Abstract
Type 1 diabetes mellitus is believed to result from destruction of the insulin-producing β-cells in pancreatic islets that is mediated by autoimmune mechanisms. The classic view is that autoreactive T cells mistakenly destroy healthy (‘innocent’) β-cells. We propose an alternative view in which the β-cell is the key contributor to the disease. By their nature and function, β-cells are prone to biosynthetic stress with limited measures for self-defence. β-Cell stress provokes an immune attack that has considerable negative effects on the source of a vital hormone. This view would explain why immunotherapy at best delays progression of type 1 diabetes mellitus and points to opportunities to use therapies that revitalize β-cells, in combination with immune intervention strategies, to reverse the disease. We present the case that dysfunction occurs in both the immune system and β-cells, which provokes further dysfunction, and present the evidence leading to the consensus that islet autoimmunity is an essential component in the pathogenesis of type 1 diabetes mellitus. Next, we build the case for the β-cell as the trigger of an autoimmune response, supported by analogies in cancer and antitumour immunity. Finally, we synthesize a model (‘connecting the dots’) in which both β-cell stress and islet autoimmunity can be harnessed as targets for intervention strategies.
Key points
-
Autoreactive T cells are part of the normal T cell repertoire.
-
β-Cells are poorly equipped to survive an inflammatory milieu and participate in their own destruction.
-
Metabolic activity drives β-cell dysfunction and destruction.
-
Inflammation triggers profound metabolic, epigenetic and autoantigenic changes, which expose β-cells to the immune system.
-
The immune response to distressed β-cells might be one with ‘good intentions’, as infected tissues or tumours provoke the immune system in similar ways.
-
Immunotherapy might be insufficient to cure type 1 diabetes mellitus; β-cell therapy might contribute to reducing β-cell immunogenicity and islet autoimmunity.
Similar content being viewed by others
Introduction
For several decades, type 1 diabetes mellitus (T1DM) was believed to be a T cell-mediated autoimmune disease1,2,3. This notion still holds, but several observations in the past few years point to a role of β-cells that goes beyond being a non-provoking victim of an autoimmune attack4,5,6. The lack of durable effects of immune-suppressive intervention therapies, islet autoimmunity occurring without the development of T1DM, a remarkably low rate of insulitis at diagnosis and the unexpectedly high proportion of β-cells that persist (although they do not always function) after the diagnosis of T1DM prompted a revision of our take on the pathogenesis of T1DM7,8,9. In this Review, we build the case for β-cells as active participants in the dialogue with the immune system. We propose that therapies targeting β-cell health, vitality and function might prove essential, in combination with immunotherapy, in changing the course of events leading to β-cell destruction.
T1DM as an autoimmune disease
A connection between the immune system and T1DM was first suggested in 1973, when HLA antigens were found to be associated with insulin-dependent diabetes mellitus but not with insulin-independent diabetes mellitus10. Since then, genome-wide association studies have confirmed that HLA genes account for up to 50% of the genetic risk of T1DM (in particular HLA class II loci), which suggests that the selective presentation of specific autoantigen peptides is involved in the pathogenesis of T1DM11,12,13. Meta-analyses have also linked non-HLA high-risk polymorphisms within INS-VNTR (variable number of tandem repeats), PTPN22, CTLA4 and IL2RA with a reduction in central and peripheral immune tolerance to self and increased T cell activation and proliferation14,15,16,17, which emphasizes the participation of the immune system in the development of T1DM18.
During the development of T1DM, seroconversion of islet autoantibodies to insulin, glutamate decarboxylase, insulinoma antigen 2 or zinc transporter 8 represents the first notable sign of autoimmunity and their combined presence in serum remains the best predictor for both loss of immune tolerance (that is, induction of autoimmunity) and clinical manifestation of T1DM, albeit that their role in β-cell destruction remains unclear19,20. During disease progression, immune cells that infiltrate the pancreas and target insulin-producing cells create an inflammatory environment characteristic of insulitis that triggers and accelerates T1DM development by increasing exposure of islet antigens presented by HLA class I molecules to the immune system7,21,22,23 (Box 1).
The presence of islet-specific autoreactive CD4+ and CD8+ T cells in peripheral blood, pancreatic draining lymph nodes and insulitic lesions7,24,25,26,27,28 provided evidence for T1DM as an autoimmune disease, where an impaired thymic education was responsible for the immune attack directed against self-proteins of insulin-producing cells29,30,31. Yet, despite their importance in T1DM pathology, the frequency of these autoreactive cells in peripheral blood is low and quite similar between patients with T1DM and healthy individuals32. Although the presence of naive autoreactive cells in healthy individuals indicates that these cells are part of the normal T cell repertoire and that ‘we are all autoimmune’, the increased frequency of CD8+ T cells (in particular resident memory cells) in the pancreata of patients with T1DM compared with those of control individuals implies a differential peripheral activation and/or regulation in patients with T1DM7,32,33. Indeed, regulatory T (Treg) cells, which have an important role in repressing these autoreactive T cells in healthy individuals, show a similar frequency in control individuals and in patients with T1DM but with a reduced suppressive capacity in patients with T1DM34,35,36.
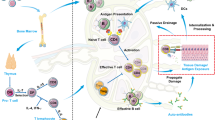
Intriguingly, islet autoreactive T cells have unusual characteristics compared with T cells that protect us from cancer and infection, such as a fairly low epitope binding affinity for HLA, low T cell receptor (TCR) avidity for HLA–epitope complexes, tilted or even reversed docking of the TCR on the HLA–peptide complex, suboptimal synapse formation in the interphase between T cells and antigen-presenting cells or target cells and abnormal expression of signalling molecules that might have contributed to incomplete thymic education and thymic selection37,38,39,40,41,42,43,44,45. Consistently, patients with cancer who are treated with immune checkpoint inhibitors (that is, anti-PD1, anti-PDL1 or anti-CTLA4 therapies) aimed at reducing immune regulation and initiating an immune response against the tumour tissue (Fig. 1) are at risk of developing adverse effects, including acute T1DM, presumably due to loss of immune regulation combined with activation of naive autoreactive T cells46,47.
a | In healthy individuals, β-cells are protected from autoimmune β-cell destruction by immune regulation exerted by regulatory T (Treg) cells and PD1 ligation. b | While advantageous in preventing autoimmunity, Treg cells impede antitumour immunity. c | In type 1 diabetes mellitus (T1DM), insufficient immune regulation can result in an autoimmune response by autoreactive T cells, particularly if these cells are provoked by β-cells. d | The response in T1DM resembles effective antitumour immunity as a result of immunotherapeutic blockade of PD1 or its ligand PDL1 that otherwise keep autoimmune responses in check. In addition to resulting in antitumour immunity, other immune and autoimmune responses might be triggered, including those against pancreatic islets. T1DM is a serious adverse effect of tumour immunotherapy. GRZB, granzyme B; TCR, T cell receptor.
In the past couple of years, it has been argued that autoimmune diabetes mellitus induced by immune checkpoint blockade and T1DM are different diseases48, but this contention is perhaps premature. Indeed, T1DM is not one disease, as can easily be appreciated by comparing T1DM diagnosed in children versus that diagnosed in adolescents or adults8,49,50,51. Some of the major differences in presentation of autoimmune diabetes mellitus induced by immune checkpoint inhibition and other types of T1DM relate to the acute manifestation and short prodromal phase of the former, leading to fairly frequent and severe ketoacidosis and paucity of autoantibodies at diagnosis46,47. After all, islet autoantibodies take time to be generated, following T cell activation. The demonstration that T1DM can be transferred with bone marrow from a donor with T1DM to an immune-suppressed recipient who did not have T1DM only when T cells are not depleted, underscores the relevance of T cells in the immunopathogenesis of T1DM52. Furthermore, pancreatitis rarely leads to T1DM, even in patients with an increased genetic risk of T1DM, which in turn emphasizes that loss of immune tolerance and induction of islet autoimmunity are a prerequisite for development of the disease53. This finding is supported by the rapid recurrence of islet autoimmunity, selective β-cell destruction and T1DM following partial pancreas transplantation from non-diabetic donors to their monozygotic twins with T1DM, as well as islet autoimmunity predicting failure or poor prognosis of allogenic islet transplantation and autologous bone marrow transplantation54,55,56,57,58. Finally, the fact that, until now, immunotherapeutic strategies have shown temporal efficacy in delaying disease progression implicates the immune system in T1DM pathology59,60.
Inconsistencies in the role of T cells
A different stand on a role of T cells in the pathogenesis of T1DM can easily be defended61 (Box 2). Islet autoreactive T cells are common in the healthy population, and nine out of ten individuals with islet autoantibodies will never develop T1DM32,62,63. Most patients with T1DM have immune regulation that is indistinguishable from that of healthy individuals, and over 99% of patients with cancer who are treated with immune checkpoint inhibitors do not develop T1DM46,47,64. Furthermore, some patients with T1DM present with negligible T cell autoimmunity51. Moreover, induction of autoimmune diabetes mellitus in mice by vaccination with islet autoantigens is very difficult, if not impossible65. Even when transduction of human islet autoreactive TCRs in humanized mice leads to high frequencies of T cell autoimmunity to islets, no diabetes mellitus was induced29. In addition, thus far, progression of T1DM has not been found to accelerate after patients with T1DM are injected with islet autoantigens59,66,67,68. Of note, HLA upregulation as an early sign of islet distress frequently occurs without inflammation, even if β-cells are still present7,69, while insulitis is a rare feature in individuals who have islet autoantibodies but not T1DM70. Furthermore, immunotherapies in T1DM have not yet shown a durable effect on disease progression60. These inconsistencies in our understanding of the critical role of islet autoimmunity, and T cells in particular, require reconciliation.
T1DM as a disease of β-cells
Given that autoreactive T cells are part of a normal T cell repertoire, it is implausible that the disease is entirely the result of dysfunctional immune cells; rather, peripheral activation of the immune system is required locally in the targeted tissue32,71. A role for β-cells in their own demise was first proposed by Bottazzo72. Different triggers that might lead β-cells to provoke an immune response have been proposed, ranging from the size of the pancreas and β-cell mass to viral infection and metabolic stress4,73,74. Indeed, the pancreata of patients with T1DM are smaller than those from unrelated control individuals73 (Box 3). Yet, at-risk individuals and patients with T1DM have pancreata of similar sizes73, and no data at this time suggest that the pancreas decreases in size with disease progression. Obviously, less β-cell mass might equal less β-cell functional capacity and increased pressure on β-cells to cope with glycaemic control. In addition to metabolic stress, viral infections or intestinal inflammatory agents ‘leaking’ into the pancreas might create a pro-inflammatory environment61,74,75. β-Cells are exposed to viral infection as they express specific receptors and adhesion molecules. Indeed, the presence of a coxsackievirus and adenovirus receptor (CAR) that is unique to β-cells, found in the insulin-containing granules, might leave β-cells vulnerable to viral infection during insulin secretion, as illustrated by studies correlating enteroviral infection by coxsackievirus B4 with islet autoimmunity (but not T1DM)76,77. Viral infection might be a risk factor in, at best, a small minority of patients with T1DM78. A viral contribution to the development of T1DM is certainly not limited to coxsackievirus; for example, rotavirus and cytomegalovirus have also been implicated78,79,80,81.
Role of diet and microbiota
Similarly, dysbiosis of the gastrointestinal tract (a ‘gut storm’) provoked by changes in intestinal microbiota and an increased Bacteroidetes to Firmicutes ratio has been correlated with seroconversion and onset of T1DM (the pancreas being an intestinal organ)82. Microbiota shape peripheral immune tolerance, modulating both migration and differentiation of immune cells to maintain intestinal homeostasis; furthermore, local inflammation is limited through short-chain fatty acids (SCFAs) generated by resident gut bacteria from fermentation of non-digestible carbohydrates83. SCFAs have a direct effect on T cell subsets via histone deacetylase inhibition and activation of mTOR and STAT3 signalling, leading to an increased proportion of regulatory T cells that produce IL-10 and express FOXP3. In addition, SCFAs can exert their anti-inflammatory effect on neutrophils, macrophages and plasmacytoid dendritic cells via antimicrobial peptides produced by innate lymphoid cells or by β-cells themselves84,85,86,87. Strong evidence from studies in mice demonstrates the protective role of these cationic antimicrobial peptides against autoimmune diabetes mellitus, and SCFAs have been used to prevent cytokine-induced cell death of human islet cells and to improve β-cell function88. Despite these positive findings, a first-in-human crossover clinical trial conducted in patients with longstanding T1DM (mean diabetes mellitus duration of 8 years) that aimed to restore epithelial integrity by short-term oral butyrate supplementation failed to show improvement in adaptive and innate immune system parameters89.
Diet can also affect the microbiome favourably or unfavourably with regard to the predisposition for developing T1DM. A low gluten diet can induce favourable changes in the intestinal microbiome of healthy adults, while low maternal gluten intake during pregnancy shows a remarkable correlation with reduced development of T1DM in the offspring90,91. While it remains to be established whether patients at risk of T1DM or patients newly diagnosed with T1DM would benefit from a low gluten diet, these data might also suggest that once initiated, local inflammation in the pancreas is sufficient to drive disease progression, given the inherent molecular fragility of β-cells (the ‘domino effect’).
Genetic risk
Genetic risk, determined by certain genetic variants in the gene encoding insulin (INS), might affect β-cell function and glycaemic control14,15,92,93. Early studies suggest that protective variants of INS result in increased INS expression in the thymus, thereby increasing the probability that the immune system will be educated to avoid immune reactivity to insulin; however, differences in INS activity in pancreatic islets have also been linked to these genetic polymorphisms, as well as effects on β-cell function and resilience14,15,92,93,94,95,96,97. Other genetic variants associated with increased risk of T1DM might affect β-cell health, vitality and self-defence98,99,100. β-Cell mass and function might have been declining for more than 10 years before clinical manifestation of T1DM, adding to increasing metabolic stress in β-cells and vulnerability to autoimmune insults101,102.
Insights from human studies of insulitis
Our understanding of the effect of insulitis on β-cells has exploded with the increased access to pancreata from donors with diabetes mellitus (Network for Pancreatic Organ Donors with Diabetes), even though the condition of the donors (factors such as cause of death, presence of brain death, stay and treatment in an intensive care unit, cold ischaemia, ketoacidosis, injury and stress) might influence some of the observations made on the pancreata, in terms of the effects of stress7,21,22. An increased expression of markers specific for the unfolded protein response to stress in β-cells during insulitis suggests that adaptive mechanisms are engaged to help β-cells deal with the environmental pressure103. Stressed β-cells have a reduced overall translation rate, initiate degradation of proteins accumulated in the endoplasmic reticulum (ER), increase the translation rate of chaperones and promote autophagy to return to cellular homeostasis104,105,106. However, the extraordinary capacities of β-cells to produce up to 1 million insulin molecules per minute and to increase production in excess of 50-fold in response to glucose106, combined with low expression of superoxide dismutase and anti-apoptotic factor BCL-2 make β-cells poorly equipped to survive the inflammatory milieu. β-Cells are more sensitive than α-cells to environmental stimuli, as illustrated by studies conducted on islets challenged by metabolic stress mimicking pathophysiological conditions in type 2 diabetes (T2DM)107,108. In addition to the cytoprotective function, activation of the ER stress sensors is known to lead to a cascade of events promoting direct apoptosis via activation of the IRF–STAT1 pathway98,109, necroptosis via activation of TNFR1–RIP1 and necrosis by increased production of reactive oxygen species as well as induction of a form of β-cell senescence108,110,111 (Fig. 2). These mechanisms might participate in the amplification of inflammation and destruction of β-cells by starting communication with other endocrine cells and resident immune cells. Stress-induced senescence drives β-cells to a senescence-associated secretory phenotype, which is correlated with intra-islet infiltration of CD45+ immune cells in patients with T1DM112.
a | In non-stressed (‘happy’) β-cells, glucose uptake via the glucose transporter GLUT1 leads to pyruvate formation through glycolysis and to increased ATP production by mitochondria. The resulting increased cytosolic level of ATP promotes the closure of the potassium channels, membrane depolarization and opening of the voltage-dependent calcium channels. The rise in intracellular levels of calcium triggers exocytosis of the insulin-containing granules. In these conditions, several genes and proteins are upregulated to restore the cellular stock of insulin. During this process, non-translocated preproinsulin, endoplasmic reticulum (ER)-resident insulin signal peptide and non-mature proinsulin molecules are degraded through the proteasome directly or after retro-translocation and are presented by HLA class I at the cell surface (normal β-cell ligandome). b | In type 1 diabetes mellitus pathophysiological conditions, cytokines lead to profound changes in gene and protein expression, mainly by activation of the STAT1, IRF1 and NF-κB downstream pathways, that ultimately drives hyper-expression of HLA class I, and also the surface expression of inhibitory receptors (that is, PDL1). In the stress condition, calcium uptake by the mitochondria is responsible for increased permeability of the mitochondria that precedes the release of pro-apoptotic factors (such as reactive oxygen species (ROS) and cytochrome c (cyt c)). Calcium depletion in the ER leads to activation of cytosolic calcium-dependent enzymes (such as transglutaminase 2 and peptidyl arginine deiminase) that are involved in the post-translational modification processes by inducing protein deamidation and citrullination, respectively. The recruitment of the ER chaperones (binding immunoglobulin protein (BiP)) in response to the accumulation of misfolded protein within the ER leads to the activation of the sensors (PERK, IRE1a and ATF6) expressed at the surface of the ER membrane. The PERK pathway attenuates mRNA translation by phosphorylation of the eIF2a translation initiation factor. Phosphorylation of IRE1a and translocation of ATF6 activate the ER accumulated protein degradation pathway and the transcription of chaperone encoding genes with protein products involved in degradation of misfolded proteins, and also restores ER homeostasis. Long-term exposure to inflammatory stress promotes additional coping mechanisms, including initiation of recycling programmes and selective secretion of proteins and small RNAs in microvesicles, and ultimately leads to the induction of an apoptosis programme mediated by the transcriptional activation of CHOP by the combined activity of PERK and ATF6. During stress, normal transcription, translation and degradation is affected, which generates alternative RNA splicing, defective ribosomal products and hybrid peptides, respectively (stressed β-cell ligandome). CAR, coxsackievirus and adenovirus receptor; DRiP, defective ribosomal product; PRR, pattern recognition receptor; SASP, senescence-associated secretory phenotype; TLR, Toll-like receptor.
Studies of human insulitis have revealed that ‘danger signals’ from stressed β-cells might precede insulitis. Among these signals, hyper-expression of HLA class I (and possibly HLA class II) was noted across pancreata from patients with newly diagnosed T1DM7. In addition, islets secrete the chemokine CXCL10, attracting leukocytes expressing its receptor CXCR3 to the lesion113. This chemokine production by stressed β-cells might present a master switch of islet inflammation and has attracted interest from the pharmaceutical industry as an opportunity for intervention therapy114. Other strategies include efforts to reduce β-cell stress with verapamil, where early studies have shown promise for delaying T1DM disease progression115. Intriguingly, high levels of insulin-specific autoreactive human T cells only precipitated insulitis and selective β-cell destruction in humanized mice in vivo after the mice had been vaccinated with insulin peptide to prime an autoimmune response and subjected to low-dose streptozotocin to stress the β-cells. This finding underscores the need for β-cell perturbation and loss of autoimmune tolerance to β-cells to create a ‘perfect storm’ that causes their destruction29.
Role of the exocrine pancreas
T1DM seems to affect both the endocrine and exocrine pancreas, as studies have shown inflammation and loss of exocrine parenchyma7,22,73. This finding is a potentially important missing link to be discussed and incorporated in any hypothesis aiming to clarify the mechanisms that lead to T1DM. Yet, in spite of efforts to prove an actual decline in total pancreas mass longitudinally, no evidence indicates that pancreas mass decreases with time in patients with T1DM. Indeed, although patients with T1DM often have a small pancreas, the pancreata of first-degree relatives of patients with T1DM, with or without islet autoimmunity, tend to be smaller than those of the general population as well, possibly pointing to inherent small pancreas sizes in individuals prone to developing T1DM73. It is tempting to speculate that a smaller pancreas and subsequent reduced endocrine mass would increase the burden on the reduced number of β-cells trying to cope with hyperglycaemia; that is, ‘size matters’. In terms of exocrine inflammation, the argument about whether this effect is secondary to the fatal condition of the pancreas donor and organ procurement has not been settled yet; however, pancreas tissue obtained from biopsy samples of living patients with newly diagnosed T1DM tends to show less pronounced or no exocrine involvement compared with samples obtained at autopsy7,22,116. Importantly, insulitic lesions early after diagnosis of T1DM point to monoclonal or oligoclonal infiltration with islet autoreactive CD8+ T cells only, with little evidence of ‘bystander’ T cells or exocrine involvement, which underscores the central role of autoimmunity in pancreas immunopathology at that stage7. In addition, islets depleted of β-cells no longer show insulitis7,22, which suggest that β-cells are the driving force of this inflammatory process characteristic of T1DM and underscores the central role of β-cells in the disease process.
Islet-resident macrophages and inflammation
In the dialogue between β-cells and the immune cell compartment, islet-resident macrophages have a mediator role as they engulf, process and present catabolic products from insulin granules or products that are carried by exosome particles secreted by β-cells117. The localization of islet-resident macrophages near blood vessels and in close contact with β-cells, forming synapse-like structures, emphasizes their role in the effector phase of T1DM as they secrete pro-inflammatory cytokines and free radicals, triggering NF-κB and STAT1 downstream signalling pathways and FAS-mediated apoptosis in β-cells118,119. Conventionally, macrophages recognize pathogen-associated molecular patterns or damaged tissue-associated molecular patterns (DAMPs) via Toll-like receptors (TLRs). DAMPs derived from β-cell-specific antigens (such as insulin and islet amyloid polypeptide (IAPP, also known as amylin)) have been described. Interestingly, in mouse models susceptible to autoimmune diabetes mellitus, members of the TLR family in the presence of β-cell DAMPs trigger T1DM, while in the absence of the corresponding ligands, the same TLRs exert tolerance; this finding shows the importance of β-cells in the balance between tolerance and autoimmunity120. In mice, therapies affecting macrophages limit T1DM progression121,122, while a study using pancreatic biopsy samples from patients with recent onset T1DM (3–9 weeks after diagnosis) showed that islet-resident macrophages (and infiltrating dendritic cells) are the main source of pro-inflammatory cytokines released during insulitis, positioning macrophages at the centre of the pathology of T1DM123.
Exposure to cytokines can cause substantial metabolic and epigenetic changes. For instance, DNA methylome profiles, histone acetylation and deacetylation levels and chromatin structure are altered in β-cells, exposing promoters and enhancers for inflammatory response factors and T1DM genetic predisposition loci, as shown in NOD mice and human islets after exposure to pro-inflammatory cytokines124,125,126,127. Combining these results with other ‘omics’ studies indicates that genes responsive to interferon, protein degradation and HLA loading machinery processes are the main factors that are disturbed during inflammation, which suggests that insulitis not only leads to β-cell dysfunction but also to increased β-cell visibility to immune surveillance99,128,129,130. We have also described how inflammation induced by ER stress can shape β-cell immunogenicity and control cytotoxic destruction by miRNA-mediated regulation of ERAP1 and its effect on preproinsulin processing104.
Peptide presentation by β-cells
The effect of cytokines on β-cells is not limited to an increased peptide–HLA density at the cell surface but also affects the nature of the peptides presented. Currently, several autoantigens have been identified and while many peptides are derived from native proteins, a new range of neoantigens (protein products from mutations, frameshifts, alternative mRNA splicing and post-translational modifications) originating from alternative splicing131, translational mistakes31, post-translational modifications5,107,132,133, peptide fusion134 and possibly immunoproteasome activation135 has emerged that strongly activate the immune system response (Fig. 2). In inflammatory conditions, the increased splicing events measured by RNA-seq in human islets combined with translation infidelity and increased activity of post-translational enzymes (such as protein arginine deiminases and tissue transglutaminase 2) contribute to the diversity of the islet proteome99,136,137,138. A β-cell ligandome landscape was presented by combining HLA class I peptidomic and transcriptomic analyses after cytokine stimulation130. While these results demonstrated that most of the presented (β-cell-specific) epitopes were derived from secretory granule components, which are hyper-immunogenic27,28,117,130, most of the alternative epitopes were not detected, despite evidence that they were able to trigger a pro-inflammatory T cell response. The low expression rate of most of these neoantigens is probably close to the sensitivity limits of proteomic analyses, so they might not be detected. Alternatively, neoantigen synthesis might require chronic rather than acute exposure to cytokines, while immune cells producing pro-inflammatory cytokines might provide additional extra stress signals to the β-cells.
Interestingly, dendritic cells can convert native islet autoantigens into immunogenic neoantigens, revealing a role for islet-resident dendritic cells in the induction or expansion of islet autoimmunity133. Yet, these results have shed light on new mechanisms implying that hybrid peptides are generated by β-cells during proteolysis in the proteasome, where some protein fragments can be retained and bound to other N-terminal peptides in a process called peptide splicing (for example, cis-peptidation reaction within PTPRN and transpeptidation reactions between IAPP and PTPRN, SLC30A8 and PCSK2, and PIK3R3 and PIK3R1), before loading on HLA134. Even though progress has been made, our knowledge of neoantigens in T1DM is still at an early stage and is limited. Furthermore, other potential mechanisms have just been presented (that is, hybrid peptides and defective ribosomal products) or have been overlooked (RNA editing). Mechanisms to create neoantigens in tumours should be investigated as additional potential sources of neoantigens in T1DM. For instance, the double-stranded RNA-specific adenosine deaminase ADAR1 switches adenine to inositol, thereby changing aspartic acid into arginine. Expression of this enzyme in breast cancer correlates with high infiltration of T cells into tumours and immune reactivity to edited antigens139. Increased expression of ADAR1 in patients with systemic lupus erythematous is associated with increased RNA editing events, indicating the possible involvement of RNA editing in the autoimmune reaction140.
All the aforementioned data highlight the importance of endogenous characteristics of β-cells and their response to exogenous inflammatory stimuli for disease progression and exacerbation. These findings also demonstrate the need for intensification of efforts to fully unravel β-cell physiology in health and autoimmunity.
Lessons from cancer
The dogma describing T1DM as a disease characterized by total destruction of the insulin-producing β-cells has been shaken by immunohistochemistry studies performed on pancreatic specimens from patients with longstanding T1DM showing the presence of β-cells and insulin microsecretion (C-peptide value of <30 pmol/l) in the majority of these patients, implying that some β-cells resist or escape the immune attack, or that new β-cells are formed141,142. The lobularity of this feature (where β-cells in certain pancreatic lobules seem unaffected, while β-cells in other lobules are depleted) might imply formation of new pancreatic lobules with unaffected islets, which increases the sense of urgency to protect β-cells after a diagnosis of T1DM. Confirming these observations, the latest single-cell analysis methods (that is, transcriptomics, mass cytometry and imaging mass spectrometry) have revealed wide heterogeneity in the β-cell population in healthy pancreata but also during disease progression, which might contribute to different sensitivities of β-cells to immune responses143,144,145,146. Evidence of this concept is found in multiple sclerosis, where different oligodendrocyte phenotypes have different levels of autoimmune reactions, potentially driving self-destruction147. Importantly, while the presence of insulin-positive cells and lack of insulitis in longstanding T1DM might suggest that ‘normal’ islets are present, the lack of detectable C-peptide and differential clustering from islets of non-diabetic donors in multidimensional mass cytometry analyses points to intrinsic differences in patient-derived islets that might reflect prodromal islet distress and prediabetic lesions145. Intriguingly, studies of insulin and proinsulin in pancreata from patients with T1DM support the existence of aetiopathological endotypes of T1DM that are associated with age at diagnosis, and point to age-related intrinsic differences in distressed β-cells during insulitis that might lead to different autoimmune reactions8,50,51.
A concept is emerging that the immune response seen in T1DM might be one with ‘good intentions’, where the immune response to distressed tissue resembles the immune response that has evolved to detect infected tissue or tumours. Indeed, people carrying T1DM risk gene variants have a hyper-inflammatory immune system148. It can be argued that patients with T1DM have an immune system that might be beneficial in patients with cancer. A clear analogy in support of this provocative contention is presented by Lambert–Eaton myasthenic syndrome, which has two different aetiologies: one associated with immune hypersensitivity and autoimmunity (a phenotype shared with T1DM) and one where an antitumour immune response against the voltage-gated calcium channels expressed by small cell lung carcinoma cells and nerve endings causes cross reactivity in the neuromuscular synapse. Patients with small cell lung carcinoma who develop Lambert–Eaton myasthenic syndrome have a better prognosis for cancer survival than patients who do not develop this syndrome149,150,151. In addition, in patients with cancer, immune responses that are initiated after antitumour immunotherapy tend to be directed to neoantigens rather than native autoantigens152.
In a similar manner to tumour cells that evade immune responses to become more invasive, β-cells have developed active self-protective mechanisms to limit further autoimmune destruction; the upregulated expression of inhibitory receptors (such as PDL1) at their cell surface and the increased expression of IDO1 after cytokine challenge illustrate these changes153,154. A correlation between loss of IDO1 expression and β-cell destruction extends proof for the participation of these protective mechanisms in the maintenance of the β-cell integrity155. In addition, several studies have suggested that increased degranulation and/or a loss of β-cell identity occurs under environmental pressure, which is supported by the defect in insulin production and the presence of polyhormonal cells in the pancreata of patients with T1DM. From these findings, a concept of a ‘β-cell identity crisis’ has emerged where β-cells dedifferentiate into other endocrine cells (α-cells or δ-cells) as a defence mechanism156,157,158. Along with this β-cell identity crisis, levels of ‘semi’ β-cells that only express chromogranin A (chromogranin-positive, hormone-negative (CPHN) cells) are increased in the pancreata from patients with T1DM and T2DM and they are scattered throughout the pancreas regardless of inflammation level158,159. The origin of these cells is still unknown; however, the mere fact that CPHN cells express the autoantigen chromogranin A without this leading to their destruction might suggest that insulin production and the inherent negative molecular effects are needed to drive autoimmunity. Similarly, not all T1DM autoantigens are β-cell-specific; chromogranin A and receptor-type tyrosine-protein phosphatase N2 are also expressed in other tissues not affected by an immune attack in patients with T1DM160.
By comparing islet and tumour microenvironments, increasing evidence supports the notion that in autoimmune diseases, as in effective tumour immunity or following antitumour immunotherapy, the immune system is acting on dysfunctional cells or tissues that have accumulated aberrant or modified proteins147.
Conclusions
The appreciation of a role for β-cells in their own demise, the importance of ER stress in T1DM pathology, the identification of residual β-cells in patients who have had T1DM for several decades and the presence of dormant (‘hibernating’) β-cells that evade immune attack suggest that β-cell dysfunction and destruction are driven by their metabolic activity, and might lie at the heart of the aetiologies of both T1DM and T2DM. Both types of diabetes mellitus are chronic inflammatory diseases, and both are β-cell diseases. Thus far, immunotherapy alone has proven insufficient to achieve lasting preservation of β-cell function, pointing to the need to combine this strategy with β-cell therapy. In T2DM, inflammatory cytokines (secreted by stressed adipocytes or stressed β-cells) and recruitment of macrophages, B cells and T cells have been found to participate in β-cell failure and pathology. Accordingly, several intervention strategies for T2DM aimed at alleviating pressure exerted on β-cells and improving glycaemic control have been evaluated in the context of T1DM: metformin, GLP1 analogues (liraglutide, exendin 4 or sitagliptin) and verapamil have shown some benefit when combined with insulin therapy in the treatment of patients with T1DM115. We favour the engagement of the immune system, rather than suppression of the immune system, to reverse the immunopathogenesis of T1DM, in combination with β-cell therapy to improve β-cell stamina and vitality and to protect these cells from metabolic and inflammatory assaults. At a time when the coronavirus disease 2019 (COVID-19) pandemic reminds us of the need for a fully functional immune system, we cannot afford to suppress it and put patients with inflammatory disorders in danger of infection or cancer60. Novel therapies are already being assessed in the clinic that ‘negotiate’ with the immune system, rather than suppress it, including ‘inverse’ vaccination strategies that aim to induce selective immune tolerance to islet autoantigens, similar to desensitization when treating allergies59,66,161,162. This strategy, in combination with β-cell therapy, is an attractive strategy to achieve durable remission in T1DM.
References
Gepts, W. Islet changes suggesting a possible immune aetiology of human diabetes mellitus. Acta Endocrinol. Suppl. 205, 95–106 (1976).
Bottazzo, G. F. et al. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N. Engl. J. Med. 313, 353–360 (1985).
Roep, B. O. The role of T-cells in the pathogenesis of type 1 diabetes: from cause to cure. Diabetologia 46, 305–321 (2003).
Eizirik, D. L., Colli, M. L. & Ortis, F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 5, 219–226 (2009).
Roep, B. O., Kracht, M. J., van Lummel, M. & Zaldumbide, A. A roadmap of the generation of neoantigens as targets of the immune system in type 1 diabetes. Curr. Opin. Immunol. 43, 67–73 (2016).
Mallone, R. & Eizirik, D. L. Presumption of innocence for beta cells: why are they vulnerable autoimmune targets in type 1 diabetes? Diabetologia 63, 1999–2006 (2020).
Coppieters, K. T. et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 209, 51–60 (2012).
Leete, P. et al. Studies of insulin and proinsulin in pancreas and serum support the existence of aetiopathological endotypes of type 1 diabetes associated with age at diagnosis. Diabetologia 63, 1258–1267 (2020).
Shields, B. M. et al. C-peptide decline in type 1 diabetes has two phases: an initial exponential fall and a subsequent stable phase. Diabetes Care 41, 1486–1492 (2018).
Nerup, J. et al. HL-A antigens and diabetes mellitus. Lancet 2, 864–866 (1974).
Barrett, J. C. et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 41, 703–707 (2009).
van Lummel, M. et al. Dendritic cells guide islet autoimmunity through a restricted and uniquely processed peptidome presented by high-risk HLA-DR. J. Immunol. 196, 3253–3263 (2016).
van Lummel, M. et al. Discovery of a selective islet peptidome presented by the highest-risk HLA-DQ8trans molecule. Diabetes 65, 732–741 (2016).
Pugliese, A. et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 15, 293–297 (1997).
Vafiadis, P. et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat. Genet. 15, 289–292 (1997).
Bottini, N. et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 36, 337–338 (2004).
Vella, A. et al. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am. J. Hum. Genet. 76, 773–779 (2005).
Gebe, J. A., Swanson, E. & Kwok, W. W. HLA class II peptide-binding and autoimmunity. Tissue Antigens 59, 78–87 (2002).
Bottazzo, G. F., Florin-Christensen, A. & Doniach, D. Islet cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 2, 1279–1282 (1974).
Bloem, S. J. & Roep, B. O. The elusive role of B lymphocytes and islet autoantibodies in (human) type 1 diabetes. Diabetologia 60, 1185–1189 (2017).
Willcox, A., Richardson, S. J., Bone, A. J., Foulis, A. K. & Morgan, N. G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 155, 173–181 (2009).
Campbell-Thompson, M. et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 65, 719–731 (2015).
Gepts, W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14, 619–663 (1965).
Velthuis, J. H. et al. Accumulation of autoreactive effector T cells and allo-specific regulatory T cells in the pancreas allograft of a type 1 diabetic recipient. Diabetologia 52, 494–503 (2009).
Michels, A. W. et al. Islet-derived CD4 T cells targeting proinsulin in human autoimmune diabetes. Diabetes 66, 722–734 (2017).
Babon, J. A. et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat. Med. 22, 1482–1487 (2016).
Roep, B. O. et al. T-cell reactivity to 38 kD insulin-secretory-granule protein in patients with recent-onset type 1 diabetes. Lancet 337, 1439–1441 (1991).
Roep, B. O., Arden, S. D., De Vries, R. R. P. & Hutton, J. C. T-cell clones from a type-1 diabetes patient respond to insulin secretory granule proteins. Nature 345, 632–634 (1990).
Tan, S. et al. Type 1 diabetes induction in humanized mice. Proc. Natl Acad. Sci. USA 114, 10954–10959 (2017).
Skowera, A. et al. CTLs are targeted to kill β cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J. Clin. Invest. 118, 3390–3402 (2008).
Kracht, M. J. et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat. Med. 23, 501–507 (2017).
Culina, S. et al. Islet-reactive CD8(+) T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci. Immunol. 3, eaao4013 (2018).
Kuric, E. et al. Demonstration of tissue resident memory CD8 T cells in insulitic lesions in adult patients with recent-onset type 1 diabetes. Am. J. Pathol. 187, 581–588 (2017).
Tree, T. I. et al. Naturally arising human CD4 T-cells that recognize islet autoantigens and secrete interleukin-10 regulate proinflammatory T-cell responses via linked suppression. Diabetes 59, 1451–1460 (2010).
Lindley, S. et al. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes 54, 92–99 (2005).
Buckner, J. H. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 10, 849–859 (2010).
Ouyang, Q. et al. Recognition of HLA class I-restricted β-cell epitopes in type 1 diabetes. Diabetes 55, 3068–3074 (2006).
Abreu, J. R. et al. CD8 T cell autoreactivity to preproinsulin epitopes with very low human leucocyte antigen class I binding affinity. Clin. Exp. Immunol. 170, 57–65 (2012).
Velthuis, J. H. et al. Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes 59, 1721–1730 (2010).
Unger, W. W. et al. Discovery of low-affinity preproinsulin epitopes and detection of autoreactive CD8 T-cells using combinatorial MHC multimers. J. Autoimmun. 37, 151–159 (2011).
Beringer, D. X. et al. T cell receptor reversed polarity recognition of a self-antigen major histocompatibility complex. Nat. Immunol. 16, 1153–1161 (2015).
Bulek, A. M. et al. Structural basis for the killing of human beta cells by CD8(+) T cells in type 1 diabetes. Nat. Immunol. 13, 283–289 (2012).
Schubert, D. A. et al. Self-reactive human CD4 T cell clones form unusual immunological synapses. J. Exp. Med. 209, 335–352 (2012).
Nicholson, M. J., Hahn, M. & Wucherpfennig, K. W. Unusual features of self-peptide/MHC binding by autoimmune T cell receptors. Immunity 23, 351–360 (2005).
Beeton, C. et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl Acad. Sci. USA 103, 17414–17419 (2006).
Stamatouli, A. M. et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 67, 1471–1480 (2018).
de Filette, J. M. K. et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur. J. Endocrinol. 181, 363–374 (2019).
Tsang, V. H. M. et al. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J. Clin. Endocrinol. Metab. 104, 5499–5506 (2019).
Leete, P. et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes 65, 1362–1369 (2016).
Battaglia, M. et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43, 5–12 (2020).
Claessens, L. A. et al. Clinical and genetic correlates of islet-autoimmune signatures in juvenile-onset type 1 diabetes. Diabetologia 63, 351–361 (2019).
Lampeter, E. F. et al. Transfer of insulin-dependent diabetes between HLA-identical siblings by bone marrow transplantation. Lancet 341, 1243–1244 (1993).
Lampeter, E. F. et al. Inflammatory islet damage in patients bearing HLA-Dr 3 and/or Dr 4 haplotypes does not lead to islet autoimmunity. Diabetologia 37, 471–475 (1994).
Sibley, R., Sutherland, D. E. R., Goetz, F. & Michael, A. F. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab. Invest. 53, 132–144 (1985).
Malmegrim, K. C. et al. Immunological balance is associated with clinical outcome after autologous hematopoietic stem cell transplantation in type 1 diabetes. Front. Immunol. 8, 167 (2017).
Hilbrands, R. et al. Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in type 1 diabetic patients. Diabetes 58, 2267–2276 (2009).
Huurman, V. A. et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS ONE 3, e2435 (2008).
Pinkse, G. G. et al. Autoreactive CD8 T cells associated with β cell destruction in type 1 diabetes. Proc. Natl Acad. Sci. USA 102, 18425–18430 (2005).
Roep, B. O., Wheeler, D. C. S. & Peakman, M. Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol. 7, 65–74 (2019).
Atkinson, M. A., Roep, B. O., Posgai, A., Wheeler, D. C. S. & Peakman, M. The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol. 7, 52–64 (2019).
Skog, O., Korsgren, S., Melhus, A. & Korsgren, O. Revisiting the notion of type 1 diabetes being a T-cell-mediated autoimmune disease. Curr. Opin. Endocrinol. Diabetes Obes. 20, 118–123 (2013).
Roep, B. O. et al. T-cell reactivity to β-cell membrane antigens associated with β-cell destruction in IDDM. Diabetes 44, 278–283 (1995).
Roep, B. O. et al. Autoreactive T cell responses in insulin-dependent (type 1) diabetes mellitus. Report of the first international workshop for standardization of T cell assays. J. Autoimmun. 13, 267–282 (1999).
Long, S. A. et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes 59, 407–415 (2010).
Gibson, V. B. et al. Proinsulin multi-peptide immunotherapy induces antigen-specific regulatory T cells and limits autoimmunity in a humanized model. Clin. Exp. Immunol. 182, 251–260 (2015).
Ludvigsson, J. et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N. Engl. J. Med. 359, 1909–1920 (2008).
Huurman, V. A., Decochez, K., Mathieu, C., Cohen, I. R. & Roep, B. O. Therapy with the hsp60 peptide DiaPep277 in C-peptide positive type 1 diabetes patients. Diabetes Metab. Res. Rev. 23, 269–275 (2007).
Thrower, S. L. et al. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man phase I safety study. Clin. Exp. Immunol. 155, 156–165 (2009).
Foulis, A. K., Jackson, R. & Farquharson, M. A. The pancreas in idiopathic Addison’s disease–a search for a prediabetic pancreas. Histopathology 12, 481–490 (1988).
In’t Veld, P. et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 56, 2400–2404 (2007).
Danke, N. A., Koelle, D. M., Yee, C., Beheray, S. & Kwok, W. W. Autoreactive T cells in healthy individuals. J. Immunol. 172, 5967–5972 (2004).
Bottazzo, G. F. Lawrence lecture. Death of a beta cell: homicide or suicide? Diabet. Med. 3, 119–130 (1986).
Campbell-Thompson, M., Wasserfall, C., Montgomery, E. L., Atkinson, M. A. & Kaddis, J. S. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA 308, 2337–2339 (2012).
Dotta, F. et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl Acad. Sci. USA 104, 5115–5120 (2007).
Krogvold, L. et al. Detection of a low-grade enteroviral infection in the islets of Langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 64, 1682–1687 (2015).
Vehik, K. et al. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat. Med. 25, 1865–1872 (2019).
Ifie, E. et al. Unexpected subcellular distribution of a specific isoform of the Coxsackie and adenovirus receptor, CAR-SIV, in human pancreatic beta cells. Diabetologia 61, 2344–2355 (2018).
Roep, B. O. A viral link for type 1 diabetes. Nat. Med. 25, 1816–1818 (2019).
Honeyman, M. C. et al. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 49, 1319–1324 (2000).
Perrett, K. P., Jachno, K., Nolan, T. M. & Harrison, L. C. Association of rotavirus vaccination with the incidence of type 1 diabetes in children. JAMA Pediatr. 173, 280–282 (2019).
Hiemstra, H. S. et al. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc. Natl Acad. Sci. USA 98, 3988–3991 (2001).
Han, H. et al. Gut microbiota and type 1 diabetes. Int. J. Mol. Sci. 19, 995–1006 (2018).
Morrison, D. J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200 (2016).
Asarat, M., Apostolopoulos, V., Vasiljevic, T. & Donkor, O. Short-Chain fatty acids regulate cytokines and Th17/Treg cells in human peripheral blood mononuclear cells in vitro. Immunol. Invest. 45, 205–222 (2016).
Nastasi, C. et al. Butyrate and propionate inhibit antigen-specific CD8(+) T cell activation by suppressing IL-12 production by antigen-presenting cells. Sci. Rep. 7, 14516 (2017).
Sun, J. et al. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 43, 304–317 (2015).
Miani, M. et al. Gut microbiota-stimulated innate lymphoid cells support β-defensin 14 expression in pancreatic endocrine cells, preventing autoimmune diabetes. Cell Metab. 28, 557–572 e6 (2018).
Pingitore, A. et al. Short chain fatty acids stimulate insulin secretion and reduce apoptosis in mouse and human islets in vitro: role of free fatty acid receptor 2. Diabetes Obes. Metab. 21, 330–339 (2019).
de Groot, P. F. et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia 63, 597–610 (2020).
Hansen, L. B. S. et al. A low-gluten diet induces changes in the intestinal microbiome of healthy Danish adults. Nat. Commun. 9, 4630 (2018).
Antvorskov, J. C. et al. Association between maternal gluten intake and type 1 diabetes in offspring: national prospective cohort study in Denmark. BMJ 362, k3547 (2018).
Barratt, B. J. et al. Remapping the insulin gene/IDDM2 locus in type 1 diabetes. Diabetes 53, 1884–1889 (2004).
Nielsen, L. B. et al. Impact of IDDM2 on disease pathogenesis and progression in children with newly diagnosed type 1 diabetes: reduced insulin antibody titres and preserved beta cell function. Diabetologia 49, 71–74 (2006).
Durinovic-Bello, I. et al. Insulin gene VNTR genotype associates with frequency and phenotype of the autoimmune response to proinsulin. Genes Immun. 11, 188–193 (2010).
Durinovic-Bello, I. et al. Class III alleles at the insulin VNTR polymorphism are associated with regulatory T-cell responses to proinsulin epitopes in HLA-DR4, DQ8 individuals. Diabetes 54 (Suppl. 2), 18–24 (2005).
Bennett, S. T. et al. IDDM2-VNTR-encoded susceptibility to type 1 diabetes: dominant protection and parental transmission of alleles of the insulin gene-linked minisatellite locus. J. Autoimmun. 9, 415–421 (1996).
Vafiadis, P. et al. Imprinted and genotype-specific expression of genes at the IDDM2 locus in pancreas and leucocytes. J. Autoimmun. 9, 397–403 (1996).
Gysemans, C., Callewaert, H., Overbergh, L. & Mathieu, C. Cytokine signalling in the β-cell: a dual role for IFNγ. Biochem. Soc. Trans. 36, 328–333 (2008).
Eizirik, D. L. et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 8, e1002552 (2012).
Davies, J. L. et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature 371, 130–136 (1994).
Ferrannini, E. et al. Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 59, 679–685 (2010).
Sosenko, J. M. et al. The acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes 62, 4179–4183 (2013).
Marhfour, I. et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 55, 2417–2420 (2012).
Thomaidou, S. et al. β-Cell stress shapes CTL immune recognition of preproinsulin signal peptide by posttranscriptional regulation of endoplasmic reticulum aminopeptidase 1. Diabetes 69, 670–680 (2020).
Marasco, M. R. & Linnemann, A. K. β-Cell autophagy in diabetes pathogenesis. Endocrinology 159, 2127–2141 (2018).
Meyerovich, K., Ortis, F., Allagnat, F. & Cardozo, A. K. Endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. J. Mol. Endocrinol. 57, R1–R17 (2016).
Storling, J. et al. Do post-translational beta cell protein modifications trigger type 1 diabetes? Diabetologia 56, 2347–2354 (2013).
Marroqui, L. et al. Pancreatic α cells are resistant to metabolic stress-induced apoptosis in type 2 diabetes. EBioMedicine 2, 378–385 (2015).
Moore, F. et al. STAT1 is a master regulator of pancreatic β-cell apoptosis and islet inflammation. J. Biol. Chem. 286, 929–941 (2011).
Li, N. et al. Aging and stress induced β cell senescence and its implication in diabetes development. Aging 11, 9947–9959 (2019).
Rojas, J. et al. Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J. Diabetes Res. 2018, 9601801 (2018).
Thompson, P. J. et al. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab. 29, 1045–1060.e10 (2019).
Roep, B. O. et al. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin. Exp. Immunol. 159, 338–343 (2010).
Bonvin, P. et al. Antibody neutralization of CXCL10 in vivo is dependent on binding to free and not endothelial-bound chemokine: implications for the design of a new generation of anti-chemokine therapeutic antibodies. J. Biol. Chem. 292, 4185–4197 (2017).
Ovalle, F. et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat. Med. 24, 1108–1112 (2018).
Krogvold, L. et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia 57, 841–843 (2014).
Wan, X. et al. Pancreatic islets communicate with lymphoid tissues via exocytosis of insulin peptides. Nature 560, 107–111 (2018).
Carrero, J. A. et al. Resident macrophages of pancreatic islets have a seminal role in the initiation of autoimmune diabetes of NOD mice. Proc. Natl Acad. Sci. USA 114, E10418–E10427 (2017).
Kolb-Bachofen, V. & Kolb, H. A role for macrophages in the pathogenesis of type 1 diabetes. Autoimmunity 3, 145–154 (1989).
Gulden, E. & Wen, L. Toll-like receptor activation in immunity vs. tolerance in autoimmune diabetes. Front. Immunol. 5, 119 (2014).
Carrero, J. A. et al. Depletion of islet resident macrophages protects mice from type 1 diabetes [abstract]. J. Immunol. 200 (Suppl. 1), 41.13 (2018).
Hutchings, P. et al. Transfer of diabetes in mice prevented by blockade of adhesion- promoting receptor on macrophages. Nature 348, 639–642 (1990).
Kent, S. C., Mannering, S. I., Michels, A. W. & Babon, J. A. B. Deciphering the pathogenesis of human type 1 diabetes (T1D) by interrogating T cells from the “scene of the crime”. Curr. Diab Rep. 17, 95 (2017).
Christensen, D. P. et al. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Mol. Med. 17, 378–390 (2011).
Lee, H. A. et al. Histone deacetylase inhibitor MGCD0103 protects the pancreas from streptozotocin-induced oxidative stress and β-cell death. Biomed. Pharmacother. 109, 921–929 (2019).
Rui, J. et al. Methylation of insulin DNA in response to proinflammatory cytokines during the progression of autoimmune diabetes in NOD mice. Diabetologia 59, 1021–1029 (2016).
Ramos-Rodriguez, M. et al. The impact of proinflammatory cytokines on the β-cell regulatory landscape provides insights into the genetics of type 1 diabetes. Nat. Genet. 51, 1588–1595 (2019).
Liu, C. W., Atkinson, M. A. & Zhang, Q. Type 1 diabetes cadaveric human pancreata exhibit a unique exocrine tissue proteomic profile. Proteomics 16, 1432–1446 (2016).
Lopes, M. et al. Temporal profiling of cytokine-induced genes in pancreatic β-cells by meta-analysis and network inference. Genomics 103, 264–275 (2014).
Gonzalez-Duque, S. et al. Conventional and neo-antigenic peptides presented by β cells are targeted by circulating naive CD8+ T cells in type 1 diabetic and healthy donors. Cell Metab. 28, 946–960.e6 (2018).
Diez, J. et al. Differential splicing of the IA-2 mRNA in pancreas and lymphoid organs as a permissive genetic mechanism for autoimmunity against the IA-2 type 1 diabetes autoantigen. Diabetes 50, 895–900 (2001).
Raposo, B. et al. T cells specific for post-translational modifications escape intrathymic tolerance induction. Nat. Commun. 9, 353 (2018).
McLaughlin, R. J. et al. Human islets and dendritic cells generate post-translationally modified islet autoantigens. Clin. Exp. Immunol. 185, 133–140 (2016).
Delong, T. et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351, 711–714 (2016).
Thomaidou, S., Zaldumbide, A. & Roep, B. O. Islet stress, degradation and autoimmunity. Diabetes Obes. Metab. 20 (Suppl. 2), 88–94 (2018).
Hutton, J. C. & Davidson, H. W. Cytokine-induced dicing and splicing in the β-cell and the immune response in type 1 diabetes. Diabetes 59, 335–336 (2010).
Alvelos, M. I., Juan-Mateu, J., Colli, M. L., Turatsinze, J. V. & Eizirik, D. L. When one becomes many–alternative splicing in β-cell function and failure. Diabetes Obes. Metab. 20 (Suppl. 2), 77–87 (2018).
Marre, M. L., James, E. A. & Piganelli, J. D. β cell ER stress and the implications for immunogenicity in type 1 diabetes. Front. Cell Dev. Biol. 3, 67 (2015).
Zhang, M. et al. RNA editing derived epitopes function as cancer antigens to elicit immune responses. Nat. Commun. 9, 3919 (2018).
Roth, S. H. et al. Increased RNA editing may provide a source for autoantigens in systemic lupus erythematosus. Cell Rep. 23, 50–57 (2018).
Morgan, N. G. & Richardson, S. J. Fifty years of pancreatic islet pathology in human type 1 diabetes: insights gained and progress made. Diabetologia 61, 2499–2506 (2018).
Oram, R. A., Sims, E. K. & Evans-Molina, C. Beta cells in type 1 diabetes: mass and function; sleeping or dead? Diabetologia 62, 567–577 (2019).
Muraro, M. J. et al. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 3, 385–394.e3 (2016).
Wang, Y. J. et al. Multiplexed in situ imaging mass cytometry analysis of the human endocrine pancreas and immune system in type 1 diabetes. Cell Metab. 29, 769–783.e4 (2019).
Damond, N. et al. A map of human type 1 diabetes progression by imaging mass cytometry. Cell Metab. 29, 755–768.e5 (2019).
Avrahami, D. et al. β-Cells are not uniform after all–novel insights into molecular heterogeneity of insulin-secreting cells. Diabetes Obes. Metab. 19 (Suppl. 1), 147–152 (2017).
Falcao, A. M. et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 24, 1837–1844 (2018).
Blanter, M., Sork, H., Tuomela, S. & Flodström-Tullberg, M. Genetic and environmental interaction in type 1 diabetes: a relationship between genetic risk alleles and molecular traits of enterovirus infection? Curr. Diab. Rep. 19, 82 (2019).
Maddison, P., Gozzard, P., Grainge, M. J. & Lang, B. Long-term survival in paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 88, 1334–1339 (2017).
Titulaer, M. J. et al. Clinical Dutch-English Lambert-Eaton myasthenic syndrome (LEMS) tumor association prediction score accurately predicts small-cell lung cancer in the LEMS. J. Clin. Oncol. 29, 902–908 (2011).
Wirtz, P. W. et al. HLA and smoking in prediction and prognosis of small cell lung cancer in autoimmune Lambert-Eaton myasthenic syndrome. J. Neuroimmunol. 159, 230–237 (2005).
Gubin, M. M. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014).
Colli, M. L. et al. PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-α and-γ via IRF1 induction. EBioMedicine 36, 367–375 (2018).
Sarkar, S. A. et al. Induction of indoleamine 2,3-dioxygenase by interferon-γ in human islets. Diabetes 56, 72–79 (2007).
Anquetil, F. et al. Loss of IDO1 expression from human pancreatic β-cells precedes their destruction during the development of type 1 diabetes. Diabetes 67, 1858–1866 (2018).
Akirav, E., Kushner, J. A. & Herold, K. C. β-Cell mass and type 1 diabetes: going, going, gone? Diabetes 57, 2883–2888 (2008).
Chen, C., Cohrs, C. M., Stertmann, J., Bozsak, R. & Speier, S. Human beta cell mass and function in diabetes: recent advances in knowledge and technologies to understand disease pathogenesis. Mol. Metab. 6, 943–957 (2017).
Moin, A. S. M. & Butler, A. E. Alterations in beta cell identity in type 1 and type 2 diabetes. Curr. Diab Rep. 19, 83 (2019).
Md Moin, A. S. et al. Increased hormone-negative endocrine cells in the pancreas in type 1 diabetes. J. Clin. Endocrinol. Metab. 101, 3487–3496 (2016).
Denroche, H. C. & Verchere, C. B. IAPP and type 1 diabetes: implications for immunity, metabolism and islet transplants. J. Mol. Endocrinol. 60, R57–R75 (2018).
Alhadj Ali, M. et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci. Transl Med. 9, eaaf7779 (2017).
Nikolic, T. et al. Safety and feasibility of intradermal injection with tolerogenic dendritic cells pulsed with proinsulin peptide–for type 1 diabetes. Lancet Diabetes Endocrinol. 8, 470–472 (2020).
Roep, B. O. et al. Auto- and alloimmune reactivity to human islet allografts transplanted to insulin-dependent diabetes mellitus patients. Diabetes 48, 484–490 (1999).
Vendrame, F. et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes 59, 947–957 (2010).
Arif, S. et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J. Clin. Invest. 113, 451–463 (2004).
Keymeulen, B. et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 352, 2598–2608 (2005).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes. N. Engl. J. Med. 346, 1692–1698 (2002).
Voltarelli, J. C. et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 297, 1568–1576 (2007).
Orban, T. et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 378, 412–419 (2011).
Rigby, M. R. et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 1, 284–294 (2013).
Schneider, A. et al. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J. Immunol. 181, 7350–7355 (2008).
Anderson, M. S. et al. Projection of an immunological self shadow within the thymus by the AIRE protein. Science 298, 1395–1401 (2002).
Wildin, R. S. et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27, 18–20 (2001).
Endl, J. et al. Identification of naturally processed T cell epitopes from glutamic acid decarboxylase presented in the context of HLA-DR alleles by T lymphocytes of recent onset IDDM patients. J. Clin. Invest. 99, 2405–2415 (1997).
Roep, B. O. et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci. Transl Med. 5, 191ra82 (2013).
Huurman, V. A. et al. Immunological efficacy of heat shock protein 60 peptide DiaPep277 therapy in clinical type I diabetes. Clin. Exp. Immunol. 152, 488–497 (2008).
van Lummel, M. et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes 63, 237–247 (2014).
de Jong, V. M. et al. Post-transcriptional control of candidate risk genes for type 1 diabetes by rare genetic variants. Genes Immun. 14, 58–61 (2013).
Acknowledgements
The authors are supported by grants from the Dutch Diabetes Research Foundation, Stichting DON, the European Commission, the Juvenile Diabetes Research Foundation and the Wanek Family Project for Type 1 Diabetes (directed by B.O.R.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Network for Pancreatic Organ Donors with Diabetes: www.jdrfnpod.org
Glossary
- Thymic education
-
The process that takes place in the thymus leading to the establishment of central immune tolerance to self-proteins and the potential for immune response against foreign proteins (such as viruses, bacteria, donor tissue and allergens).
- Self-proteins
-
Proteins normally produced by a particular organism.
- Thymic selection
-
The processes of positive and negative selection in the thymus through which T cells acquire the capacity to distinguish self-proteins from foreign proteins.
- Molecular fragility
-
The extreme sensitivity of β-cells to stress, inflammation and apoptosis.
- Translation infidelity
-
The ribosomal errors during the translation of mRNA into proteins that lead to changes in amino acid sequences of mRNA-encoded proteins (such as, alternative start site, frameshifts, readthrough of stop codons or premature termination of translation).
- Ligandome
-
The complete range of peptides presented by HLA molecules on the surface of β-cells.
Rights and permissions
About this article
Cite this article
Roep, B.O., Thomaidou, S., van Tienhoven, R. et al. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol 17, 150–161 (2021). https://doi.org/10.1038/s41574-020-00443-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-020-00443-4
This article is cited by
-
Captopril pretreatment augments diabetogenic response to streptozotocin administration: experimental in vivo rat model
Future Journal of Pharmaceutical Sciences (2024)
-
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials
BMC Medicine (2024)
-
Systematic immune cell dysregulation and molecular subtypes revealed by single-cell RNA-seq of subjects with type 1 diabetes
Genome Medicine (2024)
-
Consideration of sex as a biological variable in diabetes research across twenty years
Biology of Sex Differences (2024)
-
Metabolic switching, growth kinetics and cell yields in the scalable manufacture of stem cell-derived insulin-producing cells
Stem Cell Research & Therapy (2024)