Abstract
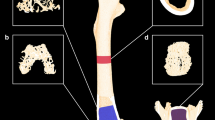
Glucocorticoids are widely used to suppress inflammation or the immune system. High doses and long-term use of glucocorticoids lead to an important and common iatrogenic complication, glucocorticoid-induced osteoporosis, in a substantial proportion of patients. Glucocorticoids mainly increase bone resorption during the initial phase (the first year of treatment) by enhancing the differentiation and maturation of osteoclasts. Glucocorticoids also inhibit osteoblastogenesis and promote apoptosis of osteoblasts and osteocytes, resulting in decreased bone formation during long-term use. Several indirect effects of glucocorticoids on bone metabolism, such as suppression of production of insulin-like growth factor 1 or growth hormone, are involved in the pathogenesis of glucocorticoid-induced osteoporosis. Fracture risk assessment for all patients with long-term use of oral glucocorticoids is required. Non-pharmacological interventions to manage the risk of fracture should be prescribed to all patients, while pharmacological management is reserved for patients who have increased fracture risk. Various treatment options can be used, ranging from bisphosphonates to denosumab, as well as teriparatide. Finally, appropriate monitoring during treatment is also important.
Key points
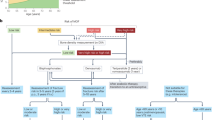
Glucocorticoid-induced osteoporosis is the most common cause of secondary osteoporosis and is an iatrogenic disease; the main pathogenesis in the long term is a reduction in bone formation.
Fracture risk is correlated with the dose and duration of glucocorticoid administration, and seems to decrease rapidly on discontinuation; the underlying disease requiring glucocorticoid therapy often contributes to bone loss.
Evaluation of fracture risk, using tools such as FRAX, is recommended in all patients treated with glucocorticoids, preferably around the time of treatment initiation.
Non-pharmacological management (such as nutrition and exercise) should be advocated in all patients receiving long-term glucocorticoid treatment.
Using the minimally effective dose and duration of glucocorticoids with steroid-sparing drugs should be considered where possible.
Pharmacological anti-osteoporotic treatment is recommended in patients at high risk of fracture; anti-resorptives are the primary option but anabolic therapy might also be considered.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Caplan, A., Fett, N., Rosenbach, M., Werth, V. P. & Micheletti, R. G. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J. Am. Acad. Dermatol. 76, 1–9 (2017).
Kaltsas, G. & Makras, P. Skeletal diseases in Cushing’s syndrome: osteoporosis versus arthropathy. Neuroendocrinology 92 (Suppl. 1), 60–64 (2010).
Devogelaer, J. P., Crabbe, J. & Nagant de Deuxchaisnes, C. Bone mineral density in Addison’s disease: evidence for an effect of adrenal androgens on bone mass. Br. Med. J. 294, 798–800 (1987).
Bjornsdottir, S. et al. Risk of hip fracture in Addison’s disease: a population-based cohort study. J. Intern. Med. 270, 187–195 (2011).
Vandewalle, J., Luypaert, A., De Bosscher, K. & Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 29, 42–54 (2018).
van Staa, T. P. et al. Use of oral corticosteroids in the United Kingdom. QJM 93, 105–111 (2000).
Overman, R. A., Yeh, J. Y. & Deal, C. L. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 65, 294–298 (2013).
Fardet, L., Petersen, I. & Nazareth, I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology 50, 1982–1990 (2011).
Compston, J. Glucocorticoid-induced osteoporosis: an update. Endocrine 61, 7–16 (2018).
Mazziotti, G., Angeli, A., Bilezikian, J. P., Canalis, E. & Giustina, A. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol. Metab. 17, 144–149 (2006).
Briot, K. & Roux, C. Glucocorticoid-induced osteoporosis. RMD Open 1, e000014 (2015).
LoCascio, V. et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner. 8, 39–51 (1990).
De Vries, F. et al. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum. 56, 208–214 (2007).
Oshagbemi, O. A. et al. Use of high-dose intermittent systemic glucocorticoids and the risk of fracture in patients with chronic obstructive pulmonary disease. Bone 110, 238–243 (2018).
van Staa, T. P., Leufkens, H. G., Abenhaim, L., Zhang, B. & Cooper, C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology 39, 1383–1389 (2000).
Seckl, J. R. 11beta-hydroxysteroid dehydrogenases: changing glucocorticoid action. Curr. Opin. Pharmacol. 4, 597–602 (2004).
Hardy, R. S., Seibel, M. J. & Cooper, M. S. Targeting 11beta-hydroxysteroid dehydrogenases: a novel approach to manipulating local glucocorticoid levels with implications for rheumatic disease. Curr. Opin. Pharmacol. 13, 440–444 (2013).
Kanis, J. A. et al. A meta-analysis of prior corticosteroid use and fracture risk. J. Bone Miner. Res. 19, 893–899 (2004).
Weinstein, R. S. Clinical practice. Glucocorticoid-induced bone disease. N. Engl. J. Med. 365, 62–70 (2011).
van Staa, T. P., Leufkens, H. G. & Cooper, C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos. Int. 13, 777–787 (2002).
Amiche, M. A. et al. Fracture risk in oral glucocorticoid users: a Bayesian meta-regression leveraging control arms of osteoporosis clinical trials. Osteoporos. Int. 27, 1709–1718 (2016).
Balasubramanian, A. et al. Glucocorticoid exposure and fracture risk in patients with new-onset rheumatoid arthritis. Osteoporos. Int. 27, 3239–3249 (2016).
Adler, R. A. & Hochberg, M. C. Glucocorticoid-induced osteoporosis in men. J. Endocrinol. Invest. 34, 481–484 (2011).
Ebeling, P. R. Clinical practice. Osteoporosis in men. N. Engl. J. Med. 358, 1474–1482 (2008).
Hoff, M. et al. Anti-osteoporosis drug use: too little, too much, or just right? The HUNT study, Norway. Osteoporos. Int. 29, 1875–1885 (2018).
Frediani, B. et al. Effects of high dose methylprednisolone pulse therapy on bone mass and biochemical markers of bone metabolism in patients with active rheumatoid arthritis: a 12-month randomized prospective controlled study. J. Rheumatol. 31, 1083–1087 (2004).
Goncalves, P. A. et al. Inhaled glucocorticoids are associated with vertebral fractures in COPD patients. J. Bone Miner. Metab. 36, 454–461 (2018).
Sosa, M. et al. Inhaled steroids do not decrease bone mineral density but increase risk of fractures: data from the GIUMO study group. J. Clin. Densitom. 9, 154–158 (2006).
Wheelock, C., Glass, J. & St Anna, L. Clinical inquiry. Do inhaled steroids reduce bone mineral density and increase fracture risk? J. Fam. Pract. 61, 493–508 (2012).
Vestergaard, P., Rejnmark, L. & Mosekilde, L. Fracture risk associated with systemic and topical corticosteroids. J. Intern. Med. 257, 374–384 (2005).
Loke, Y. K., Cavallazzi, R. & Singh, S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax 66, 699–708 (2011).
Vestergaard, P. Skeletal effects of systemic and topical corticosteroids. Curr. Drug Saf. 3, 190–193 (2008).
Loftus, J. et al. Randomized, double-blind trial of deflazacort versus prednisone in juvenile chronic (or rheumatoid) arthritis: a relatively bone-sparing effect of deflazacort. Pediatrics 88, 428–436 (1991).
Ferraris, J. R. et al. Effect of deflazacort versus methylprednisone on growth, body composition, lipid profile, and bone mass after renal transplantation. The Deflazacort Study Group. Pediatr. Nephrol. 14, 682–688 (2000).
Markham, A. & Bryson, H. M. Deflazacort. A review of its pharmacological properties and therapeutic efficacy. Drugs 50, 317–333 (1995).
Parente, L. Deflazacort: therapeutic index, relative potency and equivalent doses versus other corticosteroids. BMC Pharmacol. Toxicol. 18, 1 (2017).
Tatsuno, I. et al. Age dependence of early symptomatic vertebral fracture with high-dose glucocorticoid treatment for collagen vascular diseases. J. Clin. Endocrinol. Metab. 94, 1671–1677 (2009).
Thompson, J. M., Modin, G. W., Arnaud, C. D. & Lane, N. E. Not all postmenopausal women on chronic steroid and estrogen treatment are osteoporotic: predictors of bone mineral density. Calcif. Tissue Int. 61, 377–381 (1997).
Adler, R. A., et al. Osteoporosis (eds Marcus, R. et al.) 1191–1223 (Academic Press, 2013).
Kanis, J. A. Assessment of osteoporosis at the primary health care level. https://www.sheffield.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf (WHO, 2007).
Russcher, H. et al. Two polymorphisms in the glucocorticoid receptor gene directly affect glucocorticoid-regulated gene expression. J. Clin. Endocrinol. Metab. 90, 5804–5810 (2005).
Cooper, M. S. et al. Osteoblastic 11beta-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J. Bone Miner. Res. 17, 979–986 (2002).
Cooper, M. S. et al. Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J. Bone Miner. Res. 16, 1037–1044 (2001).
Weinstein, R. S., Jilka, R. L., Parfitt, A. M. & Manolagas, S. C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Invest. 102, 274–282 (1998).
Hofbauer, L. C. & Rauner, M. Minireview: live and let die: molecular effects of glucocorticoids on bone cells. Mol. Endocrinol. 23, 1525–1531 (2009).
den Uyl, D., Bultink, I. E. & Lems, W. F. Advances in glucocorticoid-induced osteoporosis. Curr. Rheumatol. Rep. 13, 233–240 (2011).
Shen, G. et al. Autophagy as a target for glucocorticoid-induced osteoporosis therapy. Cell Mol. Life Sci. 75, 2683–2693 (2018).
Wang, L., Heckmann, B. L., Yang, X. & Long, H. Osteoblast autophagy in glucocorticoid-induced osteoporosis. J. Cell Physiol. 234, 3207–3215 (2019).
Wu, Z., Bucher, N. L. & Farmer, S. R. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol. Cell Biol. 16, 4128–4136 (1996).
Yang, Y. J. et al. Tanshinol alleviates impaired bone formation by inhibiting adipogenesis via KLF15/PPARgamma2 signaling in GIO rats. Acta Pharmacol. Sin. 39, 633–641 (2018).
Ohnaka, K., Tanabe, M., Kawate, H., Nawata, H. & Takayanagi, R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem. Biophys. Res. Commun. 329, 177–181 (2005).
Hildebrandt, S. et al. Glucocorticoids suppress Wnt16 expression in osteoblasts in vitro and in vivo. Sci. Rep. 8, 8711 (2018).
Ohlsson, C. et al. WNT16 overexpression partly protects against glucocorticoid-induced bone loss. Am. J. Physiol. Endocrinol. Metab. 314, E597–E604 (2018).
Delany, A. M., Jeffrey, J. J., Rydziel, S. & Canalis, E. Cortisol increases interstitial collagenase expression in osteoblasts by post-transcriptional mechanisms. J. Biol. Chem. 270, 26607–26612 (1995).
Zhang, S., Liu, Y. & Liang, Q. Low-dose dexamethasone affects osteoblast viability by inducing autophagy via intracellular ROS. Mol. Med. Rep. 17, 4307–4316 (2018).
Han, Y. et al. Autophagy relieves the function inhibition and apoptosis-promoting effects on osteoblasts induced by glucocorticoid. Int. J. Mol. Med. 41, 800–808 (2018).
Pereira, R. M., Delany, A. M., Durant, D. & Canalis, E. Cortisol regulates the expression of Notch in osteoblasts. J. Cell Biochem. 85, 252–258 (2002).
Zanotti, S. & Canalis, E. Notch signaling and the skeleton. Endocr. Rev. 37, 223–253 (2016).
Zanotti, S., Yu, J., Adhikari, S. & Canalis, E. Glucocorticoids inhibit notch target gene expression in osteoblasts. J. Cell Biochem. 119, 6016–6023 (2018).
Tu, X. et al. Physiological notch signaling maintains bone homeostasis via RBPjk and Hey upstream of NFATc1. PLoS Genet. 8, e1002577 (2012).
Swanson, C., Lorentzon, M., Conaway, H. H. & Lerner, U. H. Glucocorticoid regulation of osteoclast differentiation and expression of receptor activator of nuclear factor-kappaB (NF-kappaB) ligand, osteoprotegerin, and receptor activator of NF-kappaB in mouse calvarial bones. Endocrinology 147, 3613–3622 (2006).
Hofbauer, L. C. et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 140, 4382–4389 (1999).
Piemontese, M., Xiong, J., Fujiwara, Y., Thostenson, J. D. & O’Brien, C. A. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol. Endocrinol. Metab. 311, E587–E593 (2016).
Rubin, J. et al. Dexamethasone promotes expression of membrane-bound macrophage colony-stimulating factor in murine osteoblast-like cells. Endocrinology 139, 1006–1012 (1998).
Dovio, A. et al. High-dose glucocorticoids increase serum levels of soluble IL-6 receptor alpha and its ratio to soluble gp130: an additional mechanism for early increased bone resorption. Eur. J. Endocrinol. 154, 745–751 (2006).
Takuma, A. et al. Dexamethasone enhances osteoclast formation synergistically with transforming growth factor-beta by stimulating the priming of osteoclast progenitors for differentiation into osteoclasts. J. Biol. Chem. 278, 44667–44674 (2003).
Kim, H. J. et al. Glucocorticoids suppress bone formation via the osteoclast. J. Clin. Invest. 116, 2152–2160 (2006).
Teitelbaum, S. L. Glucocorticoids and the osteoclast. Clin. Exp. Rheumatol. 33, S37–S39 (2015).
Jia, D., O’Brien, C. A., Stewart, S. A., Manolagas, S. C. & Weinstein, R. S. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology 147, 5592–5599 (2006).
Dallas, S. L., Prideaux, M. & Bonewald, L. F. The osteocyte: an endocrine cell… and more. Endocr. Rev. 34, 658–690 (2013).
Liu, Y. et al. Prevention of glucocorticoid-induced apoptosis in osteocytes and osteoblasts by calbindin-D28k. J. Bone Miner. Res. 19, 479–490 (2004).
Lane, N. E. et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J. Bone Miner. Res. 21, 466–476 (2006).
Baylink, D. J. & Wergedal, J. E. Bone formation by osteocytes. Am. J. Physiol. 221, 669–678 (1971).
Yao, W. et al. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 58, 1674–1686 (2008).
Wang, F. S., Ko, J. Y., Yeh, D. W., Ke, H. C. & Wu, H. L. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology 149, 1793–1801 (2008).
Weinstein, R. S. et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 9, 147–161 (2010).
Seeman, E. & Delmas, P. D. Bone quality — the material and structural basis of bone strength and fragility. N. Engl. J. Med. 354, 2250–2261 (2006).
Canalis, E., Centrella, M., Burch, W. & McCarthy, T. L. Insulin-like growth factor 1 mediates selective anabolic effects of parathyroid hormone in bone cultures. J. Clin. Invest. 83, 60–65 (1989).
Canalis, E. & Delany, A. M. Mechanisms of glucocorticoid action in bone. Ann. NY Acad. Sci. 966, 73–81 (2002).
Delany, A. M., Durant, D. & Canalis, E. Glucocorticoid suppression of IGF 1 transcription in osteoblasts. Mol. Endocrinol. 15, 1781–1789 (2001).
Lane, N. E. et al. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J. Clin. Invest. 102, 1627–1633 (1998).
Huybers, S., Naber, T. H., Bindels, R. J. & Hoenderop, J. G. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G92–G97 (2007).
Ritz, E., Kreusser, W. & Rambausek, M. Effects of glucocorticoids on calcium and phosphate excretion. Adv. Exp. Med. Biol. 171, 381–397 (1984).
Bonadonna, S. et al. Chronic glucocorticoid treatment alters spontaneous pulsatile parathyroid hormone secretory dynamics in human subjects. Eur. J. Endocrinol. 152, 199–205 (2005).
Urena, P. et al. Regulation of parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acid by glucocorticoids and PTH in ROS 17/2.8 and OK cells. Endocrinology 134, 451–456 (1994).
Canalis, E., Mazziotti, G., Giustina, A. & Bilezikian, J. P. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos. Int. 18, 1319–1328 (2007).
Rubin, M. R. & Bilezikian, J. P. Clinical review 151: the role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a re-examination of the evidence. J. Clin. Endocrinol. Metab. 87, 4033–4041 (2002).
Manelli, F. et al. Growth hormone in glucocorticoid-induced osteoporosis. Front. Horm. Res. 30, 174–183 (2002).
Lombardi, G. et al. The role of growth hormone in glucocorticoid-induced osteoporosis. J. Endocrinol. Invest. 31, 38–42 (2008).
van Staa, T. P. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif. Tissue Int. 79, 129–137 (2006).
MacAdams, M. R., White, R. H. & Chipps, B. E. Reduction of serum testosterone levels during chronic glucocorticoid therapy. Ann. Intern. Med. 104, 648–651 (1986).
Morrison, D. et al. Testosterone levels during systemic and inhaled corticosteroid therapy. Respir. Med. 88, 659–663 (1994).
Sato, A. Y. et al. Glucocorticoids induce bone and muscle atrophy by tissue-specific mechanisms upstream of E3 ubiquitin ligases. Endocrinology 158, 664–677 (2017).
Riso, E. M. et al. Relationship between extracellular matrix, contractile apparatus, muscle mass and strength in case of glucocorticoid myopathy. J. Steroid Biochem. Mol. Biol. 108, 117–120 (2008).
Black, R. J., Hill, C. L., Lester, S. & Dixon, W. G. The association between systemic glucocorticoid use and the risk of cataract and glaucoma in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One 11, e0166468 (2016).
Judd, L. L. et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am. J. Psychiatry 171, 1045–1051 (2014).
Compston, J. et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 12, 43 (2017).
Buckley, L. et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 69, 1095–1110 (2017).
Lekamwasam, S. et al. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos. Int. 23, 2257–2276 (2012).
Yu, S. F. et al. Beyond bone mineral density, FRAX-based tailor-made intervention thresholds for therapeutic decision in subjects on glucocorticoid: a nationwide osteoporosis survey. Medicine 96, e5959 (2017).
Hill, Q. A. et al. The prevention of glucocorticoid-induced osteoporosis in patients with immune thrombocytopenia receiving steroids: a British Society for Haematology Good Practice Paper. Br. J. Haematol. 185, 410–417 (2019).
Albaum, J. M., Youn, S., Levesque, L. E., Gershon, A. S. & Cadarette, S. M. Osteoporosis management among chronic glucocorticoid users: a systematic review. J. Popul. Ther. Clin. Pharmacol. 21, e486–e504 (2014).
Kanis, J. A. et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos. Int. 18, 1033–1046 (2007).
Kanis, J. A., Johansson, H., Oden, A. & McCloskey, E. V. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos. Int. 22, 809–816 (2011).
Johansson, H. et al. Impact of femoral neck and lumbar spine BMD discordances on FRAX probabilities in women: a meta-analysis of international cohorts. Calcif. Tissue Int. 95, 428–435 (2014).
Majumdar, S. R. et al. The disconnect between better quality of glucocorticoid-induced osteoporosis preventive care and better outcomes: a population-based cohort study. J. Rheumatol. 40, 1736–1741 (2013).
Majumdar, S. R. et al. Population-based trends in osteoporosis management after new initiations of long-term systemic glucocorticoids (1998–2008). J. Clin. Endocrinol. Metab. 97, 1236–1242 (2012).
Hernlund, E. et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 8, 136 (2013).
Rizzoli, R. & Biver, E. Glucocorticoid-induced osteoporosis: who to treat with what agent? Nat. Rev. Rheumatol. 11, 98–109 (2015).
Park, S. Y. et al. Korean guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. J. Bone Metab. 25, 195–211 (2018).
Pereira, R. M. et al. Guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis. Rev. Bras. Reumatol. 52, 580–593 (2012).
Suzuki, Y. et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J. Bone Miner. Metab. 32, 337–350 (2014).
Compston, J. Clinical question: what is the best approach to managing glucocorticoid-induced osteoporosis? Clin. Endocrinol. 74, 547–550 (2011).
Compston, J. et al. Recommendations for the registration of agents for prevention and treatment of glucocorticoid-induced osteoporosis: an update from the Group for the Respect of Ethics and Excellence in Science. Osteoporos. Int. 19, 1247–1250 (2008).
Cremers, S. C., Pillai, G. & Papapoulos, S. E. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin. Pharmacokinet. 44, 551–570 (2005).
Porras, A. G., Holland, S. D. & Gertz, B. J. Pharmacokinetics of alendronate. Clin. Pharmacokinet. 36, 315–328 (1999).
Dunn, C. J. & Goa, K. L. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs 61, 685–712 (2001).
Cryer, B. & Bauer, D. C. Oral bisphosphonates and upper gastrointestinal tract problems: what is the evidence? Mayo Clin. Proc. 77, 1031–1043 (2002).
Ghirardi, A. et al. Risk of severe upper gastrointestinal complications among oral bisphosphonate users. PLoS One 8, e73159 (2013).
Siris, E. S. et al. Association between gastrointestinal events and compliance with osteoporosis therapy. Bone Rep. 4, 5–10 (2016).
Miller, P. D., Jamal, S. A., Evenepoel, P., Eastell, R. & Boonen, S. Renal safety in patients treated with bisphosphonates for osteoporosis: a review. J. Bone Miner. Res. 28, 2049–2059 (2013).
Lipton, A. The safety of zoledronic acid. Expert Opin. Drug Saf. 6, 305–313 (2007).
Allen, C. S., Yeung, J. H., Vandermeer, B. & Homik, J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst. Rev. 10, CD001347 (2016).
Kim, D. H. et al. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS One 10, e0122646 (2015).
Sharma, A. et al. Risk of serious atrial fibrillation and stroke with use of bisphosphonates: evidence from a meta-analysis. Chest 144, 1311–1322 (2013).
Cohen, S. et al. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 42, 2309–2318 (1999).
Saag, K. G. et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N. Engl. J. Med. 339, 292–299 (1998).
Wallach, S. et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif. Tissue Int. 67, 277–285 (2000).
Adachi, J. D. et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 44, 202–211 (2001).
Reid, D. M. et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European corticosteroid-induced osteoporosis treatment study. J. Bone Miner. Res. 15, 1006–1013 (2000).
Kishimoto, M., Oishi, A. & Motojima, S. Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N. Engl. J. Med. 355, 2156–2157 (2006).
Sambrook, P. N. et al. Bisphosphonates and glucocorticoid osteoporosis in men: results of a randomized controlled trial comparing zoledronic acid with risedronate. Bone 50, 289–295 (2012).
Reid, D. M. et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 373, 1253–1263 (2009).
Gluer, C. C. et al. Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J. Bone Miner. Res. 28, 1355–1368 (2013).
Saag, K. G. et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N. Engl. J. Med. 357, 2028–2039 (2007).
Saag, K. G. et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 60, 3346–3355 (2009).
Axelsson, K. F., Nilsson, A. G., Wedel, H., Lundh, D. & Lorentzon, M. Association between alendronate use and hip fracture risk in older patients using oral prednisolone. JAMA 318, 146–155 (2017).
de Boissieu, P., Gaboriau, L., Morel, A. & Trenque, T. Bisphosphonate-related osteonecrosis of the jaw: data from the French national pharmacovigilance database. Fundam. Clin. Pharmacol. 30, 450–458 (2016).
Jadu, F., Lee, L., Pharoah, M., Reece, D. & Wang, L. A retrospective study assessing the incidence, risk factors and comorbidities of pamidronate-related necrosis of the jaws in multiple myeloma patients. Ann. Oncol. 18, 2015–2019 (2007).
Saita, Y. et al. The incidence of and risk factors for developing atypical femoral fractures in Japan. J. Bone Miner. Metab. 33, 311–318 (2015).
Takakubo, Y. et al. The incidence of atypical femoral fractures in patients with rheumatic disease: Yamagata prefectural committee of atypical femoral fractures (YamaCAFe) study. Tohoku J. Exp. Med. 242, 327–334 (2017).
Meier, R. P., Perneger, T. V., Stern, R., Rizzoli, R. & Peter, R. E. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch. Intern. Med. 172, 930–936 (2012).
Shane, E. et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 29, 1–23 (2014).
Chiu, C. T., Chiang, W. F., Chuang, C. Y. & Chang, S. W. Resolution of oral bisphosphonate and steroid-related osteonecrosis of the jaw — a serial case analysis. J. Oral Maxillofac. Surg. 68, 1055–1063 (2010).
Saag, K. G. et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 6, 445–454 (2018).
Saag, K. G. et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind, double-dummy trial. Arthritis Rheumatol. 71, 1174–1184 (2019).
Bone, H. G. et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J. Clin. Endocrinol. Metab. 96, 972–980 (2011).
McClung, M. R., Wagman, R. B., Miller, P. D., Wang, A. & Lewiecki, E. M. Observations following discontinuation of long-term denosumab therapy. Osteoporos. Int. 28, 1723–1732 (2017).
Cummings, S. R. et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J. Bone Miner. Res. 33, 190–198 (2018).
Anastasilakis, A. D. et al. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J. Bone Miner. Res. 32, 1291–1296 (2017).
Popp, A. W., Zysset, P. K. & Lippuner, K. Rebound-associated vertebral fractures after discontinuation of denosumab — from clinic and biomechanics. Osteoporos. Int. 27, 1917–1921 (2016).
Anastasilakis, A. D. & Makras, P. Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos. Int. 27, 1929–1930 (2016).
Tsourdi, E. et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 105, 11–17 (2017).
Reid, I. R. et al. Bone loss after denosumab: only partial protection with zoledronate. Calcif. Tissue Int. 101, 371–374 (2017).
Horne, A. M., Mihov, B. & Reid, I. R. Effect of zoledronate on bone loss after romosozumab/denosumab: 2-year follow-up. Calcif. Tissue Int. 105, 107–108 (2019).
Anastasilakis, A. D. et al. Zoledronate for the prevention of bone loss in women discontinuing denosumab treatment. A prospective 2-year clinical trial. J. Bone Miner. Res. 34, 2220–2228 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03396315 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03087851 (2019).
Khan, A. A. et al. Case-based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the international task force on ONJ. J. Clin. Densitom. 20, 8–24 (2017).
Devogelaer, J. P. et al. Baseline glucocorticoid dose and bone mineral density response with teriparatide or alendronate therapy in patients with glucocorticoid-induced osteoporosis. J. Rheumatol. 37, 141–148 (2010).
Caggiari, G. et al. Safety and effectiveness of teriparatide vs alendronate in postmenopausal osteoporosis: a prospective non randomized clinical study. Clin. Cases Miner. Bone Metab. 13, 200–203 (2016).
Karatoprak, C. et al. Severe hypercalcemia due to teriparatide. Indian J. Pharmacol. 44, 270–271 (2012).
Adami, G. & Saag, K. G. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos. Int. 30, 1145–1156 (2019).
Haas, A. V. & LeBoff, M. S. Osteoanabolic agents for osteoporosis. J. Endocr. Soc. 2, 922–932 (2018).
Cosman, F. et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 375, 1532–1543 (2016).
Miller, P. D. et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316, 722–733 (2016).
Adachi, J. D. et al. Management of corticosteroid-induced osteoporosis. Semin. Arthritis Rheum. 29, 228–251 (2000).
Rizzoli, R. et al. Management of glucocorticoid-induced osteoporosis. Calcif. Tissue Int. 91, 225–243 (2012).
Vasikaran, S. et al. International osteoporosis foundation and international federation of clinical chemistry and laboratory medicine position on bone marker standards in osteoporosis. Clin. Chem. Lab. Med. 49, 1271–1274 (2011).
Devogelaer, J. P. et al. Evidence-based guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis: a consensus document of the Belgian Bone Club. Osteoporos. Int. 17, 8–19 (2006).
Diez-Perez, A. et al. Treatment failure in osteoporosis. Osteoporos. Int. 23, 2769–2774 (2012).
Briot, K. et al. 2014 update of recommendations on the prevention and treatment of glucocorticoid-induced osteoporosis. Joint Bone Spine 81, 493–501 (2014).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks N. Lane and J.-P. Devogelaer for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related link
The FRAX tool: https://www.sheffield.ac.uk/FRAX/
Rights and permissions
About this article
Cite this article
Chotiyarnwong, P., McCloskey, E. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol 16, 437–447 (2020). https://doi.org/10.1038/s41574-020-0341-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-020-0341-0
This article is cited by
-
Analyzing the factors associated with efficacy among teriparatide treatment in postmenopausal women with osteoporosis
BMC Musculoskeletal Disorders (2024)
-
Insights and implications of sexual dimorphism in osteoporosis
Bone Research (2024)
-
Non-biofilm-forming Staphylococcus epidermidis planktonic cell supernatant induces alterations in osteoblast biological function
Scientific Reports (2024)
-
The interplay of rheumatoid arthritis and osteoporosis: exploring the pathogenesis and pharmacological approaches
Clinical Rheumatology (2024)
-
Safety of non-standard regimen of systemic steroid therapy in patients with Graves’ orbitopathy: a single-centre experience
Pharmacological Reports (2024)