Abstract
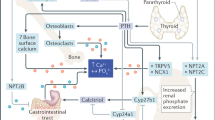
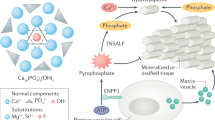
The major mineralized tissues are bone and teeth, which share several mechanisms governing their development and mineralization. This crossover includes the hormones that regulate circulating calcium and phosphate concentrations, and the genes that regulate the differentiation and transdifferentiation of cells. In developing endochondral bone and in developing teeth, parathyroid hormone-related protein (PTHrP) acts in chondrocytes to delay terminal differentiation, thereby increasing the pool of precursor cells. Chondrocytes and (in specific circumstances) pre-odontoblasts can also transdifferentiate into osteoblasts. Moreover, bone and teeth share outcomes when affected by systemic disorders of mineral homeostasis or of the extracellular matrix, and by adverse effects of treatments such as bisphosphonates and fluoride. Unlike bone, teeth have more permanent effects from systemic disorders because they are not remodelled after they are formed. This Review discusses the normal processes of bone and tooth development, followed by disorders that have effects on both bone and teeth, versus disorders that have effects in one without affecting the other. The takeaway message is that bone specialists should know when to screen for dental disorders, just as dental specialists should recognize when a tooth disorder should raise suspicions about a possible underlying bone disorder.
Key points
-
Bone and teeth, the major mineralized tissues, are regulated by many of the same genes and hormones.
-
Parathyroid hormone-related protein acts to delay terminal differentiation in chondrocytes of developing endochondral bone and in developing teeth.
-
Bone and teeth share fates when affected by systemic disorders of mineral homeostasis or of the extracellular matrix.
-
Teeth are not remodelled after they are formed, and so effects of systemic disorders are permanent, whereas bone remodelling can restore the skeleton.
-
Bone specialists and dental specialists should recognize when a disorder of one of the mineralized tissues should raise awareness of a disorder of the other.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
MacDougall, M. J. & Javed, A. in Bone and Development Ch. 11 (eds Bronner, F., Farach-Carson, M. C. & Roach, H. I.) 183–200 (Springer, 2010).
Opsahl Vital, S. et al. Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone 50, 989–997 (2012).
Roschger, P., Misof, B., Paschalis, E., Fratzl, P. & Klaushofer, K. Changes in the degree of mineralization with osteoporosis and its treatment. Curr. Osteoporos. Rep. 12, 338–350 (2014).
Berendsen, A. D. & Olsen, B. R. Bone development. Bone 80, 14–18 (2015).
Karsenty, G., Kronenberg, H. M. & Settembre, C. Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 25, 629–648 (2009).
Caplan, A. I. & Pechak, D. G. in Bone and Mineral Research (ed. Peck, W. A.) 117–183 (Elsevier, 1987).
Horton, W. A. in Extracellular Matrix and Heritable Disorders of Connective Tissue (eds Royce, P. M. & Steinman, B.) 73–84 (Alan R. Liss, 1993).
Linsenmayer, T. F. et al. Collagen types IX and X in the developing chick tibiotarsus: analyses of mRNAs and proteins. Development 111, 191–196 (1991).
Poole, A. R. in Cartilage: Molecular Aspects (eds Hall, B. K. & Newman, S. A.) 179–211 (CRC, 1991).
Ducy, P. et al. Increased bone formation in osteocalcin-deficient mice. Nature 382, 448–452 (1996).
Wolff, L. I. & Hartmann, C. A second career for chondrocytes–transformation into osteoblasts. Curr. Osteoporos. Rep. 17, 129–137 (2019).
Jahn, K. et al. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. J. Bone Min. Res. 32, 1761–1772 (2017).
Hemmatian, H., Bakker, A. D., Klein-Nulend, J. & van Lenthe, G. H. Aging, osteocytes, and mechanotransduction. Curr. Osteoporos. Rep. 15, 401–411 (2017).
Sabbagh, Y., Carpenter, T. O. & Demay, M. B. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc. Natl Acad. Sci. USA 102, 9637–9642 (2005).
Yang, Y. in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism 9th edn (ed. Bilezikian, J. P.) 3–11 (Wiley Blackwell, 2019).
Karner, C. M. & Hilton, M. J. in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism 9th edn (ed. Bilezikian, J. P.) 12–19 (Wiley Blackwell, 2019).
Lee, K. et al. In situ localization of PTH/PTHrP receptor mRNA in the bone of fetal and young rats. Bone 14, 341–345 (1993).
Lee, K., Deeds, J. D. & Segre, G. V. Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. Endocrinology 136, 453–463 (1995).
Lanske, B. et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273, 663–666 (1996).
Vortkamp, A. et al. Indian hedgehog and parathyroid hormone-related protein regulate the rate of cartilage differentiation. Science 273, 613–622 (1996).
Karaplis, A. C. et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8, 277–289 (1994).
Karaplis, A. C. et al. Inactivating mutation in the human parathyroid hormone receptor type 1 gene in Blomstrand chondrodysplasia. Endocrinology 139, 5255–5258 (1998).
Oostra, R. J. et al. Blomstrand osteochondrodysplasia: three novel cases and histological evidence for heterogeneity. Virchows Arch. 436, 28–35 (2000).
Miao, D., He, B., Karaplis, A. C. & Goltzman, D. Parathyroid hormone is essential for normal fetal bone formation. J. Clin. Invest. 109, 1173–1182 (2002).
Weir, E. C. et al. Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc. Natl Acad. Sci. USA 93, 10240–10245 (1996).
Schipani, E. et al. Targeted expression of constitutively active PTH/PTHrP receptors delays endochondral bone formation and rescues PTHrP-less mice. Proc. Natl Acad. Sci. USA 94, 13689–13694 (1997).
Kovacs, C. S. et al. Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc. Natl Acad. Sci. USA 93, 15233–15238 (1996).
Kovacs, C. S., Chafe, L. L., Fudge, N. J., Friel, J. K. & Manley, N. R. PTH regulates fetal blood calcium and skeletal mineralization independently of PTHrP. Endocrinology 142, 4983–4993 (2001).
Amizuka, N. et al. Haploinsufficiency of parathyroid hormone-related peptide (PTHrP) results in abnormal postnatal bone development. Dev. Biol. 175, 166–176 (1996).
Miao, D. et al. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J. Clin. Invest. 115, 2402–2411 (2005).
Miao, D. et al. Parathyroid hormone-related peptide is required for increased trabecular bone volume in parathyroid hormone-null mice. Endocrinology 145, 3554–3562 (2004).
Kovacs, C. S., Manley, N. R., Moseley, J. M., Martin, T. J. & Kronenberg, H. M. Fetal parathyroids are not required to maintain placental calcium transport. J. Clin. Invest. 107, 1007–1015 (2001).
Simmonds, C. S., Karsenty, G., Karaplis, A. C. & Kovacs, C. S. Parathyroid hormone regulates fetal–placental mineral homeostasis. J. Bone Min. Res. 25, 594–605 (2010).
Kovacs, C. S. Bone development and mineral homeostasis in the fetus and neonate: roles of the calciotropic and phosphotropic hormones. Physiol. Rev. 94, 1143–1218 (2014).
Halloran, B. P. & De Luca, H. F. Effect of vitamin D deficiency on skeletal development during early growth in the rat. Arch. Biochem. Biophys. 209, 7–14 (1981).
Miller, S. C., Halloran, B. P., DeLuca, H. F. & Jee, W. S. Studies on the role of vitamin D in early skeletal development, mineralization, and growth in rats. Calcif. Tissue Int. 35, 455–460 (1983).
Brommage, R. & DeLuca, H. F. Placental transport of calcium and phosphorus is not regulated by vitamin D. Am. J. Physiol. 246, F526–F529 (1984).
Glazier, J. D., Mawer, E. B. & Sibley, C. P. Calbindin-D9K gene expression in rat chorioallantoic placenta is not regulated by 1,25-dihydroxyvitamin D3. Pediatr. Res. 37, 720–725 (1995).
Kovacs, C. S., Woodland, M. L., Fudge, N. J. & Friel, J. K. The vitamin D receptor is not required for fetal mineral homeostasis or for the regulation of placental calcium transfer in mice. Am. J. Physiol. Endocrinol. Metab. 289, E133–E144 (2005).
Lieben, L., Stockmans, I., Moermans, K. & Carmeliet, G. Maternal hypervitaminosis D reduces fetal bone mass and mineral acquisition and leads to neonatal lethality. Bone 57, 123–131 (2013).
Ryan, B. A. et al. Mineral homeostasis in murine fetuses is sensitive to maternal calcitriol but not to absence of fetal calcitriol. J. Bone Min. Res. 34, 669–680 (2019).
Ma, Y. et al. FGF23 is not required to regulate fetal phosphorus metabolism but exerts effects within 12 hours after birth. Endocrinology 158, 252–263 (2017).
Ma, Y. et al. Neither absence nor excess of FGF23 disturbs murine fetal–placental phosphorus homeostasis or prenatal skeletal development and mineralization. Endocrinology 155, 1596–1605 (2014).
Rebut-Bonneton, C., Garel, J. M. & Delbarre, F. Parathyroid hormone, calcitonin, 1,25-dihydroxycholecalciferol, and basal bone resorption in the rat fetus. Calcif. Tissue Int. 35, 183–189 (1983).
Rebut-Bonneton, C., Demignon, J., Amor, B. & Miravet, L. Effect of calcitonin in pregnant rats on bone resorption in fetuses. J. Endocrinol. 99, 347–353 (1983).
Sinclair, J. G. Fetal rat parathyroids as affected by changes in maternal serum calcium and phosphorus through parathyroidectomy and dietary control. J. Nutr. 23, 141–152 (1942).
Garel, J. M. & Geloso-Meyer, A. Fetal hyperparathyroidism in rats following maternal hypoparathyroidism [French]. Rev. Eur. Etud. Clin. Biol. 16, 174–178 (1971).
Chalon, S. & Garel, J. M. Plasma calcium control in the rat fetus. I. Influence of maternal hormones. Biol. Neonate 48, 313–322 (1985).
Kovacs, C. S. in Pediatric Bone: Biology and Diseases 2nd edn (eds Glorieux, F. H., Pettifor, J. M., & Jüppner, H.) 247–275 (Elsevier, 2011).
Kovacs, C. S. et al. Regulation of murine fetal–placental calcium metabolism by the calcium-sensing receptor. J. Clin. Invest. 101, 2812–2820 (1998).
Shin, H. I. et al. Gp130-mediated signaling is necessary for normal osteoblastic function in vivo and in vitro. Endocrinology 145, 1376–1385 (2004).
Almonaitiene, R., Balciuniene, I. & Tutkuviene, J. Factors influencing permanent teeth eruption. Part one–general factors. Stomatologija 12, 67–72 (2010).
Balic, A. & Thesleff, I. Tissue interactions regulating tooth development and renewal. Curr. Top. Dev. Biol. 115, 157–186 (2015).
Jernvall, J. & Thesleff, I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 92, 19–29 (2000).
Thesleff, I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 116, 1647–1648 (2003).
Chai, Y. et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671–1679 (2000).
Balic, A. Concise review: cellular and molecular mechanisms regulation of tooth initiation. Stem Cell 37, 26–32 (2019).
McGonnell, I. M. & Graham, A. Trunk neural crest has skeletogenic potential. Curr. Biol. 12, 767–771 (2002).
Yu, T. & Klein, O. D. Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development 147, dev184754 (2020).
Pandya, M. et al. Posttranslational amelogenin processing and changes in matrix assembly during enamel development. Front. Physiol. 8, 790 (2017).
Lu, Y. et al. Functions of KLK4 and MMP-20 in dental enamel formation. Biol. Chem. 389, 695–700 (2008).
Lacruz, R. S., Habelitz, S., Wright, J. T. & Paine, M. L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 97, 939–993 (2017).
Du, C., Falini, G., Fermani, S., Abbott, C. & Moradian-Oldak, J. Supramolecular assembly of amelogenin nanospheres into birefringent microribbons. Science 307, 1450–1454 (2005).
Dunglas, C. et al. Ultrastructure of forming enamel in mouse bearing a transgene that disrupts the amelogenin self-assembly domains. Calcif. Tissue Int. 71, 155–166 (2002).
Kawashima, N. & Okiji, T. Odontoblasts: specialized hard-tissue-forming cells in the dentin–pulp complex. Congenit. Anom. 56, 144–153 (2016).
Miletich, I. & Sharpe, P. T. Normal and abnormal dental development. Hum. Mol. Genet. 12 (Suppl. 1), R69–R73 (2003).
Goldberg, M., Kulkarni, A. B., Young, M. & Boskey, A. Dentin: structure, composition and mineralization. Front. Biosci. 3, 711–735 (2011).
Kitahara, Y. et al. Disturbed tooth development in parathyroid hormone-related protein (PTHrP)-gene knockout mice. Bone 30, 48–56 (2002).
Gonçalves, P. F. et al. Dental cementum reviewed: development, structure, composition, regeneration and potential functions. Braz. J. Oral. Sci. 4, 651–658 (2015).
Philbrick, W. M., Dreyer, B. E., Nakchbandi, I. A. & Karaplis, A. C. Parathyroid hormone-related protein is required for tooth eruption. Proc. Natl Acad. Sci. USA 95, 11846–11851 (1998).
Ono, W., Sakagami, N., Nishimori, S., Ono, N. & Kronenberg, H. M. Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat. Commun. 7, 11277 (2016).
Takahashi, A. et al. Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proc. Natl Acad. Sci. USA 116, 575–580 (2019).
Yang, M. et al. Chemokine and chemokine receptor expression during colony stimulating factor-1-induced osteoclast differentiation in the toothless osteopetrotic rat: a key role for CCL9 (MIP-1γ) in osteoclastogenesis in vivo and in vitro. Blood 107, 2262–2270 (2006).
Wise, G. E., Que, B. G., Huang, H. & Lumpkin, S. J. Enhancement of gene expression in rat dental follicle cells by parathyroid hormone-related protein. Arch. Oral. Biol. 45, 903–909 (2000).
Pilz, P. et al. Differential diagnosis of primary failure of eruption (PFE) with and without evidence of pathogenic mutations in the PTHR1 gene. J. Orofac. Orthop. 75, 226–239 (2014).
Izumida, E. et al. Functional analysis of PTH1R variants found in primary failure of eruption. J. Dent. Res. 99, 429–436 (2020).
Komori, T. Regulation of osteoblast and odontoblast differentiation by RUNX2. J. Oral. Biosci. 52, 22–25 (2010).
Miyazaki, T. et al. Inhibition of the terminal differentiation of odontoblasts and their transdifferentiation into osteoblasts in Runx2 transgenic mice. Arch. Histol. Cytol. 71, 131–146 (2008).
Merrell, A. J. & Stanger, B. Z. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 17, 413–425 (2016).
Cao, Y. et al. Pulp-dentin regeneration: current state and future prospects. J. Dent. Res. 94, 1544–1551 (2015).
Vijaykumar, A., Dyrkacz, P., Vidovic-Zdrilic, I., Maye, P. & Mina, M. Expression of BSP-GFPtpz transgene during osteogenesis and reparative dentinogenesis. J. Dent. Res. 99, 89–97 (2020).
Kovacs, C. S. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol. Rev. 96, 449–547 (2016).
Mortier, G. R. et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am. J. Med. Genet. A 179, 2393–2419 (2019).
McKee, M. D. et al. Extracellular matrix mineralization in periodontal tissues: noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia. Periodontol. 63, 102–122 (2013).
Bacchetta, J., Bardet, C. & Prié, D. Physiology of FGF23 and overview of genetic diseases associated with renal phosphate wasting. Metabolism 103s, 153865 (2020).
Beck-Nielsen, S. S. et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J. Rare Dis. 14, 58 (2019).
Boukpessi, T. et al. Abnormal presence of the matrix extracellular phosphoglycoprotein-derived acidic serine- and aspartate-rich motif peptide in human hypophosphatemic dentin. Am. J. Pathol. 177, 803–812 (2010).
Boukpessi, T. et al. Osteopontin and the dento-osseous pathobiology of X-linked hypophosphatemia. Bone 95, 151–161 (2017).
Chaussain-Miller, C. et al. Dental abnormalities in patients with familial hypophosphatemic vitamin D-resistant rickets: prevention by early treatment with 1-hydroxyvitamin D. J. Pediatr. 142, 324–331 (2003).
Linglart, A. et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr. Connect. 3, R13–R30 (2014).
Biosse Duplan, M. et al. Phosphate and vitamin D prevent periodontitis in X-linked hypophosphatemia. J. Dent. Res. 96, 388–395 (2017).
Thumbigere-Math, V. et al. Hypercementosis associated with ENPP1 mutations and GACI. J. Dent. Res. 97, 432–441 (2018).
Chakhtoura, M. et al. Hyperphosphatemic familial tumoral calcinosis secondary to fibroblast growth factor 23 (FGF23) mutation: a report of two affected families and review of the literature. Osteoporos. Int. 29, 1987–2009 (2018).
Le Norcy, E. et al. Dental and craniofacial features associated with GNAS loss of function mutations. Eur. J. Orthod. 42, 525–533 (2020).
Reibel, A. et al. Orodental phenotype and genotype findings in all subtypes of hypophosphatasia. Orphanet J. Rare Dis. 4, 6 (2009).
Foster, B. L. et al. Rare bone diseases and their dental, oral, and craniofacial manifestations. J. Dent. Res. 93, 7s–19s (2014).
Linglart, A. & Biosse-Duplan, M. Hypophosphatasia. Curr. Osteoporos. Rep. 14, 95–105 (2016).
Smith, C. E. L. et al. Amelogenesis imperfecta; genes, proteins, and pathways. Front. Physiol. 8, 435 (2017).
Soliman, A. T., Ramadan, M. A., Sherif, A., Aziz Bedair, E. S. & Rizk, M. M. Pycnodysostosis: clinical, radiologic, and endocrine evaluation and linear growth after growth hormone therapy. Metabolism 50, 905–911 (2001).
Prokop, J. W. et al. Genome sequencing in the clinic: the past, present, and future of genomic medicine. Physiol. Genomics 50, 563–579 (2018).
Dallas, S. L., Xie, Y., Shiflett, L. A. & Ueki, Y. Mouse Cre models for the study of bone diseases. Curr. Osteoporos. Rep. 16, 466–477 (2018).
Klein, O. D. et al. Meeting report: a hard look at the state of enamel research. Int. J. Oral. Sci. 9, e3 (2017).
Carnovali, M., Banfi, G. & Mariotti, M. Zebrafish models of human skeletal disorders: embryo and adult swimming together. BioMed. Res. Int. 2019, 1253710 (2019).
Bruneel, B. et al. Imaging the zebrafish dentition: from traditional approaches to emerging technologies. Zebrafish 12, 1–10 (2015).
Foster, B. L., Nociti, F. H. Jr. & Somerman, M. J. The rachitic tooth. Endocr. Rev. 35, 1–34 (2014).
Houari, S., Loiodice, S., Jedeon, K., Berdal, A. & Babajko, S. Expression of steroid receptors in ameloblasts during amelogenesis in rat incisors. Front. Physiol. 7, 503 (2016).
Kovacs, C. S. in Genetics of Bone Biology and Skeletal Disease 2nd edn (eds Thakker, R. V., Whyte, M. P., Eisman, J. A., & Igarashi, T.) 329–347 (Elsevier, 2017).
Nakamura, T. et al. Transcription factor epiprofin is essential for tooth morphogenesis by regulating epithelial cell fate and tooth number. J. Biol. Chem. 283, 4825–4833 (2008).
Li, J., Parada, C. & Chai, Y. Cellular and molecular mechanisms of tooth root development. Development 144, 374–384 (2017).
Ramanathan, A., Srijaya, T. C., Sukumaran, P., Zain, R. B. & Abu Kasim, N. H. Homeobox genes and tooth development: understanding the biological pathways and applications in regenerative dental science. Arch. Oral. Biol. 85, 23–39 (2018).
Butler, W. T., Brunn, J. C. & Qin, C. Dentin extracellular matrix (ECM) proteins: comparison to bone ECM and contribution to dynamics of dentinogenesis. Connect. Tissue Res. 44 (Suppl 1), 171–178 (2003).
McKee, M. D. et al. Compounded PHOSPHO1/ALPL deficiencies reduce dentin mineralization. J. Dent. Res. 92, 721–727 (2013).
Qin, C., Baba, O. & Butler, W. T. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit. Rev. Oral. Biol. Med. 15, 126–136 (2004).
Fisher, L. W. & Fedarko, N. S. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect. Tissue Res. 44 (Suppl 1), 33–40 (2003).
Kida, M., Tsutsumi, T., Shindoh, M., Ikeda, H. & Ariga, T. De novo mutation in the DSPP gene associated with dentinogenesis imperfecta type II in a Japanese family. Eur. J. Oral. Sci. 117, 691–694 (2009).
Liang, T. et al. Mutant dentin sialophosphoprotein causes dentinogenesis imperfecta. J. Dent. Res. 98, 912–919 (2019).
Sarnat, B. G. Differential growth and healing of bones and teeth. Clin. Orthop. Relat. Res. 183, 219–237 (1984).
Kato, A., Suzuki, M., Karasawa, Y., Sugimoto, T. & Doi, K. PTHrP and PTH/PTHrP receptor 1 expression in odontogenic cells of normal and HHM model rat incisors. Toxicol. Pathol. 33, 456–464 (2005).
Zhang, X., Rahemtulla, F., Zhang, P., Beck, P. & Thomas, H. F. Different enamel and dentin mineralization observed in VDR deficient mouse model. Arch. Oral. Biol. 54, 299–305 (2009).
Zhang, X., Rahemtulla, F. G., MacDougall, M. J. & Thomas, H. F. Vitamin D receptor deficiency affects dentin maturation in mice. Arch. Oral. Biol. 52, 1172–1179 (2007).
Guimaraes, G. N. et al. Evaluation of the effects of transient or continuous PTH administration to odontoblast-like cells. Arch. Oral. Biol. 58, 638–645 (2013).
Liu, J. G. et al. Developmental role of PTHrP in murine molars. Eur. J. Oral. Sci. 106 (Suppl 1), 143–146 (1998).
Ouyang, H. et al. Parathyroid hormone-related protein regulates extracellular matrix gene expression in cementoblasts and inhibits cementoblast-mediated mineralization in vitro. J. Bone Min. Res. 15, 2140–2153 (2000).
Calvi, L. M. et al. Constitutively active PTH/PTHrP receptor in odontoblasts alters odontoblast and ameloblast function and maturation. Mech. Dev. 121, 397–408 (2004).
Acknowledgements
The authors are supported by Canadian Institutes of Health Research (C.S.K.); Fedération Hospitalo-Universitaire DDS-Paris Net and ANR Hyposkel (C.C.); Fondazione Italiana Ricerca sulle Malattie dell’Osso (M.L.B.); Wellcome Trust Investigator Award (R.V.T.); Wellcome Trust Investigator Award (R.V.T.); National Institute for Health Research (NIHR) Senior Investigator Award (R.V.T.); and NIHR Oxford Biomedical Research Centre Programme (R.V.T.). This article arose out of discussions held at a conference on Biomineralisation in Health and Disease held in Florence, Italy, in 2019 and supported by the Menarini Foundation.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks M. Somerman, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Dentin
-
A tissue produced by odontoblasts, which are derived from the neural crest. Under the enamel, dentin is similar to bone but is never remodelled under physiological conditions. Dentin surrounds the central (pulp) chamber, which contains odontoblast bodies at its periphery, and mainly consists of connective tissue, blood vessels and nerves.
- Cementum
-
A tissue produced by cementoblasts, which develop from neural crest-derived mesenchymal cells from the connective tissue of the dental follicle. Cementum is a thin layer of hard dental tissue covering the anatomic roots of the teeth.
- Enamel
-
The hardest material of the organism produced biologically by ameloblasts. It is derived from the epithelium and forms the anatomical crown of the teeth.
Rights and permissions
About this article
Cite this article
Kovacs, C.S., Chaussain, C., Osdoby, P. et al. The role of biomineralization in disorders of skeletal development and tooth formation. Nat Rev Endocrinol 17, 336–349 (2021). https://doi.org/10.1038/s41574-021-00488-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-021-00488-z
This article is cited by
-
Biomineralized synthesis of luminescent protease-(NH4)2Y3F11•H2O hybrid nanospheres and their applications as a stable and reusable enzyme reactor
Collagen and Leather (2024)
-
The role of vitamin D receptor in predentin mineralization and dental repair after injury
Cell and Tissue Research (2024)
-
Research progress of biomimetic materials in oral medicine
Journal of Biological Engineering (2023)
-
Altered Signaling and Desensitization Responses in PTH1R Mutants Associated with Eiken Syndrome
Communications Biology (2023)
-
HERC1 deficiency causes osteopenia through transcriptional program dysregulation during bone remodeling
Cell Death & Disease (2023)