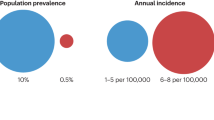
Abstract
Multiple endocrine neoplasia type 1 (MEN1) is a rare syndrome characterized by the co-occurrence of primary hyperparathyroidism, duodenopancreatic neuroendocrine tumours (NETs) and/or pituitary adenomas. MEN1 can predispose patients to other endocrine and non-endocrine tumours, such as cutaneous tumours, central nervous system tumours and breast cancer. Endocrine tumours in patients with MEN1 differ from sporadic tumours in that they have a younger age at onset, present as multiple tumours in the same organ and have a different clinical course. Therefore, patients with overt MEN1 and those who carry a MEN1 mutation should be offered tailored biochemical and imaging screening to detect tumours and evaluate their progression over time. Fortunately, over the past 10 years, knowledge about the clinical phenotype of these tumours has markedly progressed, thanks to the implementation of national registries, particularly in France and the Netherlands. This Review provides an update on the clinical management of MEN1-related tumours. Epidemiology, the clinical picture, diagnostic work-up and the main lines of treatment for MEN1-related tumours are summarized. Controversial therapeutic aspects and issues that still need to be addressed are also discussed. Moreover, special attention is given to MEN1 manifestations in children and adolescents.
Key points
-
MEN1 is a rare genetic disorder that predisposes patients to primary hyperparathyroidism, duodenopancreatic neuroendocrine tumours and anterior pituitary tumours, as well as more than 20 endocrine and non-endocrine tumours.
-
Hyperparathyroidism is the most common manifestation of MEN1 and is associated with mild to moderate hypercalcaemia but considerably reduced bone mineral density.
-
Duodenopancreatic neuroendocrine tumours are usually small in size, and multiple tumours are almost always present; they represent the main cause of death in MEN1 owing to their malignant potential.
-
Non-functioning pancreatic neuroendocrine tumours ≤2 cm could be managed by active surveillance while surgery should be proposed for large tumours and patients who progress on serial imaging studies.
-
Thymic neuroendocrine tumours occur predominantly in male individuals with MEN1, are very aggressive tumours and severely impact the prognosis of patients with MEN1.
-
MEN1 negatively influences health-related quality of life, but more data are needed about this issue.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wermer, P. Genetic aspects of adenomatosis of endocrine glands. Am. J. Med. 16, 363–371 (1954).
Dreijerink, K. M. A., Goudet, P., Burgess, J. R., Valk, G. D. & International Breast Cancer in MEN1 Study Group. Breast-cancer predisposition in multiple endocrine neoplasia type 1. N. Engl. J. Med. 371, 583–584 (2014).
Thakker, R. V. et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J. Clin. Endocrinol. Metab. 97, 2990–3011 (2012).
Carney, J. A. Familial multiple endocrine neoplasia: the first 100 years. Am. J. Surg. Pathol. 29, 254–274 (2005).
Larsson, C., Skogseid, B., Oberg, K., Nakamura, Y. & Nordenskjöld, M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature 332, 85–87 (1988).
Chandrasekharappa, S. C. et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276, 404–407 (1997).
Lemmens, I. et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum. Mol. Genet. 6, 1177–1183 (1997).
Goudet, P. et al. Gender-related differences in MEN1 lesion occurrence and diagnosis: a cohort study of 734 cases from the Groupe d’etude des tumeurs endocrines. Eur. J. Endocrinol. 165, 97–105 (2011).
Sakurai, A. et al. Multiple endocrine neoplasia type 1 in Japan: establishment and analysis of a multicentre database. Clin. Endocrinol. 76, 533–539 (2012).
Giusti, F. et al. Multiple endocrine neoplasia syndrome type 1: institution, management, and data analysis of a nationwide multicenter patient database. Endocrine 58, 349–359 (2017).
Stratakis, C. A. et al. Pituitary macroadenoma in a 5-year-old: an early expression of multiple endocrine neoplasia type 1. J. Clin. Endocrinol. Metab. 85, 4776–4780 (2000).
Trump, D. et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1). QJM 89, 653–669 (1996).
Romanet, P. et al. UMD-MEN1 Database: an overview of the 370 MEN1 variants present in 1676 patients from the French population. J. Clin. Endocrinol. Metab. 104, 753–764 (2019).
Machens, A. et al. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin. Endocrinol. 67, 613–622 (2007).
Yamazaki, M. et al. Delay in the diagnosis of multiple endocrine neoplasia type 1: typical symptoms are frequently overlooked. Endocr. J. 59, 797–807 (2012).
Goudet, P. et al. A Multiple endocrine neoplasia type-1 observatory in a French-speaking area. A tool from the Endocrine Tumor study Group (GTE). Ann. Endocrinol. 68, 154–159 (2007).
Uchino, S. et al. Screening of the Men1 gene and discovery of germ-line and somatic mutations in apparently sporadic parathyroid tumors. Cancer Res. 60, 5553–5557 (2000).
Langer, P. et al. Prevalence of multiple endocrine neoplasia type 1 in young patients with apparently sporadic primary hyperparathyroidism or pancreaticoduodenal endocrine tumours. Br. J. Surg. 90, 1599–1603 (2003).
Skandarajah, A. et al. Should routine analysis of the MEN1 gene be performed in all patients with primary hyperparathyroidism under 40 years of age? World J. Surg. 34, 1294–1298 (2010).
Goudet, P. et al. Hyperparathyroidism in multiple endocrine neoplasia type I: surgical trends and results of a 256-patient series from Groupe D’etude des Néoplasies Endocriniennes Multiples Study Group. World J. Surg. 25, 886–890 (2001).
Goudet, P. et al. MEN1 disease occurring before 21 years old: a 160-patient cohort study from the Groupe d’étude des Tumeurs Endocrines. J. Clin. Endocrinol. Metab. 100, 1568–1577 (2015).
Twigt, B. A., Scholten, A., Valk, G. D., Rinkes, I. H. M. B. & Vriens, M. R. Differences between sporadic and MEN related primary hyperparathyroidism; clinical expression, preoperative workup, operative strategy and follow-up. Orphanet J. Rare Dis. 8, 50 (2013).
Eller-Vainicher, C. et al. Sporadic and MEN1-related primary hyperparathyroidism: differences in clinical expression and severity. J. Bone Miner. Res. 24, 1404–1410 (2009).
Lourenço, D. M. et al. Early-onset, progressive, frequent, extensive, and severe bone mineral and renal complications in multiple endocrine neoplasia type 1-associated primary hyperparathyroidism. J. Bone Miner. Res. 25, 2382–2391 (2010).
Burgess, J. R., David, R., Greenaway, T. M., Parameswaran, V. & Shepherd, J. J. Osteoporosis in multiple endocrine neoplasia type 1: severity, clinical significance, relationship to primary hyperparathyroidism, and response to parathyroidectomy. Arch. Surg. 134, 1119–1123 (1999).
Christakis, I. et al. Parathyroid carcinoma and atypical parathyroid neoplasms in MEN1 patients: a clinico-pathologic challenge. The MD Anderson case series and review of the literature. Int. J. Surg. 31, 10–16 (2016).
Singh Ospina, N., Sebo, T. J., Thompson, G. B., Clarke, B. L. & Young, W. F. Prevalence of parathyroid carcinoma in 348 patients with multiple endocrine neoplasia type 1 - case report and review of the literature. Clin. Endocrinol. 84, 244–249 (2016).
Norton, J. A. et al. Prospective study of surgery for primary hyperparathyroidism (HPT) in multiple endocrine neoplasia-type 1 and Zollinger–Ellison syndrome: long-term outcome of a more virulent form of HPT. Ann. Surg. 247, 501–510 (2008).
Marx, S. J. New concepts about familial isolated hyperparathyroidism. J. Clin. Endocrinol. Metab. 104, 4058–4066 (2019).
Lassen, T., Friis-Hansen, L., Rasmussen, A. K., Knigge, U. & Feldt-Rasmussen, U. Primary hyperparathyroidism in young people. When should we perform genetic testing for multiple endocrine neoplasia 1 (MEN-1)? J. Clin. Endocrinol. Metab. 99, 3983–3987 (2014).
Green, P. et al. High prevalence of chronic kidney disease in patients with multiple endocrine neoplasia type 1 and improved kidney function after parathyroidectomy. Surgery 165, 124–128 (2019).
Schreinemakers, J. M. J. et al. The optimal surgical treatment for primary hyperparathyroidism in MEN1 patients: a systematic review. World J. Surg. 35, 1993–2005 (2011).
Lairmore, T. C. et al. A randomized, prospective trial of operative treatments for hyperparathyroidism in patients with multiple endocrine neoplasia type 1. Surgery 156, 1326–1334 (2014).
Norton, J. A., Krampitz, G. & Jensen, R. T. Multiple endocrine neoplasia: genetics and clinical management. Surg. Oncol. Clin. N. Am. 24, 795–832 (2015).
Fyrsten, E., Norlén, O., Hessman, O., Stålberg, P. & Hellman, P. Long-term surveillance of treated hyperparathyroidism for multiple endocrine neoplasia type 1: recurrence or hypoparathyroidism? World J. Surg. 40, 615–621 (2016).
Horiuchi, K. et al. Impact of ‘tailored’ parathyroidectomy for treatment of primary hyperparathyroidism in patients with multiple endocrine neoplasia type 1. World J. Surg. 42, 1772–1778 (2018).
Tonelli, F., Marcucci, T., Giudici, F., Falchetti, A. & Brandi, M. L. Surgical approach in hereditary hyperparathyroidism. Endocr. J. 56, 827–841 (2009).
Iacobone, M., Carnaille, B., Palazzo, F. F. & Vriens, M. Hereditary hyperparathyroidism—a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch. Surg. 400, 867–886 (2015).
Kluijfhout, W. P. et al. Unilateral Clearance for primary hyperparathyroidism in selected patients with multiple endocrine neoplasia type 1. World J. Surg. 40, 2964–2969 (2016).
Manoharan, J. et al. Single gland excision for MEN1-associated primary hyperparathyroidism. Clin. Endocrinol. 92, 63–70 (2020).
Montenegro, F. L. de M. et al. Could the less-than subtotal parathyroidectomy be an option for treating young patients with multiple endocrine neoplasia type 1-related hyperparathyroidism? Front. Endocrinol. 10, 123 (2019).
Filopanti, M. et al. MEN1-related hyperparathyroidism: response to cinacalcet and its relationship with the calcium-sensing receptor gene variant Arg990Gly. Eur. J. Endocrinol. 167, 157–164 (2012).
Giusti, F. et al. Cinacalcet therapy in patients affected by primary hyperparathyroidism associated to multiple endocrine neoplasia syndrome type 1 (MEN1). Endocrine 52, 495–506 (2016).
Donegan, D. et al. Long-term outcomes in patients with multiple endocrine neoplasia type 1 and pancreaticoduodenal neuroendocrine tumours. Clin. Endocrinol. 86, 199–206 (2017).
van Asselt, S. J. et al. EUS is superior for detection of pancreatic lesions compared with standard imaging in patients with multiple endocrine neoplasia type 1. Gastrointest. Endosc. 81, 159–167 (2015).
Barbe, C. et al. Magnetic resonance imaging versus endoscopic ultrasonography for the detection of pancreatic tumours in multiple endocrine neoplasia type 1. Dig. Liver Dis. 44, 228–234 (2012).
Yates, C. J., Newey, P. J. & Thakker, R. V. Challenges and controversies in management of pancreatic neuroendocrine tumours in patients with MEN1. Lancet Diabetes Endocrinol. 3, 895–905 (2015).
Anlauf, M. et al. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am. J. Surg. Pathol. 30, 560–574 (2006).
Jensen, R. T., Berna, M. J., Bingham, D. B. & Norton, J. A. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer 113, 1807–1843 (2008).
Goudet, P. et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J. Surg. 34, 249–255 (2010).
Ekeblad, S., Skogseid, B., Dunder, K., Oberg, K. & Eriksson, B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin. Cancer Res. 14, 7798–7803 (2008).
Pieterman, C. R. C. et al. Long-term natural course of small nonfunctional pancreatic neuroendocrine tumors in MEN1—results from the Dutch MEN1 Study Group. J. Clin. Endocrinol. Metab. 102, 3795–3805 (2017).
Thomas-Marques, L. et al. Prospective endoscopic ultrasonographic evaluation of the frequency of nonfunctioning pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. Am. J. Gastroenterol. 101, 266–273 (2006).
Newey, P. J. et al. Asymptomatic children with multiple endocrine neoplasia type 1 mutations may harbor nonfunctioning pancreatic neuroendocrine tumors. J. Clin. Endocrinol. Metab. 94, 3640–3646 (2009).
de Laat, J. M. et al. Low accuracy of tumor markers for diagnosing pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 patients. J. Clin. Endocrinol. Metab. 98, 4143–4151 (2013).
Qiu, W. et al. Utility of chromogranin A, pancreatic polypeptide, glucagon and gastrin in the diagnosis and follow-up of pancreatic neuroendocrine tumours in multiple endocrine neoplasia type 1 patients. Clin. Endocrinol. 85, 400–407 (2016).
van Treijen, M. J. C., van Beek, D.-J., van Leeuwaarde, R. S., Vriens, M. R. & Valk, G. D. Diagnosing nonfunctional pancreatic NETs in MEN1: the evidence base. J. Endocr. Soc. 2, 1067–1088 (2018).
Sadowski, S. M. et al. Results of (68)gallium-DOTATATE PET/CT scanning in patients with multiple endocrine neoplasia type 1. J. Am. Coll. Surg. 221, 509–517 (2015).
Kornaczewski Jackson, E. R., Pointon, O. P., Bohmer, R. & Burgess, J. R. Utility of FDG-PET imaging for risk stratification of pancreatic neuroendocrine tumors in MEN1. J. Clin. Endocrinol. Metab. 102, 1926–1933 (2017).
Triponez, F. et al. Long-term follow-up of MEN1 patients who do not have initial surgery for small ≤2 cm nonfunctioning pancreatic neuroendocrine tumors, an AFCE and GTE study: Association Francophone de Chirurgie Endocrinienne & Groupe d’Etude des Tumeurs Endocrines. Ann. Surg. 268, 158–164 (2018).
Nell, S. et al. Management of MEN1 related nonfunctioning pancreatic NETs: A shifting paradigm: results from the DutchMEN1 Study Group. Ann. Surg. 267, 1155–1160 (2018).
Vinault, S. et al. Metastatic potential and survival of duodenal and pancreatic tumors in multiple endocrine neoplasia type 1: a GTE and AFCE Cohort Study (Groupe d’étude des Tumeurs Endocrines and Association Francophone de Chirurgie Endocrinienne). Ann. Surg. 272, 1094–1101 (2020).
Nell, S. et al. Early and late complications after surgery for MEN1-related nonfunctioning pancreatic neuroendocrine tumors. Ann. Surg. 267, 352–356 (2018).
Faggiano, A. et al. Lanreotide therapy vs active surveillance in MEN1-related pancreatic neuroendocrine tumors <2 centimeters. J. Clin. Endocrinol. Metab. 105, dgz007 (2020).
Gibril, F., Schumann, M., Pace, A. & Jensen, R. T. Multiple endocrine neoplasia type 1 and Zollinger–Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine 83, 43–83 (2004).
Goudet, P. et al. Gastrinomas in multiple endocrine neoplasia type-1. A 127-case cohort study from the endocrine tumor group (ETG). Ann. Chir. 129, 149–155 (2004).
Roy, P. K. et al. Zollinger–Ellison syndrome. Clinical presentation in 261 patients. Medicine 79, 379–411 (2000).
Cadiot, G. et al. Prognostic factors in patients with Zollinger–Ellison syndrome and multiple endocrine neoplasia type 1. Groupe d’Etude des Néoplasies Endocriniennes Multiples (GENEM and Groupe de Recherche et d’Etude du Syndrome de Zollinger–Ellison (GRESZE). Gastroenterology 116, 286–293 (1999).
You, Y. N. et al. Pancreatoduodenal surgery in patients with multiple endocrine neoplasia type 1: operative outcomes, long-term function, and quality of life. Surgery 142, 829–836 (2007).
Christou, N. et al. One-year postoperative mortality in MEN1 patients operated on gastric and duodenopancreatic neuroendocrine tumors: an AFCE and GTE cohort study. World J. Surg. 43, 2856–2864 (2019).
Norton, J. A. et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger–Ellison syndrome. Ann. Surg. 234, 495–505 (2001).
Gibril, F., Venzon, D. J., Ojeaburu, J. V., Bashir, S. & Jensen, R. T. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J. Clin. Endocrinol. Metab. 86, 5282–5293 (2001).
van Beek, D.-J. et al. Prognostic factors and survival in MEN1 patients with gastrinomas: results from the DutchMEN Study Group (DMSG). J. Surg. Oncol. 120, 966–975 (2019).
Falconi, M. et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103, 153–171 (2016).
Albers, M. B., Manoharan, J. & Bartsch, D. K. Contemporary surgical management of the Zollinger–Ellison syndrome in multiple endocrine neoplasia type 1. Best Pract. Res. Clin. Endocrinol. Metab. 33, 101318 (2019).
Sadowski, S. M., Cadiot, G., Dansin, E., Goudet, P. & Triponez, F. The future: surgical advances in MEN1 therapeutic approaches and management strategies. Endocr. Relat. Cancer 24, T243–T260 (2017).
Imamura, M. et al. Biochemically curative surgery for gastrinoma in multiple endocrine neoplasia type 1 patients. World J. Gastroenterol. 17, 1343–1353 (2011).
Lopez, C. L. et al. Partial pancreaticoduodenectomy can provide cure for duodenal gastrinoma associated with multiple endocrine neoplasia type 1. Ann. Surg. 257, 308–314 (2013).
Sakurai, A. et al. Clinical features of insulinoma in patients with multiple endocrine neoplasia type 1: analysis of the database of the MEN Consortium of Japan. Endocr. J. 59, 859–866 (2012).
Zhao, Y.-P. et al. Surgical management of patients with insulinomas: result of 292 cases in a single institution. J. Surg. Oncol. 103, 169–174 (2011).
Nikfarjam, M. et al. Improved contemporary surgical management of insulinomas: a 25-year experience at the Massachusetts General Hospital. Ann. Surg. 247, 165–172 (2008).
Vezzosi, D. et al. Long-term results of the surgical management of insulinoma patients with MEN1: a Groupe d’étude des Tumeurs Endocrines (GTE) retrospective study. Eur. J. Endocrinol. 172, 309–319 (2015).
Antwi, K. et al. 68Ga-Exendin-4 PET/CT detects insulinomas in patients with endogenous hyperinsulinemic hypoglycemia in MEN-1. J. Clin. Endocrinol. Metab. 104, 5843–5852 (2019).
Tonelli, F., Giudici, F., Nesi, G., Batignani, G. & Brandi, M. L. Operation for insulinomas in multiple endocrine neoplasia type 1: when pancreatoduodenectomy is appropriate. Surgery 161, 727–734 (2017).
Lévy-Bohbot, N. et al. Prevalence, characteristics and prognosis of MEN 1-associated glucagonomas, VIPomas, and somatostatinomas: study from the GTE (Groupe des Tumeurs Endocrines) registry. Gastroenterol. Clin. Biol. 28, 1075–1081 (2004).
Soga, J. & Yakuwa, Y. Glucagonomas/diabetico-dermatogenic syndrome (DDS): a statistical evaluation of 407 reported cases. J. Hepatobiliary Pancreat. Surg. 5, 312–319 (1998).
Garbrecht, N. et al. Somatostatin-producing neuroendocrine tumors of the duodenum and pancreas: incidence, types, biological behavior, association with inherited syndromes, and functional activity. Endocr. Relat. Cancer 15, 229–241 (2008).
Garby, L. et al. Clinical characteristics and outcome of acromegaly induced by ectopic secretion of growth hormone-releasing hormone (GHRH): a French nationwide series of 21 cases. J. Clin. Endocrinol. Metab. 97, 2093–2104 (2012).
Milanesi, A. et al. Concurrent primary hyperparathyroidism and humoral hypercalcemia of malignancy in a patient with multiple endocrine neoplasia type 1. Pancreas 40, 634–637 (2011).
Berna, M. J. et al. A prospective study of gastric carcinoids and enterochromaffin-like cell changes in multiple endocrine neoplasia type 1 and Zollinger–Ellison syndrome: identification of risk factors. J. Clin. Endocrinol. Metab. 93, 1582–1591 (2008).
Lehy, T., Cadiot, G., Mignon, M., Ruszniewski, P. & Bonfils, S. Influence of multiple endocrine neoplasia type 1 on gastric endocrine cells in patients with the Zollinger–Ellison syndrome. Gut 33, 1275–1279 (1992).
Corey, B. & Chen, H. Neuroendocrine tumors of the stomach. Surg. Clin. North Am. 97, 333–343 (2017).
Tomassetti, P. et al. Treatment of type II gastric carcinoid tumors with somatostatin analogues. N. Engl. J. Med. 343, 551–554 (2000).
Vergès, B. et al. Pituitary disease in MEN type 1 (MEN1): data from the France–Belgium MEN1 multicenter study. J. Clin. Endocrinol. Metab. 87, 457–465 (2002).
de Laat, J. M. et al. Long-term natural course of pituitary tumors in patients with MEN1: results from the DutchMEN1 Study Group (DMSG). J. Clin. Endocrinol. Metab. 100, 3288–3296 (2015).
Cuny, T. et al. Genetic analysis in young patients with sporadic pituitary macroadenomas: besides AIP don’t forget MEN1 genetic analysis. Eur. J. Endocrinol. 168, 533–541 (2013).
Marques, N. V. et al. Frequency of familial pituitary adenoma syndromes among patients with functioning pituitary adenomas in a reference outpatient clinic. J. Endocrinol. Invest. 40, 1381–1387 (2017).
Nunes, V. S., Souza, G. L., Perone, D., Conde, S. J. & Nogueira, C. R. Frequency of multiple endocrine neoplasia type 1 in a group of patients with pituitary adenoma: genetic study and familial screening. Pituitary 17, 30–37 (2014).
Trouillas, J. et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am. J. Surg. Pathol. 32, 534–543 (2008).
Murray, C., Chandurkar, V. & Green, J. Clinical manifestations of multiple endocrine neoplasia type 1 in the MEN1 burin kindred. Can. J. Diabetes 32, 350 (2008).
Hao, W. et al. Multiple endocrine neoplasia type 1 variant with frequent prolactinoma and rare gastrinoma. J. Clin. Endocrinol. Metab. 89, 3776–3784 (2004).
Stratakis, C. A. et al. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin. Genet. 78, 457–463 (2010).
Lecoq, A.-L., Kamenický, P., Guiochon-Mantel, A. & Chanson, P. Genetic mutations in sporadic pituitary adenomas-what to screen for? Nat. Rev. Endocrinol. 11, 43–54 (2015).
Melmed, S. et al. A consensus statement on acromegaly therapeutic outcomes. Nat. Rev. Endocrinol. 14, 552–561 (2018).
Gatta-Cherifi, B. et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’etude des Tumeurs Endocrines database. Eur. J. Endocrinol. 166, 269–279 (2012).
Waldmann, J. et al. Adrenal involvement in multiple endocrine neoplasia type 1: results of 7 years prospective screening. Langenbecks Arch. Surg. 392, 437–443 (2007).
Schaefer, S. et al. Natural course of small adrenal lesions in multiple endocrine neoplasia type 1: an endoscopic ultrasound imaging study. Eur. J. Endocrinol. 158, 699–704 (2008).
Wang, W. et al. Adrenocortical carcinoma in patients with MEN1: a kindred report and review of the literature. Endocr. Connect. 8, 230–238 (2019).
Dénes, J. et al. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma: results from a large patient cohort. J. Clin. Endocrinol. Metab. 100, E531–E541 (2015).
Fassnacht, M. et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 175, G1–G34 (2016).
Ye, L. et al. Clinical features and prognosis of thymic neuroendocrine tumours associated with multiple endocrine neoplasia type 1: a single-centre study, systematic review and meta-analysis. Clin. Endocrinol. 87, 706–716 (2017).
Pieterman, C. R. C. et al. Thoracic and duodenopancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: natural history and function of menin in tumorigenesis. Endocr. Relat. Cancer 21, R121–R142 (2014).
Goudet, P. et al. Thymic neuroendocrine tumors in multiple endocrine neoplasia type 1: a comparative study on 21 cases among a series of 761 MEN1 from the GTE (Groupe des Tumeurs Endocrines). World J. Surg. 33, 1197–1207 (2009).
Christakis, I. et al. Clinical features, treatments, and outcomes of patients with thymic carcinoids and multiple endocrine neoplasia type 1 syndrome at MD Anderson Cancer Center. Horm. Cancer 7, 279–287 (2016).
Singh Ospina, N., Thompson, G. B., Nichols, F. C., Cassivi, S. D. & Young, Jr,W. F. Thymic and bronchial carcinoid tumors in multiple endocrine neoplasia type 1: the Mayo Clinic experience from 1977 to 2013. Horm. Cancer 6, 247–253 (2015).
Boix, E., Picó, A., Pinedo, R., Aranda, I. & Kovacs, K. Ectopic growth hormone-releasing hormone secretion by thymic carcinoid tumour. Clin. Endocrinol. 57, 131–134 (2002).
Li, X. et al. Familial Cushing syndrome due to thymic carcinoids in a multiple endocrine neoplasia type 1 kindred. Endocrine 47, 183–190 (2014).
Sachithanandan, N., Harle, R. A. & Burgess, J. R. Bronchopulmonary carcinoid in multiple endocrine neoplasia type 1. Cancer 103, 509–515 (2005).
de Laat, J. M. et al. Natural course and survival of neuroendocrine tumors of thymus and lung in MEN1 patients. J. Clin. Endocrinol. Metab. 99, 3325–3333 (2014).
Lecomte, P. et al. Histologically proven bronchial neuroendocrine tumors in MEN1: a GTE 51-case cohort study. World J. Surg. 42, 143–152 (2018).
Vidal, A., Iglesias, M. J., Fernández, B., Fonseca, E. & Cordido, F. Cutaneous lesions associated to multiple endocrine neoplasia syndrome type 1. J. Eur. Acad. Dermatol. Venereol. 22, 835–838 (2008).
Asgharian, B. et al. Cutaneous tumors in patients with multiple endocrine neoplasm type 1 (MEN1) and gastrinomas: prospective study of frequency and development of criteria with high sensitivity and specificity for MEN1. J. Clin. Endocrinol. Metab. 89, 5328–5336 (2004).
Pack, S. et al. Cutaneous tumors in patients with multiple endocrine neoplasia type 1 show allelic deletion of the MEN1 gene. J. Invest. Dermatol. 110, 438–440 (1998).
Nord, B. et al. Malignant melanoma in patients with multiple endocrine neoplasia type 1 and involvement of the MEN1 gene in sporadic melanoma. Int. J. Cancer 87, 463–467 (2000).
Fang, M. et al. MEN1 is a melanoma tumor suppressor that preserves genomic integrity by stimulating transcription of genes that promote homologous recombination-directed DNA repair. Mol. Cell. Biol. 33, 2635–2647 (2013).
Gao, S.-B. et al. Menin represses malignant phenotypes of melanoma through regulating multiple pathways. J. Cell. Mol. Med. 15, 2353–2363 (2011).
Marchand, L. et al. Hibernoma and multiple endocrine neoplasia type 1 syndrome: a non-fortuitous association? A case report and literature review. Ann. Endocrinol. 78, 194–197 (2017).
Gisselsson, D., Höglund, M., Mertens, F., Dal Cin, P. & Mandahl, N. Hibernomas are characterized by homozygous deletions in the multiple endocrine neoplasia type I region. Metaphase fluorescence in situ hybridization reveals complex rearrangements not detected by conventional cytogenetics. Am. J. Pathol. 155, 61–66 (1999).
Nord, K. H. et al. Concomitant deletions of tumor suppressor genes MEN1 and AIP are essential for the pathogenesis of the brown fat tumor hibernoma. Proc. Natl Acad. Sci. USA 107, 21122–21127 (2010).
Garbe, C. et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment – Update 2019. Eur. J. Cancer 126, 159–177 (2020).
Swetter, S. M. et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 80, 208–250 (2019).
Giraud, S. et al. A large multiple endocrine neoplasia type 1 family with clinical expression suggestive of anticipation. J. Clin. Endocrinol. Metab. 82, 3487–3492 (1997).
Cuevas-Ocampo, A. K. et al. Genetic confirmation that ependymoma can arise as part of multiple endocrine neoplasia type 1 (MEN1) syndrome. Acta Neuropathol. 133, 661–663 (2017).
Asgharian, B. et al. Meningiomas may be a component tumor of multiple endocrine neoplasia type 1. Clin. Cancer Res. 10, 869–880 (2004).
van Leeuwaarde, R. S. et al. MEN1-dependent breast cancer: indication for early screening? results from the Dutch MEN1 Study Group. J. Clin. Endocrinol. Metab. 102, 2083–2090 (2017).
van Leeuwaarde, R. S. et al. The future: medical advances in MEN1 therapeutic approaches and management strategies. Endocr. Relat. Cancer 24, T179–T193 (2017).
McKeeby, J. L. et al. Multiple leiomyomas of the esophagus, lung, and uterus in multiple endocrine neoplasia type 1. Am. J. Pathol. 159, 1121–1127 (2001).
de Laat, J. M. et al. Predicting the risk of multiple endocrine neoplasia type 1 for patients with commonly occurring endocrine tumors. Eur. J. Endocrinol. 167, 181–187 (2012).
Cardinal, J. W. et al. A report of a national mutation testing service for the MEN1 gene: clinical presentations and implications for mutation testing. J. Med. Genet. 42, 69–74 (2005).
Frederiksen, A. et al. Clinical features of multiple endocrine neoplasia type 4: novel pathogenic variant and review of published cases. J. Clin. Endocrinol. Metab. 104, 3637–3646 (2019).
Agarwal, S. K., Mateo, C. M. & Marx, S. J. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J. Clin. Endocrinol. Metab. 94, 1826–1834 (2009).
Burgess, J. R. et al. Phenotype and phenocopy: the relationship between genotype and clinical phenotype in a single large family with multiple endocrine neoplasia type 1 (MEN 1). Clin. Endocrinol. 53, 205–211 (2000).
Turner, J. J. O., Christie, P. T., Pearce, S. H. S., Turnpenny, P. D. & Thakker, R. V. Diagnostic challenges due to phenocopies: lessons from multiple endocrine neoplasia type1 (MEN1). Hum. Mutat. 31, E1089–E1101 (2010).
Cetani, F., Saponaro, F., Borsari, S. & Marcocci, C. Familial and hereditary forms of primary hyperparathyroidism. Front. Horm. Res. 51, 40–51 (2019).
Pontikides, N. et al. Genetic basis of familial isolated hyperparathyroidism: a case series and a narrative review of the literature. J. Bone Miner. Metab. 32, 351–366 (2014).
Dean, P. G. et al. Are patients with multiple endocrine neoplasia type I prone to premature death? World J. Surg. 24, 1437–1441 (2000).
Geerdink, E. A. M., Van der Luijt, R. B. & Lips, C. J. M. Do patients with multiple endocrine neoplasia syndrome type 1 benefit from periodical screening? Eur. J. Endocrinol. 149, 577–582 (2003).
Wilson, S. D. et al. The influence of surgery in MEN-1 syndrome: observations over 150 years. Surgery 144, 695–701; discussion 701–702 (2008).
Ito, T., Igarashi, H., Uehara, H., Berna, M. J. & Jensen, R. T. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger–Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine 92, 135–181 (2013).
Revicki, D. A., Kleinman, L. & Cella, D. A history of health-related quality of life outcomes in psychiatry. Dialogues Clin. Neurosci. 16, 127–135 (2014).
Berglund, G. et al. Quality of life in patients with multiple endocrine neoplasia type 1 (MEN 1). Fam. Cancer 2, 27–33 (2003).
Goswami, S., Peipert, B. J., Helenowski, I., Yount, S. E. & Sturgeon, C. Disease and treatment factors associated with lower quality of life scores in adults with multiple endocrine neoplasia type I. Surgery 162, 1270–1277 (2017).
Peipert, B. J., Goswami, S., Yount, S. E. & Sturgeon, C. Health-related quality of life in MEN1 patients compared with other chronic conditions and the United States general population. Surgery 163, 205–211 (2018).
Peipert, B. J., Goswami, S., Helenowski, I., Yount, S. E. & Sturgeon, C. Financial burden is associated with worse health-related quality of life in adults with multiple endocrine neoplasia type 1. Surgery 162, 1278–1285 (2017).
van Leeuwaarde, R. S. et al. High fear of disease occurrence is associated with low quality of life in patients with multiple endocrine neoplasia type 1: results from the Dutch MEN1 Study Group. J. Clin. Endocrinol. Metab. 103, 2354–2361 (2018).
Herath, M., Parameswaran, V., Thompson, M., Williams, M. & Burgess, J. Paediatric and young adult manifestations and outcomes of multiple endocrine neoplasia type 1. Clin. Endocrinol. 91, 633–638 (2019).
Marx, S. J. & Lourenço, D. M. Questions and controversies about parathyroid pathophysiology in children with multiple endocrine neoplasia type 1. Front. Endocrinol. 9, 359 (2018).
Gonçalves, T. D. et al. Penetrance of functioning and nonfunctioning pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 in the second decade of life. J. Clin. Endocrinol. Metab. 99, E89–E96 (2014).
Makri, A. et al. Children with MEN1 gene mutations may present first (and at a young age) with Cushing disease. Clin. Endocrinol. 89, 437–443 (2018).
Salenave, S. et al. Macroprolactinomas in children and adolescents: factors associated with the response to treatment in 77 patients. J. Clin. Endocrinol. Metab. 100, 1177–1186 (2015).
García García, E., Mangas Cruz, M. Á., Guerrero Vázquez, R. & Navarro González, E. Hyperandrogenism in a child with multiple endocrine neoplasia type 1. Endocrinol. Diabetes Nutr. 66, 132–133 (2019).
Manoharan, J. et al. Is routine screening of young asymptomatic MEN1 patients necessary? World J. Surg. 41, 2026–2032 (2017).
Nozières, C. et al. p.Ala541Thr variant of MEN1 gene: a non deleterious polymorphism or a pathogenic mutation? Ann. Endocrinol. 75, 133–140 (2014).
Giraud, S. et al. Germ-line mutation analysis in patients with multiple endocrine neoplasia type 1 and related disorders. Am. J. Hum. Genet. 63, 455–467 (1998).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
A.A.-S. has worked as sub-investigator for Alnylam Pharmaceuticals, Ionis (Isis) Pharmaceuticals, Novartis, MedDay Pharmaceuticals, Auris Medical, Gilead Sciences, Euroscreen (Ogeda), Alexion, Faron Pharmaceuticals, Actelion (Idorsia) Pharmaceuticals and IPSEN. G.C. is member of advisory boards of AAA, Keocyt, Ipsen, Novartis and Pfizer. P.G. received research grant from IPSEN. P.C. has received unrestricted research and educational grants from Ipsen, Novartis, Novo-Nordisk and Pfizer as Head of the Department of Endocrinology and Reproductive Diseases, APHP Paris-Saclay. P.C. has served as investigator (principal or coordinator) for clinical trials funded by Novartis, Pfizer, Ipsen, Antisense and Prolor Biotech. P.C. is a member of advisory boards from Ipsen, Novartis, Pfizer and Tiburio. P.C. gave lectures for Ipsen, Novartis and Pfizer. All the fees and honoraria are paid to his Institution. A.C. declares no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks B. Skogseid, F. Tonelli, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Salameh, A., Cadiot, G., Calender, A. et al. Clinical aspects of multiple endocrine neoplasia type 1. Nat Rev Endocrinol 17, 207–224 (2021). https://doi.org/10.1038/s41574-021-00468-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-021-00468-3
This article is cited by
-
Genetics of hereditary forms of primary hyperparathyroidism
Hormones (2024)
-
Familial states of primary hyperparathyroidism: an update
Journal of Endocrinological Investigation (2024)
-
Performance of [18F]fluorocholine PET/CT in MEN1-related primary hyperparathyroidism before initial surgery or for persistent/recurrent disease
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Pathogenic variants among females with breast cancer and a non-breast cancer reveal opportunities for cancer interception
Breast Cancer Research and Treatment (2023)
-
Familial parathyroid tumours—comparison of clinical profiles between syndromes
Journal of Endocrinological Investigation (2023)