 (2020).
Barry, J. A., Azizia, M. M. & Hardiman, P. J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update 20, 748–758 (2014).
Yin, W., Falconer, H., Yin, L., Xu, L. & Ye, W. Association between polycystic ovary syndrome and cancer risk. JAMA Oncol. 5, 106–107 (2019).
Cesta, C. E. et al. Polycystic ovary syndrome and psychiatric disorders: co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology 73, 196–203 (2016).
Chen, S. F., Yang, Y. C., Hsu, C. Y. & Shen, Y. C. Risk of bipolar disorder in patients with polycystic ovary syndrome: a nationwide population-based cohort study. J. Affect. Disord. 263, 458–462 (2020).
Dokras, A. et al. Androgen excess — Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil. Steril. 109, 888–899 (2018).
Jedel, E. et al. Anxiety and depression symptoms in women with polycystic ovary syndrome compared with controls matched for body mass index. Hum. Reprod. 25, 450–456 (2010).
Risal, S. et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 25, 1894–1904 (2019).
Vink, J. M., Sadrzadeh, S., Lambalk, C. B. & Boomsma, D. I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J. Clin. Endocrinol. Metab. 91, 2100–2104 (2006).
Legro, R. S., Driscoll, D., Strauss, J. F. 3rd, Fox, J. & Dunaif, A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc. Natl Acad. Sci. USA 95, 14956–14960 (1998).
Escobar-Morreale, H. F. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 14, 270–284 (2018).
Stener-Victorin, E. et al. Are there any sensitive and specific sex steroid markers for polycystic ovary syndrome? J. Clin. Endocrinol. Metab. 95, 810–819 (2010).
Gilling-Smith, C., Story, H., Rogers, V. & Franks, S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin. Endocrinol. 47, 93–99 (1997).
Nisenblat, V. & Norman, R. J. Androgens and polycystic ovary syndrome. Curr. Opin. Endocrinol. Diab Obes. 16, 224–231 (2009).
Webber, L. J. et al. Formation and early development of follicles in the polycystic ovary. Lancet 362, 1017–1021 (2003).
Lenie, S. & Smitz, J. Functional AR signaling is evident in an in vitro mouse follicle culture bioassay that encompasses most stages of folliculogenesis. Biol. Reprod. 80, 685–695 (2009).
Taylor, A. E. et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 82, 2248–2256 (1997).
Baillargeon, J. P. & Carpentier, A. Role of insulin in the hyperandrogenemia of lean women with polycystic ovary syndrome and normal insulin sensitivity. Fertil. Steril. 88, 886–893 (2007).
Barbieri, R. L. et al. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J. Clin. Endocrinol. Metab. 62, 904–910 (1986).
Barbieri, R. L. & Hornstein, M. D. Hyperinsulinemia and ovarian hyperandrogenism. Cause and effect. Endocrinol. Metab. Clin. N. Am. 17, 685–703 (1988).
Barbieri, R. L., Smith, S. & Ryan, K. J. The role of hyperinsulinemia in the pathogenesis of ovarian hyperandrogenism. Fertil. Steril. 50, 197–212 (1988).
Thong, E. P., Codner, E., Laven, J. S. E. & Teede, H. Diabetes: a metabolic and reproductive disorder in women. Lancet Diab Endocrinol. 8, 134–149 (2019).
Ruth, K. S. et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 26, 252–258 (2020).
Day, F. et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 14, e1007813 (2018).
Gorsic, L. K. et al. Pathogenic anti-mullerian hormone variants in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 102, 2862–2872 (2017).
Gorsic, L. K., Dapas, M., Legro, R. S., Hayes, M. G. & Urbanek, M. Functional genetic variation in the anti-mullerian hormone pathway in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 104, 2855–2874 (2019).
Dapas, M. et al. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: an unsupervised, phenotypic clustering analysis. PLoS Med. 17, e1003132 (2020).
Manolio, T. A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009).
Li, J. et al. Transmission of polycystic ovary syndrome susceptibility single-nucleotide polymorphisms and their association with phenotype changes in offspring. Hum. Reprod. 35, 1711–1718 (2020).
Waddington, C. H. The epigenotype. 1942. Reprinted. Int. J. Epidemiol. 41, 10–13 (2012).
Slack, J. M. Conrad Hal Waddington: the last Renaissance biologist? Nat. Rev. Genet. 3, 889–895 (2002).
Nanney, D. L. Epigenetic control systems. Proc. Natl Acad. Sci. USA 44, 712–717 (1958).
Holliday, R. & Pugh, J. E. DNA modification mechanisms and gene activity during development. Science 187, 226–232 (1975).
Riggs, A. D. X inactivation, differentiation, and DNA methylation. Cytogenet. Cell Genet. 14, 9–25 (1975).
Lappalainen, T. & Greally, J. M. Associating cellular epigenetic models with human phenotypes. Nat. Rev. Genet. 18, 441–451 (2017).
Goldberg, A. D., Allis, C. D. & Bernstein, E. Epigenetics: a landscape takes shape. Cell 128, 635–638 (2007).
Bird, A. Perceptions of epigenetics. Nature 447, 396–398 (2007).
Cavalli, G. & Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 571, 489–499 (2019).
Sharif, J. et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nat. Rev. Genet. 450, 908–912 (2007).
Bostick, M. et al. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science 317, 1760–1764 (2007).
Song, J., Rechkoblit, O., Bestor, T. H. & Patel, D. J. Structure of DNMT1-DNA complex reveals a role for autoinhibition in maintenance DNA methylation. Science 331, 1036–1040 (2011).
Okano, M., Xie, S. & Li, E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet 19, 219–220 (1998).
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999).
Bourc’his, D., Xu, G. L., Lin, C. S., Bollman, B. & Bestor, T. H. Dnmt3L and the establishment of maternal genomic imprints. Science 294, 2536–2539 (2001).
Barau, J. et al. The DNA methyltransferase DNMT3C protects male germ cells from transposon activity. Science 354, 909–912 (2016).
Kriaucionis, S. & Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324, 929–930 (2009).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Ito, S. et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333, 1300–1303 (2011).
Greenberg, M. V. C. & Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 20, 590–607 (2019).
Wang, L. et al. Programming and inheritance of parental DNA methylomes in mammals. Cell 157, 979–991 (2014).
Tang, W. W. et al. A unique gene regulatory network resets the human germline epigenome for development. Cell 161, 1453–1467 (2015).
Skvortsova, K., Iovino, N. & Bogdanovic´, O. Functions and mechanisms of epigenetic inheritance in animals. Nat. Rev. Mol. Cell Biol. 19, 774–790 (2018).
Steger, K. & Balhorn, R. Sperm nuclear protamines: a checkpoint to control sperm chromatin quality. Anatom Histol. Embryol. 47, 273–279 (2018).
Hammoud, S. S. et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature 460, 473–478 (2009).
Inoue, A., Jiang, L., Lu, F., Suzuki, T. & Zhang, Y. Maternal H3K27me3 controls DNA methylation-independent imprinting. Nat. Rev. Genet. 547, 419–424 (2017).
Dahl, J. A. et al. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nat. Rev. Genet. 537, 548–552 (2016).
Peters, A. F. M. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 (2001).
Brykczynska, U. et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 17, 679–687 (2010).
Erkek, S. et al. Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat. Struct. Mol. Biol. 20, 868–875 (2013).
Duempelmann, L., Skribbe, M. & Buhler, M. Small RNAs in the transgenerational inheritance of epigenetic information. Trends Genet. 36, 203–214 (2020).
Zhang, Y., Shi, J., Rassoulzadegan, M., Tuorto, F. & Chen, Q. Sperm RNA code programmes the metabolic health of offspring. Nat. Rev. Endocrinol. 15, 489–498 (2019).
Gapp, K. et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17, 667–669 (2014).
Chen, Q. et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016).
Zhang, Y. et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat. Cell Biol. 20, 535–540 (2018).
Sharma, U. et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351, 391–396 (2016).
Rodgers, A. B., Morgan, C. P., Leu, N. A. & Bale, T. L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl Acad. Sci. USA 112, 13699–13704 (2015).
Jonkhout, N. et al. The RNA modification landscape in human disease. RNA 23, 1754–1769 (2017).
Mannerås-Holm, L. et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J. Clin. Endocrinol. Metab. 96, E304–E311 (2011).
Kokosar, M. et al. Epigenetic and transcriptional alterations in human adipose tissue of polycystic ovary syndrome. Sci. Rep. 6, 22883 (2016).
Jones, M. R. et al. Systems genetics reveals the functional context of PCOS loci and identifies genetic and molecular mechanisms of disease heterogeneity. PLoS Genet. 11, e1005455 (2015).
Dahlman, I. et al. The fat cell epigenetic signature in post-obese women is characterized by global hypomethylation and differential DNA methylation of adipogenesis genes. Int. J. Obes. 39, 910–919 (2015).
Kokosar, M. et al. A single bout of electroacupuncture remodels epigenetic and transcriptional changes in adipose tissue in polycystic ovary syndrome. Sci. Rep. 8, 1878 (2018).
Carbone, L. et al. Synergistic effects of hyperandrogenemia and obesogenic western-style diet on transcription and DNA methylation in visceral adipose tissue of nonhuman primates. Sci. Rep. 9, 19232 (2019).
Xu, N., Azziz, R. & Goodarzi, M. O. Epigenetics in polycystic ovary syndrome: a pilot study of global DNA methylation. Fertil. Steril. 94, 781–783 (2010).
Chen, Y.-H. et al. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 62, 2278–2286 (2013).
Wu, H.-L. et al. The expression of the miR-25/93/106b family of micro-RNAs in the adipose tissue of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 99, E2754–E2761 (2014).
Nilsson, E. et al. Transcriptional and epigenetic changes influencing skeletal muscle metabolism in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 103, 4465–4477 (2018).
Benrick, A. et al. Electroacupuncture mimics exercise-induced changes in skeletal muscle gene expression in polycystic ovary syndrome women. J. Clin. Endocrinol. Metab. 105, 2027–2041 (2020).
Yu, Y. Y. et al. Genome-wide screen of ovary-specific DNA methylation in polycystic ovary syndrome. Fertil. Steril. 104, 145–153 (2015).
Wang, X. X. et al. Genome-wide DNA methylation and gene expression patterns provide insight into polycystic ovary syndrome development. Oncotarget 5, 6603–6610 (2014).
Yu, Y. Y. et al. Promoter methylation of CYP19A1 gene in Chinese polycystic ovary syndrome patients. Gynecol. Obstet. Invest. 76, 209–213 (2013).
Qu, F. et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. 90, 911–923 (2012).
Wang, P. et al. Hypomethylation of the LH/choriogonadotropin receptor promoter region is a potential mechanism underlying susceptibility to polycystic ovary syndrome. Endocrinology 155, 1445–1452 (2014).
Sagvekar, P., Mangoli, V., Desai, S., Patil, A. & Mukherjee, S. LINE1 CpG-DNA hypomethylation in granulosa cells and blood leukocytes is associated with PCOS and related traits. J. Clin. Endocrinol. Metab. 102, 1396–1405 (2017).
Xu, J. et al. Comprehensive analysis of genome-wide DNA methylation across human polycystic ovary syndrome ovary granulosa cell. Oncotarget 7, 27899–27909 (2016).
Sagvekar, P., Kumar, P., Mangoli, V., Desai, S. & Mukherjee, S. DNA methylome profiling of granulosa cells reveals altered methylation in genes regulating vital ovarian functions in polycystic ovary syndrome. Clin. Epigenet. 11, 61 (2019).
Imbar, T. & Eisenberg, I. Regulatory role of microRNAs in ovarian function. Fertil. Steril. 101, 1524–1530 (2014).
Nagaraja, A. K. et al. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol. Endocrinol. 22, 2336–2352 (2008).
Lei, L., Jin, S., Gonzalez, G., Behringer, R. R. & Woodruff, T. K. The regulatory role of Dicer in folliculogenesis in mice. Mol. Cell Endocrinol. 315, 63–73 (2010).
Naji, M. et al. Expression of miR-15a, miR-145, and miR-182 in granulosa-lutein cells, follicular fluid, and serum of women with polycystic ovary syndrome (PCOS). Arch. Gynecol. Obstet. 297, 221–231 (2018).
Naji, M. et al. Differential expression of miR-93 and miR-21 in granulosa cells and follicular fluid of polycystic ovary syndrome associating with different phenotypes. Sci. Rep. 7, 14671 (2017).
Wang, M. et al. MicroRNA-27a-3p affects estradiol and androgen imbalance by targeting Creb1 in the granulosa cells in mouse polycytic ovary syndrome model. Reprod. Biol. 17, 295–304 (2017).
Barker, D. J. The fetal and infant origins of adult disease. BMJ 301, 1111 (1990).
de Rooij, S. R., Wouters, H., Yonker, J. E., Painter, R. C. & Roseboom, T. J. Prenatal undernutrition and cognitive function in late adulthood. Proc. Natl Acad. Sci. USA 107, 16881–16886 (2010).
Roseboom, T., de Rooij, S. & Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 82, 485–491 (2006).
Schulz, L. C. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl Acad. Sci. USA 107, 16757–16758 (2010).
Bygren, L. O. et al. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet. 15, 12 (2014).
Pembrey, M., Saffery, R. & Bygren, L. O., Network in Epigenetic Epidemiology & Network in Epigenetic Epidemiology. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J. Med. Genet. 51, 563–572 (2014).
Hanson, M. A. & Gluckman, P. D. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol. Rev. 94, 1027–1076 (2014).
Cesta, C. E. et al. Maternal polycystic ovary syndrome and risk of neuropsychiatric disorders in offspring: prenatal androgen exposure or genetic confounding? Psychol. Med. 50, 616–624 (2019).
Crisosto, N. et al. Higher luteinizing hormone levels associated with antimullerian hormone in postmenarchal daughters of women with polycystic ovary syndrome. Fertil. Steril. 111, 381–388 (2019).
Crisosto, N. et al. Reproductive and metabolic features during puberty in sons of women with polycystic ovary syndrome. Endocr. Connect. 6, 607–613 (2017).
Torchen, L. C. et al. Increased antimullerian hormone levels and other reproductive endocrine changes in adult male relatives of women with polycystic ovary syndrome. Fertil. Steril. 106, 50–55 (2016).
Kent, J. et al. Gestational weight gain in women with polycystic ovary syndrome: a controlled study. J. Clin. Endocrinol. Metab. 103, 4315–4323 (2018).
Tata, B. et al. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 24, 834–846 (2018).
Barrett, E. S. et al. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J. Dev. Orig. Health Dis. 9, 307–314 (2018).
Sir-Petermann, T. et al. Increased anti-Mullerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 91, 3105–3109 (2006).
Detti, L. et al. Serum anti-mullerian hormone (AMH) in mothers with polycystic ovary syndrome (PCOS) and their term fetuses. Syst. Biol. Reprod. Med. 65, 147–154 (2019).
Manti, M. et al. Maternal androgen excess induces cardiac hypertrophy and left ventricular dysfunction in female mice offspring. Cardiovasc Res. 116, 619–632 (2019).
Hu, M. et al. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc. Natl Acad. Sci. USA 112, 14348–14353 (2015).
Rusche, L. N., Kirchmaier, A. L. & Rine, J. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Ann. Rev. Biochem. 72, 481–516 (2003).
Quadrana, L. & Colot, V. Plant transgenerational epigenetics. Ann. Rev. Genet. 50, 467–491 (2016).
Serobyan, V. & Sommer, R. J. Developmental systems of plasticity and trans-generational epigenetic inheritance in nematodes. Curr. Opin. Genet. Dev. 45, 51–57 (2017).
Morgan, H. D., Sutherland, H. G. E., Martin, D. I. K. & Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 23, 314–318 (1999).
Saitou, M., Barton, S. C. & Surani, M. A. A molecular programme for the specification of germ cell fate in mice. Nature 418, 293–300 (2002).
Ohinata, Y. et al. A signaling principle for the specification of the germ cell lineage in mice. Cell 137, 571–584 (2009).
Hill, P. W. S. et al. Epigenetic reprogramming enables the transition from primordial germ cell to gonocyte. Nature 555, 392–396 (2018).
Sasaki, H. & Matsui, Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat. Rev. Genet. 9, 129–140 (2008).
Hargan-Calvopina, J. et al. Stage-specific demethylation in primordial germ cells safeguards against precocious differentiation. Dev. Cell 39, 75–86 (2016).
Gkountela, S. et al. DNA demethylation dynamics in the human prenatal germline. Cell 161, 1425–1436 (2015).
Skvortsova, K., Iovino, N. & Bogdanovic, O. Functions and mechanisms of epigenetic inheritance in animals. Nat. Rev. Mol. Cell Biol. 19, 774–790 (2018).
Ginno, P. A. et al. A genome-scale map of DNA methylation turnover identifies site-specific dependencies of DNMT and TET activity. Nat. Commun. 11, 2680 (2020).
Heard, E. & Martienssen, R. A. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157, 95–109 (2014).
Radford, E. J. et al. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 345, 1255903 (2014).
Tobi, E. W. et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat. Commun. 5, 5592 (2014).
Painter, R. C. et al. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 115, 1243–1249 (2008).
Veenendaal, M. et al. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG 120, 548–554 (2013).
Perez, M. F. & Lehner, B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 21, 143–151 (2019).
Chen, Q., Yan, W. & Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 17, 733–743 (2016).
Nilsson, E. E., Sadler-Riggleman, I. & Skinner, M. K. Environmentally induced epigenetic transgenerational inheritance of disease. Env. Epigenet. 4, dvy016 (2018).
Skinner, M. K. Endocrine disruptors in 2015: epigenetic transgenerational inheritance. Nat. Rev. Endocrinol. 12, 68–70 (2016).
Kaspar, D., Hastreiter, S., Irmler, M., de Angelis, M. H. & Beckers, J. Nutrition and its role in epigenetic inheritance of obesity and diabetes across generations. Mamm. Genome 2, 1–15 (2020).
Bohacek, J. & Mansuy, I. M. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat. Rev. Genet. 16, 641–652 (2015).
Mimouni, N. E. H. et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 33, 513–530 (2021).
Stener-Victorin, E. et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr. Rev. 41, 538–576 (2020).
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 (2008).
Acknowledgements
The authors’ research is supported by the Swedish Medical Research Council (Project No. 2018-02435: E.S.V., 2018-02557: Q.D.), Novo Nordisk Foundation (NNF19OC0056647: E.S.V.), the Strategic Research Programme in Diabetes at Karolinska Institutet (E.S.V.), Adlerbert Research Foundation (E.S.V.), Åke Wibergs Stiftelse (Q.D.) and faculty funding at Karolinska Institutet (Q.D.).
Author information
Authors and Affiliations
Contributions
E.S.V. and Q.D. contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks E. Diamanti-Kandarakis, H. Teede, who co-reviewed with A. Johan, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Mendelian randomization analyses
-
A genetic approach that determines the causal effects of putative risk factors in disease by using genetic variants as instrumental variables to infer whether a risk factor causally affects a health outcome.
Rights and permissions
About this article
Cite this article
Stener-Victorin, E., Deng, Q. Epigenetic inheritance of polycystic ovary syndrome — challenges and opportunities for treatment. Nat Rev Endocrinol 17, 521–533 (2021). https://doi.org/10.1038/s41574-021-00517-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-021-00517-x
This article is cited by
-
High-fat and high-sucrose diet impairs female reproduction by altering ovarian transcriptomic and metabolic signatures
Journal of Translational Medicine (2024)
-
Polycystic ovary syndrome
Nature Reviews Disease Primers (2024)
-
Role of circular RNA/miRNA axes in the pathophysiology of polycystic ovary syndrome
Molecular Biology Reports (2024)
-
Modulation of the RAC1/MAPK/ERK signalling pathway by farnesyl diphosphate synthase regulates granulosa cells proliferation in polycystic ovary syndrome
Human Cell (2024)
-
Effects and mechanisms of acupuncture on women related health
Frontiers of Medicine (2024)