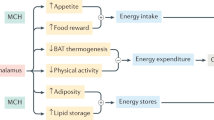
Abstract
The bioactive peptides galanin, spexin and kisspeptin have a common ancestral origin and their pathophysiological roles are increasingly the subject of investigation. Evidence suggests that these bioactive peptides play a role in the regulation of metabolism, pancreatic β-cell function, energy homeostasis, mood and behaviour in several species, including zebrafish, rodents and humans. Galanin signalling suppresses insulin secretion in animal models (but not in humans), is potently obesogenic and plays putative roles governing certain evolutionary behaviours and mood modulation. Spexin decreases insulin secretion and has potent anorectic, analgesic, anxiolytic and antidepressive-like effects in animal models. Kisspeptin modulates glucose-stimulated insulin secretion, food intake and/or energy expenditure in animal models and humans. Furthermore, kisspeptin is implicated in the control of reproductive behaviour in animals, modulation of human sexual and emotional brain processing, and has antidepressive and fear-suppressing effects. In addition, galanin-like peptide is a further member of the galaninergic family that plays emerging key roles in metabolism and behaviour. Therapeutic interventions targeting galanin, spexin and/or kisspeptin signalling pathways could therefore contribute to the treatment of conditions ranging from obesity to mood disorders. However, many gaps and controversies exist, which must be addressed before the therapeutic potential of these bioactive peptides can be established.
Key points
-
The bioactive peptides galanin, spexin and kisspeptin have a common ancestral genetic origin and similar peptide sequences and have wide central and peripheral tissue distribution.
-
Galanin and spexin act through galanin receptors 1–3, whereas kisspeptin acts through the kisspeptin receptor.
-
These bioactive peptides control key metabolic, emotional and behavioural processes in species ranging from zebrafish to humans.
-
Although some actions are well described in animal models, controversies remain about their roles in several aspects of human physiology.
-
Further study of therapeutic agents targeting the signalling pathways of these bioactive peptides could identify a possible role for these in the management of type 2 diabetes mellitus, obesity, and behavioural and mood disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Simon, G. E. et al. Association between obesity and psychiatric disorders in the us adult population. Arch. Gen. Psychiatry 63, 824–830 (2006).
Dallman, M. F. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 21, 159–165 (2010).
Lu, X. Y. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr. Opin. Pharmacol. 7, 648–652 (2007).
Kim, D. K. et al. Coevolution of the spexin/galanin/kisspeptin family: spexin activates galanin receptor type II and III. Endocrinology 155, 1864–1873 (2014).
Yun, S. et al. Does kisspeptin belong to the proposed RF-amide peptide family? Front Endocrinol. 5, 134 (2014).
Ohtaki, T. et al. Isolation and cDNA cloning of a novel galanin-like peptide (GALP) from porcine hypothalamus. J. Biol. Chem. 274, 370451–370455 (1999).
Burbach, J. P. H. What are neuropeptides? Methods Mol. Biol. 789, 1–36 (2011).
Tatemoto, K., Rökaeus, Å., Jörnvall, H., McDonald, T. J. & Mutt, V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 164, 124–128 (1983).
Burgevin, M. C., Loquet, I., Quarteronet, D. & Habert-Ortoli, E. Cloning, pharmacological characterization, and anatomical distribution of a rat cDNA encoding for a galanin receptor. J. Mol. Neurosci. 6, 33–41 (1995).
Lang, R., Gundlach, A. L. & Kofler, B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol. Ther. 115, 177–207 (2007).
Lang, R. et al. Pharmacological and functional characterization of galanin-like peptide fragments as potent galanin receptor agonists. Neuropeptides 39, 179–184 (2005).
Santic, R. et al. Gangliocytes in neuroblastic tumors express alarin, a novel peptide derived by differential splicing of the galanin-like peptide gene. J. Mol. Neurosci. 29, 145–152 (2006).
Santic, R. et al. Alarin is a vasoactive peptide. Proc. Natl Acad. Sci. USA 104, 10217–10222 (2007).
Waters, S. M. & Krause, J. E. Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience 95, 265–271 (2000).
Webling, K. E. B., Runesson, J., Bartfai, T. & Langel, Ü. Galanin receptors and ligands. Front. Endocrinol. 3, 146 (2012).
Anglade, I. et al. Characterization of trout galanin and its distribution in trout brain and pituitary. J. Comp. Neurol. 350, 63–74 (1994).
Olivereau, M. & Olivereau, J. M. Immunocytochemical localization of a galanin-like peptidergic system in the brain of two urodele and two anuran species (Amphibia). Histochemistry 98, 51–66 (1992).
Jiménez, A.-J., Mancera, J.-M., Pérez-Fígares, J.-M. & Fernández-Llebrez, P. Distribution of galanin-like immunoreactivity in the brain of the turtle Mauremys caspica. J. Comp. Neurol. 349, 73–84 (1994).
Klein, S., Jurkevich, A. & Grossmann, R. Sexually dimorphic immunoreactivity of galanin and colocalization with arginine vasotocin in the chicken brain (Gallus gallus domesticus). J. Comp. Neurol. 499, 828–839 (2006).
Takatsu, Y. et al. Distribution of galanin-like peptide in the rat brain. Endocrinology 142, 1626–1634 (2001).
Kordower, J. H. & Mufson, E. J. Galanin-like immunoreactivity within the primate basal forebrain: differential staining patterns between humans and monkeys. J. Comp. Neurol. 294, 281–292 (1990).
Gentleman, S. M. et al. Distribution of galanin-like immunoreactivity in the human brain. Brain Res. 505, 311–315 (1989).
Lang, R. et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharmacol. Rev. 67, 118–175 (2015).
Koshiyama, H. et al. Central galanin stimulates pituitary prolactin secretion in rats: possible involvement of hypothalamic vasoactive intestinal polypeptide. Neurosci. Lett. 75, 49–54 (1987).
Murakami, Y. et al. Galanin stimulates growth hormone (GH) secretion via GH-releasing factor (GRF) in conscious rats. Eur. J. Pharmacol. 136, 415–418 (1987).
Sahu, A., Crowley, W. R., Tatemoto, K., Balasubramaniam, A. & Kalra, S. P. Effects of neuropeptide Y, NPY analog (norleucine4-NPY), galanin and neuropeptide K on LH release in ovariectomized (Ovx) and Ovx estrogen, progesterone-treated rats. Peptides 8, 921–926 (1987).
Mirabeau, O. et al. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 17, 320–327 (2007).
Sonmez, K. et al. Evolutionary sequence modeling for discovery of peptide hormones. PLoS Comput. Biol. 5, e1000258 (2009).
Porzionato, A. et al. Spexin expression in normal rat tissues. J. Histochem. Cytochem. 58, 825–837 (2010).
Liu, Y. et al. A novel neuropeptide in suppressing luteinizing hormone release in goldfish, carassius auratus. Mol. Cell. Endocrinol. 374, 65–72 (2013).
Sassek, M., Kolodziejski, P. A., Szczepankiewicz, D. & Pruszynska-Oszmalek, E. Spexin in the physiology of pancreatic islets-mutual interactions with insulin. Endocrine 63, 513–519 (2019).
Gu, L. et al. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides 71, 232–239 (2015).
Kotani, M. et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 276, 34631–34636 (2001).
Lee, D. K. et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 446, 103–107 (1999).
de Roux, N. et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl Acad. Sci. USA 100, 10972–10976 (2003).
Seminara, S. B. et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 349, 1614–1627 (2003).
Topaloglu, A. K. et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N. Engl. J. Med. 366, 629–635 (2012).
Gottsch, M. L. et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073–4077 (2004).
Clarkson, J., d’Anglemont de Tassigny, X., Colledge, W. H., Caraty, A. & Herbison, A. E. Distribution of kisspeptin neurones in the adult female mouse brain. J. Neuroendocrinol. 21, 673–682 (2009).
Rometo, A. M., Krajewski, S. J., Voytko, M. L. & Rance, N. E. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J. Clin. Endocrinol. Metab. 92, 2744–2750 (2007).
Kim, J. et al. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 152, 2020–2030 (2011).
Stephens, S. B. Z., Chahal, N., Munaganuru, N., Parra, R. A. & Kauffman, A. S. Estrogen stimulation of Kiss1 expression in the medial amygdala involves estrogen receptor-α but not estrogen receptor-β. Endocrinology 157, 4021–4031 (2016).
Di Giorgio, N. P. et al. Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology 155, 1033–1044 (2014).
Stephens, S. B. Z. et al. Estradiol-dependent and -independent stimulation of Kiss1 expression in the amygdala, BNST, and lateral septum of mice. Endocrinology 159, 3389–3402 (2018).
Muir, A. I. et al. AXOR12, a novel human g protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem. 276, 28969–28975 (2001).
Ohtaki, T. et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411, 613–617 (2001).
Dudek, M. et al. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic-pituitary-gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides 56, 41–49 (2016).
Song, W. J. et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 19, 667–681 (2014).
Tolson, K. P., Marooki, N., Wolfe, A., Smith, J. T. & Kauffman, A. S. Cre/lox generation of a novel whole-body Kiss1r KO mouse line recapitulates a hypogonadal, obese, and metabolically-impaired phenotype. Mol. Cell. Endocrinol. 498, 110559 (2019).
Ly, T., Harihar, S. & Welch, D. R. KISS1 in metastatic cancer research and treatment: potential and paradoxes. Cancer Metastasis Rev. 39, 739–754 (2020).
Kanazawa, T. et al. Promoter methylation of galanin receptors as epigenetic biomarkers for head and neck squamous cell carcinomas. Expert. Rev. Mol. Diagn. 19, 137–148 (2019).
Olkowicz, M. et al. New galanin(1-15) analogues modified in positions 9, 10 and 11 act as galanin antagonists on glucose-induced insulin secretion. J. Physiol. Pharmacol. 58, 859–872 (2007).
Ahrén, B., Pacini, G., Wynick, D., Wierup, N. & Sundler, F. Loss-of-function mutation of the galanin gene is associated with perturbed islet function in mice. Endocrinology 145, 3190–3196 (2004).
Barreto, S. G. et al. Galanin receptor 3 — a potential target for acute pancreatitis therapy. Neurogastroenterol. Motil. 23, e141–e151 (2011).
Pham, T., Guerrini, S., Wong, H., Reeve, J. & Sternini, C. Distribution of galanin receptor 1 immunoreactivity in the rat stomach and small intestine. J. Comp. Neurol. 450, 292–302 (2002).
Zorrilla, E. P., Brennan, M., Sabino, V., Lu, X. & Bartfai, T. Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. Physiol. Behav. 91, 479–485 (2007).
Kumar, S., Hossain, M. J., Javed, A., Kullo, I. J. & Balagopal, P. B. Relationship of circulating spexin with markers of cardiovascular disease: a pilot study in adolescents with obesity. Pediatr. Obes. 13, 374–380 (2018).
Tolson, K. P. et al. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J. Clin. Invest. 124, 3075–3079 (2014).
Velasco, I. et al. Gonadal hormone-dependent vs. -independent effects of kisspeptin signaling in the control of body weight and metabolic homeostasis. Metabolism 98, 84–94 (2019).
Bowe, J. E. et al. A role for placental kisspeptin in β cell adaptation to pregnancy. JCI Insight 4, e124540 (2019).
Beck, B., Burlet, A., Nicolas, J. P. & Burlet, C. Galanin in the hypothalamus of fed and fasted lean and obese zucker rats. Brain Res. 623, 124–130 (1993).
Leibowitz, S. F., Akabayashi, A. & Wang, J. Obesity on a high-fat diet: role of hypothalamic galanin in neurons of the anterior paraventricular nucleus projecting to the median eminence. J. Neurosci. 18, 2709–2719 (1998).
Akabayashi, A., Koenig, J. I., Watanabe, Y., Alexander, J. T. & Leibowitz, S. F. Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc. Natl Acad. Sci. USA 91, 10375–10379 (1994).
Idelevich, A. et al. ΔFosB requires galanin, but not leptin, to increase bone mass via the hypothalamus, but both are needed to increase energy expenditure. J. Bone Miner. Res. 34, 1707–1720 (2019).
Idelevich, A. et al. Neuronal hypothalamic regulation of body metabolism and bone density is galanin dependent. J. Clin. Invest. 128, 2626–2641 (2018).
Karatayev, O., Baylan, J. & Leibowitz, S. F. Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol 43, 571–580 (2009).
Hohmann, J. G. et al. Neuroendocrine profiles in galanin-overexpressing and knockout mice. Neuroendocrinology 77, 354–366 (2003).
Jungnickel, S. R. F. & Gundlach, A. L. 125I-galanin binding in brain of wildtype, and galanin- and GalR1-knockout mice: strain and species differences in GalR1 density and distribution. Neuroscience 131, 407–421 (2005).
Schäuble, N. et al. Human galanin (GAL) and galanin 1 receptor (GALR1) variations are not involved in fat intake and early onset obesity. J. Nutr. 135, 1387–1392 (2005).
Al-Daghri, N. M. et al. Favorable changes in fasting glucose in a 6-month self-monitored lifestyle modification programme inversely affects spexin levels in females with prediabetes. Sci. Rep. 9, 9454 (2019).
Akbas, M. et al. Serum levels of spexin are increased in the third trimester pregnancy with gestational diabetes mellitus. Gynecol. Endocrinol. 35, 1050–1053 (2019).
Karaca, A., Bakar-Ates, F. & Ersoz-Gulcelik, N. Decreased spexin levels in patients with type 1 and type 2 diabetes. Med. Princ. Pract. 27, 549–554 (2018).
Hodges, S. K., Teague, A. M., Dasari, P. S. & Short, K. R. Effect of obesity and type 2 diabetes, and glucose ingestion on circulating spexin concentration in adolescents. Pediatr. Diabetes 19, 212–216 (2018).
Chen, T. et al. Circulating spexin decreased and negatively correlated with systemic insulin sensitivity and pancreatic β cell function in obese children. Ann. Nutr. Metab. 74, 125–131 (2019).
Kołodziejski, P. A. et al. Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol. Res. 67, 45–56 (2018).
Kumar, S., Hossain, M. J., Inge, T. & Balagopal, P. B. Roux-en-Y gastric bypass surgery in youth with severe obesity: 1-year longitudinal changes in spexin. Surg. Obes. Relat. Dis. 14, 1537–1543 (2018).
Lin, C. et al. Circulating spexin levels negatively correlate with age, BMI, fasting glucose, and triglycerides in healthy adult women. J. Endocr. Soc. 2, 409–419 (2018).
Zheng, B. et al. Spexin suppress food intake in zebrafish: evidence from gene knockout study. Sci. Rep. 7, 14643 (2017).
Wu, H. et al. Ya-fish (Schizothorax prenanti) spexin: identification, tissue distribution and mRNA expression responses to periprandial and fasting. Fish Physiol. Biochem. 42, 39–49 (2016).
Deng, S.-P. et al. Molecular cloning, characterization and expression analysis of spexin in spotted scat (Scatophagus argus). Gen. Comp. Endocrinol. 266, 60–66 (2018).
Wang, S., Wang, B. & Chen, S. Spexin in the half-smooth tongue sole (Cynoglossus semilaevis): molecular cloning, expression profiles, and physiological effects. Fish Physiol. Biochem. 44, 829–839 (2018).
Li, S. et al. Molecular cloning and functional characterization of spexin in orange-spotted grouper (Epinephelus Coioides). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 196–197, 85–91 (2016).
Ma, A. et al. Dual role of insulin in spexin regulation: functional link between food intake and spexin expression in a fish model. Endocrinology 158, 560–577 (2017).
Walewski, J. L. et al. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity 22, 1643–1652 (2014).
Kumar, S. et al. Decreased circulating levels of spexin in obese children. J. Clin. Endocrinol. Metab. 101, 2931–2936 (2016).
Horikoshi, Y. et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J. Clin. Endocrinol. Metab. 88, 914–919 (2003).
Izzi-Engbeaya, C. et al. The effects of kisspeptin on β-cell function, serum metabolites and appetite in humans. Diabetes Obes. Metab. 20, 2800–2810 (2018).
Jayasena, C. N. et al. Reduced levels of plasma kisspeptin during the antenatal booking visit are associated with increased risk of miscarriage. J. Clin. Endocrinol. Metab. 99, e2652–e2660 (2014).
Ćetković, A. et al. Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocr. Res. 37, 78–88 (2012).
True, C., Verma, S., Grove, K. L. & Smith, M. S. Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology 154, 2821–2832 (2013).
Padilla, S. L. et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc. Natl Acad. Sci. USA 114, 2413–2418 (2017).
Tolson, K. P. et al. Conditional knockout of kisspeptin signaling in brown adipose tissue increases metabolic rate and body temperature and lowers body weight. FASEB J. 34, 107–121 (2020).
Kuteeva, E., Hökfelt, T., Wardi, T. & Ogren, S. O. Galanin, galanin receptor subtypes and depression-like behaviour. Cell. Mol. Life Sci. 65, 1854–1863 (2008).
Unschuld, P. G. et al. Gender-specific association of galanin polymorphisms with HPA-axis dysregulation, symptom severity, and antidepressant treatment response. Neuropsychopharmacology 35, 1583–1592 (2010).
Juhasz, G. et al. Brain galanin system genes interact with life stresses in depression-related phenotypes. Proc. Natl Acad. Sci. USA 111, E1666–E1673 (2014).
Barde, S. et al. Alterations in the neuropeptide galanin system in major depressive disorder involve levels of transcripts, methylation, and peptide. Proc. Natl Acad. Sci. USA 113, E8472–E8481 (2016).
Wang, Y. J. et al. Plasma galanin is a biomarker for severity of major depressive disorder. Int. J. Psychiatry Med. 48, 109–119 (2014).
Li, H. et al. Inhibition of GALR1 in PFC alleviates depressive-like behaviors in postpartum depression rat model by upregulating CREB-BNDF and 5-HT Levels. Front. Psychiatry 9, 588 (2018).
Brunner, S. M. et al. GAL3 receptor KO mice exhibit an anxiety-like phenotype. Proc. Natl Acad. Sci. USA 111, 7138–7143 (2014).
Kozyrev, N. & Coolen, L. M. Activation of galanin and cholecystokinin receptors in the lumbosacral spinal cord is required for ejaculation in male rats. Eur. J. Neurosci. 45, 846–858 (2017).
Tripp, J. A. & Bass, A. H. Galanin immunoreactivity is sexually polymorphic in neuroendocrine and vocal-acoustic systems in a teleost fish. J. Comp. Neurol. 528, 433–452 (2020).
Kohl, J. et al. Functional circuit architecture underlying parental behaviour. Nature 556, 326–331 (2018).
Wu, Z., Autry, A. E., Bergan, J. F., Watabe-Uchida, M. & Dulac, C. G. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330 (2014).
Cservenák, M. et al. Maternally involved galanin neurons in the preoptic area of the rat. Brain Struct. Funct. 222, 781–798 (2017).
Galbally, M., Lewis, A. J., van Ijzendoorn, M. & Permezel, M. The role of oxytocin in mother-infant relations: a systematic review of human studies. Harv. Rev. Psychiatry 19, 1–14 (2011).
Ciosek, J. & Drobnik, J. Galanin modulates oxytocin release from rat hypothalamo-neurohypophysial explant in vitro - the role of acute or prolonged osmotic stimulus. Endokrynol. Pol. 64, 139–148 (2013).
Eskova, A., Frohnhöfer, H. G., Nüsslein-Volhard, C. & Irion, U. Galanin signaling in the brain regulates color pattern formation in zebrafish. Curr. Biol. 30, 298–303 (2020).
McMenamin, S. K. et al. Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science 345, 1358–1361 (2014).
Hökfelt, T., Zhang, X. & Wiesenfeld-Hallin, Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 17, 22–30 (1994).
Yang, Y. et al. Involvements of galanin and its receptors in antinociception in nucleus accumbens of rats with inflammatory pain. Neurosci. Res. 97, 20–25 (2015).
Zhang, Y. et al. Galanin plays a role in antinociception via binding to galanin receptors in the nucleus accumbens of rats with neuropathic pain. Neurosci. Lett. 706, 93–98 (2019).
Zhuang, M. et al. Spexin as an anxiety regulator in mouse hippocampus: mechanisms for transcriptional regulation of spexin gene expression by corticotropin releasing factor. Biochem. Biophys. Res. Commun. 525, 326–333 (2020).
Jeong, I., Kim, E., Seong, J. Y. & Park, H.-C. Overexpression of spexin 1 in the dorsal habenula reduces anxiety in zebrafish. Front. Neural Circuits 13, 53 (2019).
Kim, E. et al. Distribution and neuronal circuit of spexin 1/2 neurons in the zebrafish CNS. Sci. Rep. 9, 5025 (2019).
Okamoto, H., Agetsuma, M. & Aizawa, H. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev. Neurobiol. 72, 386–394 (2012).
Lim, C. H., Soga, T., Levavi-Sivan, B. & Parhar, I. S. Chronic social defeat stress up-regulates spexin in the brain of Nile tilapia (Oreochromis niloticus). Sci. Rep. 10, 7666 (2020).
Adekunbi, D. A. et al. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J. Neuroendocrinol. 30, e12572 (2018).
Delmas, S., Porteous, R., Bergin, D. H. & Herbison, A. E. Altered aspects of anxiety-related behavior in kisspeptin receptor-deleted male mice. Sci. Rep. 8, 2794 (2018).
Pineda, R., Plaisier, F., Millar, R. P. & Ludwig, M. Amygdala kisspeptin neurons: putative mediators of olfactory control of the gonadotropic axis. Neuroendocrinology 104, 223–228 (2017).
Aggarwal, S. et al. Medial amygdala kiss1 neurons mediate female pheromone stimulation of luteinizing hormone in male mice. Neuroendocrinology 108, 172–189 (2019).
Bakker, J., Pierman, S. & González-Martínez, D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm. Behav. 57, 390–395 (2010).
Watanabe, Y. et al. Enhancement of the luteinising hormone surge by male olfactory signals is associated with anteroventral periventricular KISS1 cell activation in female rats. J. Neuroendocrinol. https://doi.org/10.1111/jne.12505 (2017).
Yang, L. et al. Kisspeptin enhances brain responses to olfactory and visual cues of attraction in men. JCI Insight 5, e133633 (2020).
Kauffman, A. S. et al. The kisspeptin receptor GPR54 Is required for sexual differentiation of the brain and behavior. J. Neurosci. 27, 8826–8835 (2007).
Hellier, V. et al. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat. Commun. 9, 400 (2018).
Liu, X. & Herbison, A. Kisspeptin regulation of arcuate neuron excitability in kisspeptin receptor knockout mice. Endocrinology 156, 1815–1827 (2015).
Manabe, T. et al. Effect of galanin on plasma glucose, insulin and pancreatic glucagon in dogs. J. Int. Med. Res. 31, 126–132 (2003).
Lindskog, S. & Ahren, B. Galanin: effects on basal and stimulated insulin and glucagon secretion in the mouse. Acta Physiol. Scand. 129, 305–309 (1987).
Lindskog, S. et al. Galanin of the homologous species inhibits insulin secretion in the rat and in the pig. Acta Physiol. Scand. 139, 591–596 (1990).
Abot, A. et al. Galanin enhances systemic glucose metabolism through enteric nitric oxide synthase-expressed neurons. Mol. Metab. 10, 100–108 (2018).
Amiranoff, B., Lorinet, A. M., Yanaihara, N. & Laburthe, M. Structural requirement for galanin action in the pancreatic beta cell line Rin M 5F. Eur. J. Pharmacol. 163, 205–207 (1989).
Sharp, G. W. et al. Galanin can inhibit insulin release by a mechanism other than membrane hyperpolarization or inhibition of adenylate cyclase. J. Biol. Chem. 264, 7302–7309 (1989).
Tang, G. et al. Go2 G protein mediates galanin inhibitory effects on insulin release from pancreatic β cells. Proc. Natl Acad. Sci. USA 109, 2636–2641 (2012).
Yun, R. et al. PVN galanin increases fat storage and promotes obesity by causing muscle to utilize carbohydrate more than fat. Peptides 26, 2265–2273 (2005).
White, H. S. et al. Developing novel antiepileptic drugs: characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics 6, 372–380 (2009).
Flynn, S. P. & White, H. S. Regulation of glucose and insulin release following acute and repeated treatment with the synthetic galanin analog NAX-5055. Neuropeptides 50, 35–42 (2014).
Gilbey, S. G., Stephenson, J., O’Halloran, D. J., Burrin, J. M. & Bloom, S. R. High-dose porcine galanin infusion and effect on intravenous glucose tolerance in humans. Diabetes 38, 1114–1116 (1989).
Carey, D. G. et al. Potent effects of human galanin in man: growth hormone secretion and vagal blockade. J. Clin. Endocrinol. Metab. 77, 90–93 (1993).
Holst, J. J. et al. On the effects of human galanin in man. Diabetologia 36, 653–657 (1993).
McDonald, T. J. et al. Canine, human, and rat plasma insulin responses to galanin administration: species response differences. Am. J. Physiol. Endocrinol. Metab. 266, e612–e617 (1994).
Ahrén, B., Ar’Rajab, A., Böttcher, G., Sundler, F. & Dunning, B. E. Presence of galanin in human pancreatic nerves and inhibition of insulin secretion from isolated human islets. Cell Tissue Res. 264, 263–267 (1991).
Kyrkouli, S. E., Stanley, B. G., Seirafi, R. D. & Leibowitz, S. F. Stimulation of feeding by galanin: Anatomical localization and behavioral specificity of this peptide’s effects in the brain. Peptides 11, 995–1001 (1990).
Tempel, D. L., Leibowitz, K. J. & Leibowitz, S. F. Effects of PVN galanin on macronutrient selection. Peptides 9, 309–314 (1988).
de Pedro, N., Céspedes, M. V., Delgado, M. J. & Alonso-Bedate, M. The galanin-induced feeding stimulation is mediated via alpha 2-adrenergic receptors in goldfish. Regul. Pept. 57, 77–84 (1995).
Tachibana, T. et al. Central administration of galanin stimulates feeding behavior in chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 637–640 (2008).
Psichas, A., Glass, L. L., Sharp, S. J., Reimann, F. & Gribble, F. M. Galanin inhibits GLP-1 and GIP secretion via the GAL1 receptor in enteroendocrine L and K cells. Br. J. Pharmacol. 173, 888–898 (2016).
Saar, I. et al. Novel galanin receptor subtype specific ligands in feeding regulation. Neurochem. Int. 58, 714–720 (2011).
Gottsch, M. L. et al. Phenotypic analysis of mice deficient in the type 2 galanin receptor (GALR2). Mol. Cell. Biol. 25, 4804–4811 (2005).
Corwin, R. L., Robinson, J. K. & Crawley, J. N. Galanin antagonists block galanin-induced feeding in the hypothalamus and amygdala of the rat. Eur. J. Neurosci. 5, 1528–1533 (1993).
Schusdziarra, V., Zimmermann, J. P., Erdmann, J., Bader, U. & Schick, R. R. Differential inhibition of galanin- and ghrelin-induced food intake by i.c.v. GLP-1(7-36)-amide. Regul. Pept. 147, 29–32 (2008).
Smith, B. K., York, D. A. & Bray, G. A. Chronic cerebroventricular galanin does not induce sustained hyperphagia or obesity. Peptides 15, 1267–1272 (1994).
Li, R. Y. et al. Galanin inhibits leptin expression and secretion in rat adipose tissue and 3T3-L1 adipocytes. J. Mol. Endocrinol. 33, 11–19 (2004).
Bauer, F. E. et al. Inhibitory effect of galanin on postprandial gastrointestinal motility and gut hormone release in humans. Gastroenterology 97, 260–264 (1989).
Blissett, J., Harris, G. & Kirk, J. Effect of growth hormone therapy on feeding problems and food intake in children with growth disorders. Acta Paediatr. 89, 644–649 (2000).
Batterham, R. L. et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418, 650–654 (2002).
Bagger, J. I. et al. Effect of oxyntomodulin, glucagon, GLP-1, and combined glucagon +GLP-1 infusion on food intake, appetite, and resting energy expenditure. J. Clin. Endocrinol. Metab. 100, 4541–4552 (2015).
Sassek, M. et al. Spexin modulates functions of rat endocrine pancreatic cells. Pancreas 47, 904–909 (2018).
Wong, M. K. H. et al. Goldfish spexin: solution structure and novel function as a satiety factor in feeding control. Am. J. Physiol. Endocrinol. Metab. 305, e348–e366 (2013).
Silvestre, R. A., Egido, E. M., Hernández, R. & Marco, J. Kisspeptin-13 inhibits insulin secretion without affecting glucagon or somatostatin release: study in the perfused rat pancreas. J. Endocrinol. 196, 283–290 (2008).
Vikman, J. & Ahrén, B. Inhibitory effect of kisspeptins on insulin secretion from isolated mouse islets. Diabetes Obes. Metab. 11, 197–201 (2009).
Chen, J. et al. LIM-homeodomain transcription factor Isl-1 mediates kisspeptin’s effect on insulin secretion in mice. Mol. Endocrinol. 28, 1276–1290 (2014).
Hauge-Evans, A. C. et al. A role for kisspeptin in islet function. Diabetologia 49, 2131–2135 (2006).
Bowe, J. E. et al. Kisspeptin stimulation of insulin secretion: mechanisms of action in mouse islets and rats. Diabetologia 52, 855–862 (2009).
Wahab, F., Riaz, T. & Shahab, M. Study on the effect of peripheral kisspeptin administration on basal and glucose-induced insulin secretion under fed and fasting conditions in the adult male rhesus monkey (Macaca Mulatta). Horm. Metab. Res. 43, 37–42 (2011).
Bowe, J. E. et al. GPR54 peptide agonists stimulate insulin secretion from murine, porcine and human islets. Islets 4, 20–23 (2012).
Schwetz, T. A., Reissaus, C. A. & Piston, D. W. Differential stimulation of insulin secretion by GLP-1 and kisspeptin-10. PLoS ONE 9, e113020 (2014).
Gilon, P. & Henquin, J. C. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr. Rev. 22, 565–604 (2001).
Huang, C., Wang, H., Wang, M., Hsu, M. & Wu, Y. S. Kisspeptin-activated autophagy independently suppresses non-glucose-stimulated insulin secretion from pancreatic β-cells. Sci. Rep. 9, 17451 (2019).
Backholer, K. et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 151, 2233–2243 (2010).
Fu, L. Y. & Van Den Pol, A. N. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J. Neurosci. 30, 10205–10219 (2010).
Orlando, G. et al. Effects of kisspeptin-10 on hypothalamic neuropeptides and neurotransmitters involved in appetite control. Molecules 23, 3071 (2018).
Pruszyńska-Oszmałek, E., Kołodziejski, P. A., Sassek, M. & Sliwowska, J. H. Kisspeptin-10 inhibits proliferation and regulates lipolysis and lipogenesis processes in 3T3-L1 cells and isolated rat adipocytes. Endocrine 56, 54–64 (2017).
Stengel, A., Wang, L., Goebel-Stengel, M. & Taché, Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 22, 253–257 (2011).
Dong, T. S. et al. Intraperitoneal treatment of kisspeptin suppresses appetite and energy expenditure and alters gastrointestinal hormones in mice. Dig. Dis. Sci. 65, 2254–2263 (2020).
Saito, R. et al. Centrally administered kisspeptin suppresses feeding via nesfatin-1 and oxytocin in male rats. Peptides 112, 114–124 (2019).
Thompson, E. L. et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am. J. Physiol. Endocrinol. Metab. 291, e1074–e1082 (2006).
Castellano, J. M. et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146, 3917–3925 (2005).
Jayasena, C. N. et al. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J. Clin. Invest. 124, 3667–3677 (2014).
Scott, G. et al. Double-blind, randomized, placebo-controlled study of safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-683, an investigational metastin analogue in healthy men. Br. J. Clin. Pharmacol. 75, 381–391 (2013).
Jayasena, C. N. et al. Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of kisspeptin-54. J. Clin. Endocrinol. Metab. 99, e953–e961 (2014).
Kuteeva, E., Wardi, T., Hökfelt, T. & Ögren, S. O. Galanin enhances and a galanin antagonist attenuates depression-like behaviour in the rat. Eur. Neuropsychopharmacol. 17, 64–69 (2007).
Kuteeva, E. et al. Differential role of galanin receptors in the regulation of depression-like behavior and monoamine/stress-related genes at the cell body level. Neuropsychopharmacology 33, 2573–2585 (2008).
Weiss, J. M., Bonsall, R. W., Demetrikopoulos, M. K., Emery, M. S. & West, C. H. Galanin: a significant role in depression? Ann. N. Y. Acad. Sci. 863, 364–382 (1998).
Ögren, S. O., Kuteeva, E., Ḧokfelt, T. & Kehr, J. Galanin receptor antagonists: a potential novel pharmacological treatment for mood disorders. CNS Drugs 20, 633–654 (2006).
Silote, G. P., Rosal, A. B., de Souza, M. M. & Beijamini, V. Infusion of galanin into the mid-caudal portion of the dorsal raphe nucleus has an anxiolytic effect on rats in the elevated T-maze. Behav. Brain Res. 252, 312–317 (2013).
de Souza, M. M. et al. The antidepressant-like effect of galanin in the dorsal raphe nucleus of rats involves GAL2 receptors. Neurosci. Lett. 681, 26–30 (2018).
Wardi Le Maître, T. et al. Galanin receptor 2 overexpressing mice display an antidepressive-like phenotype: possible involvement of the subiculum. Neuroscience 190, 270–288 (2011).
Saar, I. et al. Novel systemically active galanin receptor 2 ligands in depression-like behavior. J. Neurochem. 127, 114–123 (2013).
Narváez, M. et al. Galanin receptor 2-neuropeptide Y Y1 receptor interactions in the dentate gyrus are related with antidepressant-like effects. Brain Struct. Funct. 221, 4129–4139 (2016).
Wang, P. et al. Depression-like behavior in rat: involvement of galanin receptor subtype 1 in the ventral periaqueductal gray. Proc. Natl Acad. Sci. USA 113, e4726–e4735 (2016).
Christiansen, S. H., Olesen, M. V., Wörtwein, G. & Woldbye, D. P. D. Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behav. Brain Res. 216, 585–591 (2011).
Yamada, M. et al. Induction of galanin after chronic sertraline treatment in mouse ventral dentate gyrus. Brain Res. 1516, 76–82 (2013).
Murck, H. et al. Intravenous administration of the neuropeptide galanin has fast antidepressant efficacy and affects the sleep EEG. Psychoneuroendocrinology 29, 1205–1211 (2004).
Thom, G. et al. Enhanced delivery of galanin conjugates to the brain through bioengineering of the anti-transferrin receptor antibody OX26. Mol. Pharm. 15, 1420–1431 (2018).
Klenerova, V., Flegel, M., Skopek, P., Sida, P. & Hynie, S. Galanin modulating effect on restraint stress-induced short- and long-term behavioral changes in Wistar rats. Neurosci. Lett. 502, 147–151 (2011).
Broadwell, R. D. & Sofroniew, M. V. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp. Neurol. 120, 245–263 (1993).
Begley, D. J. Peptides and the blood-brain barrier: the status of our understanding. Ann. N. Y. Acad. Sci. 739, 89–100 (1994).
Soares, F. R. C. et al. Galanin microinjection into the dorsal periaqueductal gray matter produces paradigm-dependent anxiolytic effects. Brain Res. Bull. 121, 42–47 (2016).
Lyudyno, V. I., Tsikunov, S. G., Abdurasulova, I. N., Kusov, A. G. & Klimenko, V. M. Modification of anxious behavior after psychogenic trauma and treatment with galanin receptor antagonist. Bull. Exp. Biol. Med. 159, 344–347 (2015).
Möller, C., Sommer, W., Thorsell, A. & Heilig, M. Anxiogenic-like action of galanin after intra-amygdala administration in the rat. Neuropsychopharmacology 21, 507–512 (1999).
Poggioli, R., Rasori, E. & Bertolini, A. Galanin inhibits sexual behavior in male rats. Eur. J. Pharmacol. 213, 87–90 (1992).
Bloch, G. J., Butler, P. C., Kohlert, J. G. & Bloch, D. A. Microinjection of galanin into the medial preoptic nucleus facilitates copulatory behavior in the male rat. Physiol. Behav. 54, 615–624 (1993).
Bloch, G. J., Butler, P. C. & Kohlert, J. G. Galanin microinjected into the medial preoptic nucleus facilitates female- and male-typical sexual behaviors in the female rat. Physiol. Behav. 59, 1147–1154 (1996).
Zhang, M. L., Wang, H. B., Fu, F. H. & Yu, L. C. Involvement of galanin and galanin receptor 2 in nociceptive modulation in anterior cingulate cortex of normal rats and rats with mononeuropathy. Sci. Rep. 7, 45930 (2017).
Li, S. Y. et al. Involvement of galanin and galanin receptor 1 in nociceptive modulation in the central nucleus of amygdala in normal and neuropathic rats. Sci. Rep. 7, 15317 (2017).
Amorim, D. et al. Galanin-mediated behavioural hyperalgesia from the dorsomedial nucleus of the hypothalamus involves two independent descending pronociceptive pathways. PLoS ONE 10, e0142919 (2015).
Xu, S. L., Li, J., Zhang, J. J. & Yu, L. C. Antinociceptive effects of galanin in the nucleus accumbens of rats. Neurosci. Lett. 520, 43–46 (2012).
Reyes-Alcaraz, A. et al. Development of spexin-based human galanin receptor type II-specific agonists with increased stability in serum and anxiolytic effect in mice. Sci. Rep. 6, 21453 (2016).
Yun, S. et al. Spexin-based galanin receptor type 2 agonist for comorbid mood disorders and abnormal body weight. Front. Neurosci. 13, 391 (2019).
Reyes-Alcaraz, A., Lee, Y. N., Yun, S., Hwang, J. I. & Seong, J. Y. Conformational signatures in β-arrestin2 reveal natural biased agonism at a G-protein-coupled receptor. Commun. Biol. 1, 128 (2018).
Pałasz, A. et al. Effect of long-term treatment with classical neuroleptics on NPQ/spexin, kisspeptin and POMC mRNA expression in the male rat amygdala. J. Neural Transm. 125, 1099–1105 (2018).
Temmingh, H. & Stein, D. J. Anxiety in patients with schizophrenia: epidemiology and management. CNS Drugs 29, 819–832 (2015).
Pałasz, A. et al. Escitalopram affects spexin expression in the rat hypothalamus, hippocampus and striatum. Pharmacol. Rep. 68, 1326–1331 (2016).
Uher, R. et al. Changes in body weight during pharmacological treatment of depression. Int. J. Neuropsychopharmacol. 14, 367–375 (2011).
Toll, L. et al. Peptides derived from the prohormone proNPQ/spexin are potent central modulators of cardiovascular and renal function and nociception. FASEB J. 26, 947–954 (2012).
Coronel, M. F. et al. Progesterone modulates pro-inflammatory cytokine expression profile after spinal cord injury: implications for neuropathic pain. J. Neuroimmunol. 292, 85–92 (2016).
Lv, S. Y. et al. Spexin/NPQ induces FBJ osteosarcoma oncogene (Fos) and produces antinociceptive effect against inflammatory pain in the mouse model. Am. J. Pathol. 189, 886–899 (2019).
Gregory, N. S. et al. An overview of animal models of pain: disease models and outcome measures. J. Pain 14, 1255–1269 (2013).
Moazen, P., Taherianfard, M., Ahmadi Soleimani, M. & Norozpor, M. Synergistic effect of spexin and progesterone on pain sensitivity attenuation in ovariectomized rats. Clin. Exp. Pharmacol. Physiol. 45, 349–354 (2018).
Pirzeh, L. & Taherianfard, M. Effect of intra hippocampal CA1 injection of spexin on pain sensitivity in female rat. Bull. Env. Pharmacol. Life Sci. 3, 71–74 (2014).
Tanaka, M., Csabafi, K. & Telegdy, G. Neurotransmissions of antidepressant-like effects of kisspeptin-13. Regul. Pept. 180, 1–4 (2013).
Comninos, A. N. et al. Kisspeptin modulates sexual and emotional brain processing in humans. J. Clin. Invest. 127, 709–719 (2017).
Ogawa, S., Nathan, F. M. & Parhar, I. S. Habenular kisspeptin modulates fear in the zebrafish. Proc. Natl Acad. Sci. USA 111, 3841–3846 (2014).
Nathan, F. M., Ogawa, S. & Parhar, I. S. Kisspeptin1 modulates odorant-evoked fear response via two serotonin receptor subtypes (5-HT1A and 5-HT2) in zebrafish. J. Neurochem. 133, 870–878 (2015).
Csabafi, K., Jászberényi, M., Bagosi, Z., Lipták, N. & Telegdy, G. Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behav. Brain Res. 241, 56–61 (2013).
Rao, Y. S., Mott, N. N. & Pak, T. R. Effects of kisspeptin on parameters of the HPA axis. Endocrine 39, 220–228 (2011).
Thomson, E. L. et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J. Neuroendocrinol. 16, 850–858 (2004).
Gresham, R. et al. Kisspeptin in the medial amygdala and sexual behavior in male rats. Neurosci. Lett. 627, 13–17 (2016).
Comninos, A. N. et al. Modulations of human resting brain connectivity by kisspeptin enhance sexual and emotional functions. JCI Insight 3, e121958 (2018).
Messager, S. et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl Acad. Sci. 102, 1761–1766 (2005).
Mills, E. G. A., Dhillo, W. S. & Comninos, A. N. Kisspeptin and the control of emotions, mood and reproductive behaviour. J. Endocrinol. 239, R1–R12 (2018).
Comninos, A. N. & Dhillo, W. S. Emerging roles of kisspeptin in sexual and emotional brain processing. Neuroendocrinology 106, 195–202 (2018).
Yang, L., Comninos, A. N. & Dhillo, W. S. Intrinsic links among sex, emotion, and reproduction. Cell. Mol. Life Sci. 75, 2197–2210 (2018).
Yamaguchi, T., Ikeda, Y., Tashiro, K. & Ohkawa, Y. The role of galanin in the differentiation of mucosal mast cells in mice. Eur. J. Immunol. 50, 110–118 (2020).
Gambaro, S. E. et al. Spexin improves adipose tissue inflammation and macrophage recruitment in obese mice. Bioquim. Biophys. Acta Mol. Cell Biol. Lipids 1856, 158700 (2020).
Iwasa, T. et al. Decreased expression of kisspeptin mediates acute immune/inflammatory stress-induced suppression of gonadotropin secretion in female rat. J. Endocrinol. Invest. 31, 656–659 (2008).
Cunningham, M. J., Scarlett, J. M. & Steiner, R. A. Cloning and distribution of galanin-like peptide mRNA in the hypothalamus and pituitary of the macaque. Endocrinology 143, 755 (2002).
Kumano, S. et al. Changes in hypothalamic expression levels of galanin-like peptide in rat and mouse models support that it is a leptin-target peptide. Endocrinology 144, 2634–2643 (2003).
Kerr, N. C., Holmes, F. E. & Wynick, D. Galanin-like peptide (GALP) is expressed in rat hypothalamus and pituitary, but not in DRG. Neuroreport 11, 3909–3913 (2000).
Krasnow, S. M. et al. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology 144, 813–822 (2003).
Castellano, J. M. et al. Effects of galanin-like peptide on luteinizing hormone secretion in the rat: sexually dimorphic responses and enhanced sensitivity at male puberty. Am. J. Physiol. Endocrinol. Metab. 291, e1281–e1289 (2006).
Cunningham, M. J. et al. Galanin-like peptide as a possible link between metabolism and reproduction in the macaque. J. Clin. Endocrinol. Metab. 89, 1760–1766 (2004).
Fraley, G. S., Thomas-Smith, S. E., Acohido, B. V., Steiner, R. A. & Clifton, D. K. Stimulation of sexual behavior in the male rat by galanin-like peptide. Horm. Behav. 46, 551–557 (2004).
Taylor, A., Madison, F. N. & Fraley, G. S. Galanin-like peptide stimulates feeding and sexual behavior via dopaminergic fibers within the medial preoptic area of adult male rats. J. Chem. Neuroanat. 37, 105–111 (2009).
Kauffman, A. S., Buenzle, J., Fraley, G. S. & Rissman, E. F. Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Horm. Behav. 48, 141–151 (2005).
Lawrence, C. B., Baudoin, F. M. H. & Luckman, S. M. Centrally administered galanin-like peptide modifies food intake in the rat: a comparison with galanin. J. Neuroendocrinol. 14, 853–860 (2002).
Matsumoto, Y. et al. Galanin-like peptide stimulates food intake in the rat. Neurosci. Lett. 322, 67–69 (2002).
Seth, A. et al. Effects of galanin-like peptide on food intake and the hypothalamo-pituitary-thyroid axis. Neuroendocrinology 77, 125–131 (2003).
Juréus, A., Cunningham, M. J., Mcclain, M. E., Clifton, D. K. & Steiner, R. A. Galanin-like peptide (GALP) is a target for regulation by leptin in the hypothalamus of the rat. Endocrinology 141, 2703–2706 (2000).
Ito, K. et al. Interactive effect of galanin-like peptide (GALP) and spontaneous exercise on energy metabolism. Peptides 49, 109–116 (2013).
Hirako, S. et al. Autonomic nervous system-mediated effects of galanin-like peptide on lipid metabolism in liver and adipose tissue. Sci. Rep. 6, 21481 (2016).
Acknowledgements
The authors acknowledge the support of the National Institute for Health Research (NIHR), the NIHR Imperial Clinical Research Facility and NIHR Imperial Biomedical Research Centre (BRC) at Imperial College Healthcare NHS Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. The Section of Endocrinology and Investigative Medicine is funded by grants from the UK Medical Research Council (MRC) and NIHR. E.G.M. acknowledges the support of an MRC Clinical Research Training Fellowship (MR/T006242/1). C.I.-E. acknowledges the support of an Imperial College-BRC IPPRF Fellowship (P79696). A.A. acknowledges the support of an NIHR Clinician Scientist Award (CS-2018-18-ST2-002). A.N.C. acknowledges the support of the NHS. W.S.D. acknowledges the support of an NIHR Professorship (RP-2014-05-001).
Author information
Authors and Affiliations
Contributions
All authors contributed to writing the article, reviewing/editing the manuscript before submission, and made substantial contributions to discussions of the content. E.G.M. and C.I-E. researched data for the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Corticotropin releasing factor
-
(CRF). A neuropeptide that acts as the central driver of the hormonal stress response by activating the synthesis and release of adrenocorticotropic hormone from the anterior pituitary.
- Rostral periventricular region of the third ventricle
-
A group of cells residing in the preoptic area of the hypothalamus, encompassing the anteroventral periventricular and the periventricular nuclei.
- Circumventricular organs
-
These are specialized neuroepithelial midline structures that are not protected by the blood–brain barrier.
- Periaqueductal grey matter
-
A complex nucleus that coordinates the antinociceptive, behavioural and autonomic responses to stress and injury.
Rights and permissions
About this article
Cite this article
Mills, E.G., Izzi-Engbeaya, C., Abbara, A. et al. Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour. Nat Rev Endocrinol 17, 97–113 (2021). https://doi.org/10.1038/s41574-020-00438-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-020-00438-1
This article is cited by
-
Normalization of Spexin Levels in Patients with Obesity Submitted to Bariatric Surgery
Obesity Surgery (2024)
-
The role of central neurotransmitters in appetite regulation of broilers and layers: similarities and differences
Veterinary Research Communications (2024)
-
Unveiling adcyap1 as a protective factor linking pain and nerve regeneration through single-cell RNA sequencing of rat dorsal root ganglion neurons
BMC Biology (2023)
-
Alleviation of Limosilactobacillus reuteri in polycystic ovary syndrome protects against circadian dysrhythmia-induced dyslipidemia via capric acid and GALR1 signaling
npj Biofilms and Microbiomes (2023)
-
Maternal neuropeptide galanin levels in pregnancies with intra-uterine growth restriction (IUGR): neurohormonal regulation of fetal weight
Irish Journal of Medical Science (1971 -) (2023)