Abstract
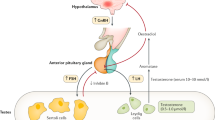
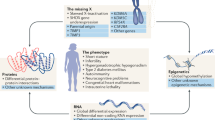
Differences of sex development are conditions with discrepancies between chromosomal, gonadal and phenotypic sex. In congenital hypogonadotropic hypogonadism, a lack of gonadotropin activity results primarily in the absence of pubertal development with prenatal sex development being (almost) unaffected in most patients. To expedite progress in the care of people affected by differences of sex development and congenital hypogonadotropic hypogonadism, the European Union has funded a number of scientific networks. Two Actions of the Cooperation of Science and Technology (COST) programmes — DSDnet (BM1303) and GnRH Network (BM1105) — provided the framework for ground-breaking research and allowed the development of position papers on diagnostic procedures and special laboratory analyses as well as clinical management. Both Actions developed educational programmes to increase expertise and promote interest in this area of science and medicine. In this Perspective article, we discuss the success of the COST Actions DSDnet and GnRH Network and the European Reference Network for Rare Endocrine Conditions (Endo–ERN), and provide recommendations for future research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hughes, I. A. et al. Consensus statement on management of intersex disorders. Arch. Dis. Child. 91, 554–563 (2006).
Flück, C. E. et al. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am. J. Hum. Genet. 89, 201–18 (2011).
Croft, B. et al. Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nat. Commun. 9, 5319 (2018).
Cox, K. et al. Novel associations in disorders of sex development: findings from the I-DSD Registry. J. Clin. Endocrinol. Metab. 99, E348–E355 (2014).
Lucas-Herald, A. et al. The long-term outcome of boys with partial androgen insensitivity syndrome and a mutation in the androgen receptor gene. J. Clin. Endocrinol. Metab. 101, 3959–3967 (2016).
Schweizer, K., Brunner, F., Gedrose, B., Handford, C. & Richter-Appelt, H. Coping with diverse sex development: treatment experiences and psychosocial support during childhood and adolescence and adult well-being. J. Pediatr. Psychol. 42, 504–519 (2016).
Ahmed, S. F. et al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015). Clin. Endocrinol. 84, 771–788 (2016).
Boehm, U. et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 11, 547–564 (2015).
Bonomi, M. et al. Characteristics of a nationwide cohort of patients presenting with isolated hypogonadotropic hypogonadism (IHH). Eur. J. Endocrinol. 178, 23–32 (2018).
Cools, M. et al. Caring for individuals with a difference of sex development (DSD): a Consensus Statement. Nat. Rev. Endocrinol. 14, 415–429 (2018).
Ahmed, S. F., Khwaja, O. & Hughes, I. A. The role of a clinical score in the assessment of ambiguous genitalia. BJU Int. 85, 120–124 (2000).
Dwyer, A. A. et al. Trial of recombinant follicle-stimulating hormone pretreatment for GnRH-induced fertility in patients with congenital hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 98, E1790–1795 (2013).
Dwyer, A. A., Raivio, T. & Pitteloud, N. Gonadotrophin replacement for induction of fertility in hypogonadal men. Best Pract. Res. Clin. Endocrinol. Metab. 29, 91–103 (2015).
Dwyer, A. A., Quinton, R., Pitteloud, N. & Morin, D. Psychosexual development in men with congenital hypogonadotropic hypogonadism on long-term treatment: a mixed methods study. Sex. Med. 3, 32–41 (2015).
Audi, L. et al. Genetics in endocrinology: approaches to molecular genetic diagnosis in the management of differences/disorders of sex development (DSD): position paper of EU COST Action BM 1303 ‘DSDnet’. Eur. J. Endocrinol. https://doi.org/10.1530/EJE-18-0256 (2018).
Cariboni, A. et al. Dysfunctional SEMA3E signalling underlies gonadotropin-releasing hormone neuron deficiency in Kallmann syndrome. J. Clin. Invest. 125, 2413–2428 (2015).
Parkash, J. et al. Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nat. Commun. 6, 6385 (2015).
Miraoui, H. et al. Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. Am. J. Hum. Genet. 92, 725–743 (2013).
Xu, C. et al. KLB, encoding β-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol. Med. 9, 1379–1397 (2017).
Bouilly, J. et al. DCC/NTN1 complex mutations in patients with congenital hypogonadotropic hypogonadism impair GnRH neuron development. Hum. Mol. Genet. 27, 359–372 (2018).
Harris, A. et al. ZNRF3 functions in mammalian sex determination by inhibiting canonical WNT signalling. Proc. Natl Acad. Sci. U S A 115, 5474–5479 (2018).
Bashamboo, A. et al. Loss of function of the nuclear receptor NR2F2, encoding COUP-TF2, causes testis development and cardiac defects in 46,XX children. Am. J. Hum. Genet. 102, 487–493 (2018).
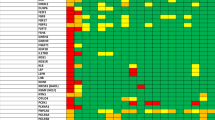
Eggers, S. et al. Disorders of sex development: insights from targeted gene sequencing of a large international patient cohort. Genome Biol. 17, 243 (2016).
Ridder, J. D. et al. SDgeneMatch, a new tool to aid the identification of the genetic causes of DSD. ESPE Abstracts. 89, P-P1-216 (2018).
Kulle, A. et al. Steroid hormone analysis in diagnosis and treatment of DSD: position paper of EU COST Action BM 1303 ‘DSDnet’. Eur. J. Endocrinol. 176, P1–P9 (2017).
Segal, T. Y., Mehta, A., Anazodo, A., Hindmarsh, P. C. & Dattani, M. T. Role of gonadotropin-releasing hormone and human chorionic gonadotropin stimulation tests in differentiating patients with hypogonadotropic hypogonadism from those with constitutional delay of growth and puberty. J. Clin. Endocrinol. Metab. 94, 780–785 (2009).
Trabado, S. et al. Insulin-like peptide 3 (INSL3) in men with congenital hypogonadotropic hypogonadism/Kallmann syndrome and effects of different modalities of hormonal treatment: a single-centre study of 281 patients. J. Clin. Endocrinol. Metab. 99, E268–275 (2014).
Greaves, R. F. et al. Current state and recommendations for harmonization of serum/plasma 17-hydroxyprogesterone mass spectrometry methods. Clin. Chem. Lab. Med. 56, 1685–1697 (2018).
Cools, M. et al. Response to the Council of Europe Human Rights Commissioner’s Issue Paper on Human Rights and Intersex People. Eur. Urol. 70, 407–409 (2016).
Bertalan, R. et al. Evaluation of DSD training schools organized by cost action BM1303 ‘DSDnet’. Orphanet J. Rare Dis. 13, 227 (2018).
Kyriakou, A. et al. Current models of care for disorders of sex development - results from an international survey of specialist centres. Orphanet J. Rare Dis. 11, 155 (2016).
Dessens, A. et al. Understanding the needs of professionals who provide psychosocial care for children and adults with disorders of sex development. BMJ Paediatr. Open 1, e000132 (2017).
Minto, C. L., Liao, L. M., Woodhouse, C. R., Ransley, P. G. & Creighton, S. M. The effect of clitoral surgery on sexual outcome in individuals who have intersex conditions with ambiguous genitalia: a cross-sectional study. Lancet 361, 1252–1257 (2003).
Frisen, L. et al. Gender role behaviour, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J. Clin. Endocrinol. Metab. 94, 3432–3439 (2009).
van der Zwan, Y. G. et al. Severity of virilization is associated with cosmetic appearance and sexual function in women with congenital adrenal hyperplasia: a cross-sectional study. J. Sex. Med. 10, 866–875 (2013).
Callens, N. et al. Recalled and current gender role behaviour, gender identity and sexual orientation in adults with disorders/differences of sex development. Horm. Behav. 86, 8–20 (2016).
Sanders, C. et al. Involving individuals with disorders of sex development and their parents in exploring new models of shared learning: proceedings from a DSDnet COST Action Workshop. Sex. Dev. https://doi.org/10.1159/000490081 (2018).
Dwyer, A. A., Quinton, R., Morin, D. & Pitteloud, N. Identifying the unmet health needs of patients with congenital hypogonadotropic hypogonadism using a web-based needs assessment: implications for online interventions and peer-to-peer support. Orphanet J. Rare Dis. 9, 83 (2014).
COST Action BM1105, Badiu, C. et al. Developing and evaluating rare disease educational materials co-created by expert clinicians and patients: the paradigm of congenital hypogonadotropic hypogonadism. Orphanet J. Rare Dis. 12, 57 (2017).
Ali, S. R. et al. The current landscape of European registries for rare endocrine conditions. Eur. J. Endocrinol. 180, 89–98 (2019).
Bick, D., Jones, M., Taylor, S. L., Taft, R. J., Belmont, J. Case for genome sequencing in infants and children with rare, undiagnosed or genetic diseases. J. Med. Genet. https://doi.org/10.1136/jmedgenet-2019-106111(2019).
Carre, G. A. & Greenfield, A. Characterizing novel pathways in testis determination using mouse genetics. Sex. Dev. 8, 199–207 (2014).
Carré, G. A. et al. Loss of p300 and CBP disrupts histone acetylation at the mouse Sry promoter and causes XY gonadal sex reversal. Hum. Mol. Genet. 27, 190–198 (2017).
Greenfield, A. Editing mammalian genomes: ethical perspectives. Mamm. Genome 28, 388–393 (2017).
Warr, N. et al. Transgenic overexpression of Map3k4 rescues T-associated sex reversal (Tas) in mice. Hum. Mol. Genet. 23, 3035–3044 (2014).
Cassatella, D. et al. Congenital hypogonadotropic hypogonadism and constitutional delay of growth and puberty have distinct genetic architectures. Eur. J. Endocrinol. 178, 377–388 (2018).
Acknowledgements
The authors thank all members and participants of both COST Actions for their continuous support and active input.
Author information
Authors and Affiliations
Consortia
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks C. H. Gravholt and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CHUV: www.chuv.ch/en/hhn/hhn-home/
COST Action DSDnet: www.dsdnet.eu
DSD-Life: https://www.dsd-life.eu/
DSDnet: www.dsdnet.eu
Endo–ERN: https://endo-ern.eu/
European Commission European Reference Networks: https://ec.europa.eu/health/ern_en
GnRH Network: www.gnrhnetwork.eu
I-CAH Registry: www.i-cah.org
I-DSD Registry: www.i-dsd.org
Rights and permissions
About this article
Cite this article
Hiort, O., Cools, M., Springer, A. et al. Addressing gaps in care of people with conditions affecting sex development and maturation. Nat Rev Endocrinol 15, 615–622 (2019). https://doi.org/10.1038/s41574-019-0238-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0238-y
This article is cited by
-
Advancing qualitative rare disease research methodology: a comparison of virtual and in-person focus group formats
Orphanet Journal of Rare Diseases (2022)
-
Single-cell roadmap of human gonadal development
Nature (2022)
-
Validating online approaches for rare disease research using latent class mixture modeling
Orphanet Journal of Rare Diseases (2021)
-
Assessing the health-related management of people with differences of sex development
Endocrine (2021)
-
Collaboration for rare diabetes: understanding new treatment options for Wolfram syndrome
Endocrine (2021)