Abstract
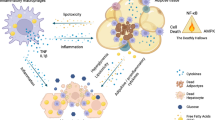
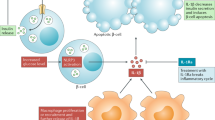
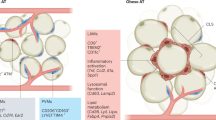
Chronic, unresolved tissue inflammation is a well-described feature of obesity, type 2 diabetes mellitus (T2DM) and other insulin-resistant states. In this context, adipose tissue and liver inflammation have been particularly well studied; however, abundant evidence demonstrates that inflammatory processes are also activated in pancreatic islets from obese animals and humans with obesity and/or T2DM. In this Review, we focus on the characteristics of immune cell-mediated inflammation in islets and the consequences of this with respect to β-cell function. In contrast to type 1 diabetes mellitus, the dominant immune cell type causing inflammation in obese and T2DM islets is the macrophage. The increased macrophage accumulation in T2DM islets primarily arises through local proliferation of resident macrophages, which then provide signals (such as platelet-derived growth factor) that drive β-cell hyperplasia (a classic feature of obesity). In addition, islet macrophages also impair the insulin secretory capacity of β-cells. Through these mechanisms, islet-resident macrophages underlie the inflammatory response in obesity and mechanistically participate in the β-cell hyperplasia and dysfunction that characterizes this insulin-resistant state. These findings point to the possibility of therapeutics that target islet inflammation to elicit beneficial effects on β-cell function and glycaemia.
Key points
-
Macrophages are the primary immune cells involved in obesity-associated islet inflammation in both mice and humans.
-
Obesity reprogrammes the islet immune microenvironment by inducing the local replication of islet-resident macrophages or by recruiting circulating monocytes.
-
Islet macrophages in obese mice display multiple functions, including decreasing β-cell insulin secretion and stimulating β-cell proliferation.
-
In the normal, lean state, islet macrophages promote islet development and maintenance of normal glucose-stimulated insulin secretion.
-
Islet macrophages are potential therapeutic targets to modulate β-cell function in individuals with obesity and/or type 2 diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
da Rocha Fernandes, J. et al. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res. Clin. Pract. 117, 48–54 (2016).
Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346, 393–403 (2002).
Magkos, F. et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 23, 591–601 (2016).
Saisho, Y. Importance of beta cell function for the treatment of type 2 diabetes. J. Clin. Med. 3, 923–943 (2014).
Kitamura, T. The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 9, 615–623 (2013).
Biden, T. J., Boslem, E., Chu, K. Y. & Sue, N. Lipotoxic endoplasmic reticulum stress, beta cell failure, and type 2 diabetes mellitus. Trends Endocrinol. Metab. 25, 389–398 (2014).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 (2011).
Lackey, D. E. & Olefsky, J. M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 12, 15–28 (2016).
Lee, Y. S., Wollam, J. & Olefsky, J. M. An integrated view of immunometabolism. Cell 172, 22–40 (2018).
Eguchi, K. et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell. Metab. 15, 518–533 (2012). This study shows that SFAs induce β-cells to produce chemokines that recruit pro-inflamamtory monocytes and macrophages, which impair β-cell function.
Boni-Schnetzler, M. & Meier, D. T. Islet inflammation in type 2 diabetes. Semin. Immunopathol. 41, 501–513 (2019).
Ying, W. et al. Expansion of islet-resident macrophages leads to inflammation affecting beta cell proliferation and function in obesity. Cell Metab. 29, 457–474 e455 (2019). This study reports that resident macrophages drive obesity-associated islet inflammation through local proliferation and that the accumulated islet macrophages affect both β-cell proliferation and function.
Ginhoux, F. & Guilliams, M. Tissue-resident macrophage ontogeny and homeostasis. Immunity 44, 439–449 (2016).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Geutskens, S. B., Otonkoski, T., Pulkkinen, M. A., Drexhage, H. A. & Leenen, P. J. Macrophages in the murine pancreas and their involvement in fetal endocrine development in vitro. J. Leukoc. Biol. 78, 845–852 (2005).
Banaei-Bouchareb, L. et al. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J. Leukoc. Biol. 76, 359–367 (2004).
Calderon, B. et al. The pancreas anatomy conditions the origin and properties of resident macrophages. J. Exp. Med. 212, 1497–1512 (2015). This study provides a comprehensive view of the origin, phenotype, turnover and gene expression of macrophages in exocrine pancreas and the islets.
Mathis, D. Immunological goings-on in visceral adipose tissue. Cell Metab. 17, 851–859 (2013).
McLaughlin, T., Ackerman, S. E., Shen, L. & Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Invest. 127, 5–13 (2017).
Sell, H., Habich, C. & Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 8, 709–716 (2012).
Shalapour, S. & Karin, M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Invest. 125, 3347–3355 (2015).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Ehses, J. A. et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 (2007). This study provides clear evidence showing an increase in the number of macrophages in the islets of humans with T2DM and animal models of obesity and T2DM.
Kamata, K. et al. Islet amyloid with macrophage migration correlates with augmented beta-cell deficits in type 2 diabetic patients. Amyloid 21, 191–201 (2014).
Zhao, H. L. et al. Prevalence and clinicopathological characteristics of islet amyloid in Chinese patients with type 2 diabetes. Diabetes 52, 2759–2766 (2003).
Richardson, S. J., Willcox, A., Bone, A. J., Foulis, A. K. & Morgan, N. G. Islet-associated macrophages in type 2 diabetes. Diabetologia 52, 1686–1688 (2009).
Marselli, L. et al. Beta-cell inflammation in human type 2 diabetes and the role of autophagy. Diabetes Obes. Metab. 15, 130–136 (2013).
Butcher, M. J. et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia 57, 491–501 (2014).
Donath, M. Y., Storling, J., Berchtold, L. A., Billestrup, N. & Mandrup-Poulsen, T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr. Rev. 29, 334–350 (2008).
Eguchi, K. & Nagai, R. Islet inflammation in type 2 diabetes and physiology. J. Clin. Invest. 127, 14–23 (2017).
Cucak, H., Grunnet, L. G. & Rosendahl, A. Accumulation of M1-like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. J. Leukoc. Biol. 95, 149–160 (2014).
Perdiguero, E. G. & Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 17, 2–8 (2016).
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Braune, J. et al. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J. Immunol. 198, 2927–2934 (2017).
Haase, J. et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia 57, 562–571 (2014).
Amano, S. U. et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 19, 162–171 (2014).
Tardelli, M. et al. Osteopontin is a key player for local adipose tissue macrophage proliferation in obesity. Mol. Metab. 5, 1131–1137 (2016).
Hamilton, J. A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 (2008).
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2010).
Maedler, K. et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 110, 851–860 (2002).
Boni-Schnetzler, M. et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150, 5218–5229 (2009).
Ye, R. et al. Intracellular lipid metabolism impairs beta cell compensation during diet-induced obesity. J. Clin. Invest. 128, 1178–1189 (2018).
Amar, S., Zhou, Q., Shaik-Dasthagirisaheb, Y. & Leeman, S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc. Natl Acad. Sci. USA 104, 20466–20471 (2007).
Zhang, Q. et al. NF-kappaB dynamics discriminate between TNF doses in single cells. Cell Syst. 5, 638–645.e5 (2017).
Turner, D. A. et al. Physiological levels of TNFalpha stimulation induce stochastic dynamics of NF-kappaB responses in single living cells. J. Cell Sci. 123, 2834–2843 (2010).
Stephens, J. M., Lee, J. & Pilch, P. F. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J. Biol. Chem. 272, 971–976 (1997).
McGillicuddy, F. C. et al. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes 60, 1688–1698 (2011).
Aamodt, K. I. & Powers, A. C. Signals in the pancreatic islet microenvironment influence beta-cell proliferation. Diabetes Obes. Metab. 19, 124–136 (2017).
Weitz, J. R. et al. Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia 61, 182–192 (2018).
Westwell-Roper, C. Y., Ehses, J. A. & Verchere, C. B. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1beta production and beta-cell dysfunction. Diabetes 63, 1698–1711 (2014).
Kratz, M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014).
Lumeng, C. N., DelProposto, J. B., Westcott, D. J. & Saltiel, A. R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246 (2008).
Li, P. et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem. 285, 15333–15345 (2010).
Rodriguez-Calvo, T., Ekwall, O., Amirian, N., Zapardiel-Gonzalo, J. & von Herrath, M. G. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes 63, 3880–3890 (2014).
Dalmas, E. et al. Interleukin-33-activated islet-resident innate lymphoid cells promote insulin secretion through myeloid cell retinoic acid production. Immunity 47, 928–942 e7 (2017).
Brissova, M. et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes beta cell regeneration. Cell Metab. 19, 498–511 (2014). This study demonstrates the importance of endothelial cell-derived VEGF in regulating β-cell proliferation and insulin secretion.
Porte, D. Jr., Baskin, D. G. & Schwartz, M. W. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes 54, 1264–1276 (2005).
Halban, P. A. et al. Beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 37, 1751–1758 (2014). This paper briefly summarizes recent knowledge on the mechanisms underlying β-cell failure in T2DM.
Donath, M. Y., Boni-Schnetzler, M., Ellingsgaard, H., Halban, P. A. & Ehses, J. A. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol. Metab. 21, 261–267 (2010).
Burns, S. M. et al. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic beta-cell function. Cell Metab. 21, 126–137 (2015).
Westwell-Roper, C., Denroche, H. C., Ehses, J. A. & Verchere, C. B. Differential activation of innate immune pathways by distinct islet amyloid polypeptide (IAPP) aggregates. J. Biol. Chem. 291, 8908–8917 (2016).
Dasu, M. R., Devaraj, S. & Jialal, I. High glucose induces IL-1beta expression in human monocytes: mechanistic insights. Am. J. Physiol. Endocrinol. Metab. 293, E337–E346 (2007).
Riera-Borrull, M. et al. Palmitate conditions macrophages for enhanced responses toward inflammatory stimuli via JNK activation. J. Immunol. 199, 3858–3869 (2017).
Kim, H. E. et al. Tumour necrosis factor-alpha-induced glucose-stimulated insulin secretion inhibition in INS-1 cells is ascribed to a reduction of the glucose-stimulated Ca2+ influx. J. Endocrinol. 198, 549–560 (2008).
Cardozo, A. K. et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54, 452–461 (2005).
Major, C. D. & Wolf, B. A. Interleukin-1beta stimulation of c-Jun NH2-terminal kinase activity in insulin-secreting cells: evidence for cytoplasmic restriction. Diabetes 50, 2721–2728 (2001).
Ammendrup, A. et al. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes 49, 1468–1476 (2000).
Bonny, C., Oberson, A., Negri, S., Sauser, C. & Schorderet, D. F. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes 50, 77–82 (2001).
Welsh, N. Interleukin-1 beta-induced ceramide and diacylglycerol generation may lead to activation of the c-Jun NH2-terminal kinase and the transcription factor ATF2 in the insulin-producing cell line RINm5F. J. Biol. Chem. 271, 8307–8312 (1996).
Bouzakri, K. et al. Rab GTPase-activating protein AS160 is a major downstream effector of protein kinase B/Akt signaling in pancreatic beta-cells. Diabetes 57, 1195–1204 (2008).
Kawamori, D. et al. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J. Biol. Chem. 281, 1091–1098 (2006).
Kaneto, H. et al. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J. Biol. Chem. 277, 30010–30018 (2002).
Kim-Muller, J. Y. et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metab. 20, 593–602 (2014).
Grunnet, L. G. et al. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes 58, 1807–1815 (2009).
Cardozo, A. K., Kruhoffer, M., Leeman, R., Orntoft, T. & Eizirik, D. L. Identification of novel cytokine-induced genes in pancreatic beta-cells by high-density oligonucleotide arrays. Diabetes 50, 909–920 (2001).
Weir, G. C., Laybutt, D. R., Kaneto, H., Bonner-Weir, S. & Sharma, A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes 50, S154–S159 (2001).
Welsh, N. et al. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes 54, 3238–3244 (2005).
Vomund, A. N. et al. Beta cells transfer vesicles containing insulin to phagocytes for presentation to T cells. Proc. Natl Acad. Sci. USA 112, E5496–E5502 (2015). This study shows evidence that intra-islet macrophages take up intact insulin-containing vesicles from β-cells.
Yamashita, Y. M., Inaba, M. & Buszczak, M. Specialized intercellular communications via cytonemes and nanotubes. Annu. Rev. Cell. Dev. Biol. 34, 59–84 (2018).
Baeyens, L., Hindi, S., Sorenson, R. L. & German, M. S. Beta-cell adaptation in pregnancy. Diabetes Obes. Metab. 18, 63–70 (2016).
Hull, R. L. et al. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia 48, 1350–1358 (2005).
Peyot, M. L. et al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 59, 2178–2187 (2010).
Ebato, C. et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 8, 325–332 (2008).
Stamateris, R. E., Sharma, R. B., Hollern, D. A. & Alonso, L. C. Adaptive beta-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. Am. J. Physiol. Endocrinol. Metab. 305, E149–E159 (2013).
Mosser, R. E. et al. High-fat diet-induced beta-cell proliferation occurs prior to insulin resistance in C57BL/6J male mice. Am. J. Physiol. Endocrinol. Metab. 308, E573–E582 (2015).
Alonso, L. C. et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 56, 1792–1801 (2007).
Levitt, H. E. et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia 54, 572–582 (2011).
Porat, S. et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 13, 440–449 (2011).
Assmann, A., Ueki, K., Winnay, J. N., Kadowaki, T. & Kulkarni, R. N. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol. Cell Biol. 29, 3219–3228 (2009).
Garcia-Ocana, A. et al. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J. Biol. Chem. 275, 1226–1232 (2000).
Araujo, T. G. et al. Hepatocyte growth factor plays a key role in insulin resistance-associated compensatory mechanisms. Endocrinol. 153, 5760–5769 (2012).
Demirci, C. et al. Loss of HGF/c-Met signaling in pancreatic beta-cells leads to incomplete maternal beta-cell adaptation and gestational diabetes mellitus. Diabetes 61, 1143–1152 (2012).
Xiao, X. et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc. Natl Acad. Sci. USA 111, E1211–E1220 (2014). This study reveals that M2-like macrophages and the SMAD7 pathway can increase β-cell mass.
Riley, K. G. et al. Macrophages are essential for CTGF-mediated adult beta-cell proliferation after injury. Mol. Metab. 4, 584–591 (2015).
Chen, H. et al. PDGF signalling controls age-dependent proliferation in pancreatic beta-cells. Nature 478, 349–355 (2011).
Jaguin, M., Fardel, O. & Lecureur, V. AhR-dependent secretion of PDGF-BB by human classically activated macrophages exposed to DEP extracts stimulates lung fibroblast proliferation. Toxicol. Appl. Pharmacol. 285, 170–178 (2015).
Onogi, Y. et al. PDGFRbeta regulates adipose tissue expansion and glucose metabolism via vascular remodeling in diet-induced obesity. Diabetes 66, 1008–1021 (2017).
Shimokado, K. et al. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell 43, 277–286 (1985).
Zhou, X. et al. Circuit design features of a stable two-cell system. Cell 172, 744–757.e17 (2018).
Donath, M. Y., Dalmas, E., Sauter, N. S. & Boni-Schnetzler, M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 17, 860–872 (2013).
Burke, S. J. et al. Pancreatic deletion of the interleukin-1 receptor disrupts whole body glucose homeostasis and promotes islet beta-cell de-differentiation. Mol. Metab. 14, 95–107 (2018). This study reports that knockout of the β-cell IL-1β receptor in mice can result in dedifferentiation of β-cells.
Maedler, K. et al. Low concentration of interleukin-1 beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes 55, 2713–2722 (2006). This study shows an improvement in β-cell proliferation by a low concentration of IL-1β.
Nordmann, T. M. et al. The role of inflammation in beta-cell dedifferentiation. Sci. Rep. 7, 6285 (2017).
Talchai, C., Xuan, S., Lin, H. V., Sussel, L. & Accili, D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 150, 1223–1234 (2012).
Boni-Schnetzler, M. et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta-cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J. Clin. Endocrinol. Metab. 93, 4065–4074 (2008).
Boni-Schnetzler, M. et al. Beta cell-specific deletion of the IL-1 receptor antagonist impairs beta cell proliferation and insulin secretion. Cell Rep. 22, 1774–1786 (2018). This study provides evidence supporting an important role of β-cell-derived IL-1Ra on β-cell function and maintenance.
Dror, E. et al. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 18, 283–292 (2017). This study indicates the physiological role of macrophage-derived IL-1β in mediating postprandial β-cell insulin secretion.
Hajmrle, C. et al. Interleukin-1 signaling contributes to acute islet compensation. JCI Insight 1, e86055 (2016).
Kelpe, C. L. et al. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J. Biol. Chem. 278, 30015–30021 (2003).
Lang, F., Ullrich, S. & Gulbins, E. Ceramide formation as a target in beta-cell survival and function. Expert Opin. Ther. Targets 15, 1061–1071 (2011).
Cunha, D. A. et al. Death protein 5 and p53-upregulated modulator of apoptosis mediate the endoplasmic reticulum stress-mitochondrial dialog triggering lipotoxic rodent and human beta-cell apoptosis. Diabetes 61, 2763–2775 (2012).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Thompson, P. J. et al. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab. 29, 1045–1060.e10 (2019). This study demonstrates the critical role of senescent β-cells in triggering the occurrence of T1DM.
Sone, H. & Kagawa, Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48, 58–67 (2005).
Aguayo-Mazzucato, C. et al. Beta cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab. 25, 898–910.e5 (2017).This study reveals the development of senescent β-cells that affect insulin secretion in obesity and/or T2DM.
Donath, M. Y. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat. Rev. Drug Discov. 13, 465–476 (2014).
Goldfine, A. B. et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann. Intern. Med. 159, 1–12 (2013).
Larsen, C. M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J. Am. Coll. Cardiol. 71, 2392–2401 (2018). This study provides a critical assessment of the effect of canakinumab on major cardiovascular events among individuals with and without diabetes mellitus.
Kataria, Y., Ellervik, C. & Mandrup-Poulsen, T. Treatment of type 2 diabetes by targeting interleukin-1: a meta-analysis of 2921 patients. Semin. Immunopathol. 41, 413–425 (2019).
Gordon, S. & Pluddemann, A. Tissue macrophages: heterogeneity and functions. BMC Biol. 15, 53 (2017).
Acknowledgements
The authors acknowledge the support of the US National Institute of Diabetes and Digestive and Kidney Diseases (DK063491 and DK101395 to J.M.O., and DK114427 to W.F.), the US National Institute of Diabetes and Digestive and Kidney Diseases K99/R00 award (1K99DK115998 to W.Y.), the University of California San Diego (UCSD)/ University of California Los Angeles (UCLA) Diabetes Research Center Pilot and Feasibility grants (to W.Y., Y.S.L, and W.F.), and the UCSD Clinical and Translational Research Institute (CTRI) UL1 TR000100 (to W.F.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Yolk sac-derived primitive haematopoiesis
-
The generation of blood-lineage cells, including primitive erythroid cells, platelets and macrophages, in the extra-embryonic yolk sac during early embryonic development.
- Osteopetrosis
-
A rare inherited syndrome characterized by increased bone density due to a defect of osteoclasts, a specialized population of macrophages that can resorb bone.
- Insulitis
-
Islet-specific inflammation characterized by the infiltration of various types of immune cell into pancreatic islets, more commonly used to describe the islet inflammation preceding or accompanying type 1 diabetes mellitus.
- Islet amyloid polypeptide
-
(IAPP). An islet hormone that is co-secreted with insulin from β-cells and forms islet amyloids.
- Metaflammation
-
Low-grade inflammation induced by overnutrition, which occurs in metabolic tissue (primarily adipose tissue, liver, muscle and pancreatic islets), causes dysregulation of immune cells and inflammatory responses.
- Type 2 innate lymphoid cells
-
(ILC2s). A subgroup of innate lymphoid cells characterized by the lack of rearranged receptors and production of type 2 cytokines such as IL-5 and IL-13.
- Transwell plate chambers
-
Devices designed to study cell migration and cell–cell interaction, which are permeable to soluble factors but prevent the migration or contact of cells between the upper and lower chambers.
- IL-1 receptor antagonist
-
(IL-1Ra). A member of the IL-1 family of cytokines that binds to the IL-1 receptor but does not induce intracellular signalling.
Rights and permissions
About this article
Cite this article
Ying, W., Fu, W., Lee, Y.S. et al. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol 16, 81–90 (2020). https://doi.org/10.1038/s41574-019-0286-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0286-3
This article is cited by
-
Didymin protects pancreatic beta cells by enhancing mitochondrial function in high-fat diet-induced impaired glucose tolerance
Diabetology & Metabolic Syndrome (2024)
-
Macrophage-specific FGFR1 deletion alleviates high-fat-diet-induced liver inflammation by inhibiting the MAPKs/TNF pathways
Acta Pharmacologica Sinica (2024)
-
STING signaling in islet macrophages impairs insulin secretion in obesity
Science China Life Sciences (2024)
-
Forkhead box P3 gene polymorphisms predispose to type 2 diabetes and diabetic nephropathy in the Han Chinese populations: a genetic-association and gender-based evaluation study
Hereditas (2023)
-
Association of remnant cholesterol with hypertension, type 2 diabetes, and their coexistence: the mediating role of inflammation-related indicators
Lipids in Health and Disease (2023)