Abstract
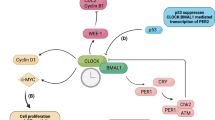
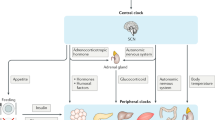
Circadian rhythmicity is an approximately 24-h cell-autonomous period driven by transcription–translation feedback loops of specific genes, which are referred to as ‘circadian clock genes’. In mammals, the central circadian pacemaker, which is located in the hypothalamic suprachiasmatic nucleus, controls peripheral circadian clocks. The circadian system regulates virtually all physiological processes, which are further modulated by changes in the external environment, such as light exposure and the timing of food intake. Chronic circadian disruption caused by shift work, travel across time zones or irregular sleep–wake cycles has long-term consequences for our health and is an important lifestyle factor that contributes to the risk of obesity, type 2 diabetes mellitus and cancer. Although the hypothalamic–pituitary–thyroid axis is under the control of the circadian clock via the suprachiasmatic nucleus pacemaker, daily TSH secretion profiles are disrupted in some patients with hypothyroidism and hyperthyroidism. Disruption of circadian rhythms has been recognized as a perturbation of the endocrine system and of cell cycle progression. Expression profiles of circadian clock genes are abnormal in well-differentiated thyroid cancer but not in the benign nodules or a healthy thyroid. Therefore, the characterization of the thyroid clock machinery might improve the preoperative diagnosis of thyroid cancer.
Key points
-
The hypothalamic–pituitary–thyroid axis is controlled by the central circadian pacemaker located in the suprachiasmatic nucleus.
-
Daily TSH secretion profiles are often disrupted in patients with hypothyroidism or hyperthyroidism.
-
Circadian dysfunction caused by shift work, travel across time zones or irregular sleep–wake cycles might be a novel lifestyle risk factor for disturbances in thyroid homeostasis in modern societies.
-
Disruption of circadian clock genes in vivo and in vitro disturbs cell cycle progression.
-
The circadian clock is thought to be disrupted in well-differentiated thyroid cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fekete, C. & Lechan, R. M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 35, 159–194 (2014).
Ortiga-Carvalho, T. M., Chiamolera, M. I., Pazos-Moura, C. C. & Wondisford, F. E. Hypothalamus-pituitary-thyroid axis. Compr. Physiol. 6, 1387–1428 (2016).
Mohawk, J. A., Green, C. B. & Takahashi, J. S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 35, 445–462 (2012).
Scheer, F. A. J. L., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458 (2009).
Davidson, A. J. et al. Chronic jet- lag increases mortality in aged mice. Curr. Biol. 16, 914–916 (2006).
Buxton, O. M. et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl Med. 4, 129ra43 (2012).
Kettner, N. M., Katchy, C. A. & Fu, L. Circadian gene variants in cancer. Ann. Med. 46, 208–220 (2014).
Bellastella, A. et al. Endocrine secretions under abnormal light-dark cycles and in the blind. Horm. Res. 49, 153–157 (1998).
Kalsbeek, A. & Fliers, E. Daily regulation of hormone profile. Handb. Exp. Pharmacol. 217, 185–226 (2013).
Pierce, J. G. Eli lilly lecture: The subunits of pituitary thyrotropin—their relationship to other glycoprotein hormones. Endocrinology 89, 1331–1344 (1971).
Shupnik, M. A., Greenspan, S. L. & Ridgway, E. C. Transcriptional regulation of thyrotropin subunit genes by thyrotropin-releasing hormone and dopamine in pituitary cell culture. J. Biol. Chem. 261, 12675–12679 (1986).
Vassart, G. & Dumont, J. E. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr. Rev. 13, 596–611 (1992).
Friesema, E. C. H. et al. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem. 278, 40128–40135 (2003).
Pizzagalli, F. et al. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol. Endocrinol. 16, 2283–2296 (2002).
Schweizer, U., Weitzel, J. M. & Schomburg, L. Think globally: act locally. New insights into the local regulation of thyroid hormone availability challenge long accepted dogmas. Mol. Cell. Endocrinol. 289, 1–9 (2008).
Gereben, B. et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 29, 898–938 (2008).
Hollenberg, A. N. et al. The human thyrotropin-releasing hormone gene is regulated by thyroid hormone through two distinct classes of negative thyroid hormone response elements. Mol. Endocrinol. 9, 540–550 (1995).
Franklyn, J. A., Wood, D. F., Balfour, N. J. & Sheppard, M. C. Effect of triiodothyronine treatment on thyrotrophin β- and α-messenger RNAs in the pituitary of the euthyroid rat. Mol. Cell. Endocrinol. 60, 1–5 (1988).
Cohen, O., Flynn, T. R. & Wondisford, F. E. Ligand-dependent antagonism by retinoid X receptors of inhibitory thyroid hormone response elements. J. Biol. Chem. 270, 13899–13905 (1995).
Ralph, M. R., Foster, R. G., Davis, F. C. & Menaker, M. Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978 (1990).
Brancaccio, M. et al. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 192, 187–192 (2019).
Panda, S. et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527 (2003).
Hattar, S. et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 (2003).
Balsalobre, A., Damiola, F. & Schibler, U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93, 929–937 (1998).
Yamazaki, S. et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 (2000).
Sehgal, A. Physiology flies with time. Cell 171, 1232–1235 (2017).
Takahashi, J. S. Molecular components of the circadian clock in mammals. Diabetes Obes. Metab. 17, 6–11 (2015).
Gekakis, N. et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 (1998).
Hogenesch, J. B., Gu, Y.-Z., Jain, S. & Bradfield, C. A. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl Acad. Sci. USA 95, 5474–5479 (1998).
Cho, H. et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485, 123–127 (2012).
Ueda, H. R. et al. A transcription factor response element for gene expression during circadian night. Nature 418, 534–539 (2002).
Preitner, N. et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002).
Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E. & Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111, 16219–16224 (2014).
Weeke, J. & Gundersen, H. J. Circadian and 30 minutes variations in serum TSH and thyroid hormones in normal subjects. Acta. Endocrinol. (Copenh) 89, 659–672 (1978).
Copinschi, G. & Challet, E. Endocrine rhythms, the sleep-wake cycle, and biological clocks, in Endocrinology: Adult and Pediatric (Jameson, J. L. & Groot, L. De (eds)) 147-173.e9 (Elsevier, 2016).
Van Cauter, E. & Spiegel, K. Circadian and sleep control of hormonal secretions, in Regulation of sleep and circadian rhythms (Turek, F. W. & Zee, P. C. (eds)) 397–426 (Marcel Dekker, Inc., 1999).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999).
Gronfier, C. & Brandenberger, G. Ultradian rhythms in pituitary and adrenal hormones: their relations to sleep. Sleep Med. Rev. 2, 17–29 (1998).
Romijn, J. A. et al. Pulsatile secretion of thyrotropin during fasting: a decrease of thyrotropin pulse amplitude. J. Clin. Endocrinol. Metab. 70, 1631–1636 (1990).
Brabant, G. et al. Circadian and pulsatile thyrotropin secretion in euthyroid man under the influence of thyroid hormone and glucocorticoid administration. J. Clin. Endocrinol. Metab. 65, 83–88 (1987).
Samuels, M. H., Veldhuis, J. D., Henry, P. & Ridgway, E. C. Pathophysiology of pulsatile and copulsatile release of thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating hormone, and α-subunit. J. Clin. Endocrinol. Metab. 71, 425–432 (1990).
Samuels, M. H., Henry, P., Luther, M. & Ridgway, E. C. Pulsatile TSH secretion during 48-hour continuous TRH infusions. Thyroid 3, 201–206 (1993).
Brabant, G. et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. J. Clin. Endocrinol. Metab. 70, 403–409 (1990).
Roelfsema, F. et al. Thyrotropin secretion profiles are not different in men and women. J. Clin. Endocrinol. Metab. 94, 3964–3967 (2009).
Ehrenkranz, J. et al. Circadian and circannual rhythms in thyroid hormones: determining the TSH and free T4 reference intervals based upon time of day, age, and sex. Thyroid 25, 954–961 (2015).
Lucke, C., Hehrmann, R., von Mayersbach, K. & von zur Muhlen, A. Studies on circadian variations of plasma TSH, thyroxine and triiodothyronine in man. Acta Endocrinol. 86, 81–88 (1977).
Jordan, D., Rousset, B., Perrin, F., Fournier, M. & Orgiazzi, J. Evidence for circadian variations in serum thyrotropin, 3,5,3′-triiodothyronine, and thyroxine in the rat. Endocrinology 107, 1245–1248 (1980).
Fukuda, H. et al. Nyctohemeral and sex-related variations in plasma thyrotropin, thyroxine, and triiodothyronine. Endocrinology 97, 1424–1431 (1975).
Azukizawa, M., Eugene Pekary, A., Hershman, J. M. & Parker, D. C. Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J. Clin. Endocrinol. Metab. 43, 533–542 (1976).
Roelfsema, F. & Veldhuis, J. D. Thyrotropin secretion patterns in health and disease. Endocr. Rev. 34, 619–657 (2013).
Mazzoccoli, G. et al. The hypothalamic-pituitary-thyroid axis and melatonin: possible interactions in the control of body temperature. Neuroendocrinol. Lett. 25, 368–372 (2004).
Covarrubias, L. et al. In vitro TRH release from hypothalamus slices varies during the diurnal cycle. Neurochem. Res. 19, 845–850 (1994).
Kalsbeek, A., Fliers, E., Franke, A. N., Wortel, J. & Buijs, R. M. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 141, 3832–3841 (2000).
Zandieh Doulabi, B. et al. Diurnal variation in rat liver thyroid hormone receptor (TR)-α messenger ribonucleic acid (mRNA) is dependent on the biological clock in the suprachiasmatic nucleus, whereas diurnal variation of TRβ1 mRNA is modified by food intake. Endocrinology 145, 1284–1289 (2004).
Vakili, H., Jin, Y. & Cattini, P. A. Evidence for a circadian effect on the reduction of human growth hormone gene expression in response to excess caloric intake. J. Biol. Chem. 291, 13823–13833 (2016).
Aninye, I. O., Matsumoto, S., Sidhaye, A. R. & Wondisford, F. E. Circadian regulation of Tshb gene expression by Rev-Erbα (NR1D1) and nuclear corepressor 1 (NCOR1). J. Biol. Chem. 289, 17070–17077 (2014).
Fahrenkrug, J., Georg, B., Hannibal, J. & Jørgensen, H. L. Hypophysectomy abolishes rhythms in rat thyroid hormones but not in the thyroid clock. J. Endocrinol. 233, 209–216 (2017).
Yang, X. et al. Nuclear receptor expression links the circadian clock to metabolism. Cell 126, 801–810 (2006).
Hatanaka, F. et al. Genome-wide profiling of the core clock protein BMAL1 targets reveals a strict relationship with metabolism. Mol. Cell. Biol. 30, 5636–5648 (2010).
Nikolaeva, S. et al. The circadian clock modulates renal sodium handling. J. Am. Soc. Nephrol. 23, 1019–1026 (2012).
Munroe, S. H. & Lazar, M. A. Inhibition of c-erbA mRNA splicing by a naturally occurring antisense RNA. J. Biol. Chem. 266, 22083–22086 (1991).
Vollmers, C. et al. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab. 16, 833–845 (2012).
Benvenga, S., Klose, M., Vita, R. & Feldt-Rasmussen, U. Less known aspects of central hypothyroidism: part 2 – congenital etiologies. J. Clin. Transl Endocrinol. 14, 5–11 (2018).
Benvenga, S., Klose, M., Vita, R. & Feldt-Rasmussen, U. Less known aspects of central hypothyroidism: part 1 – acquired etiologies. J. Clin. Transl. Endocrinol. 14, 25–33 (2018).
Biondi, B. & Cooper, D. S. The clinical significance of subclinical thyroid dysfunction. Endocr. Rev. 29, 76–131 (2008).
Adriaanse, R., Brabant, G., Prank, K., Endert, E. & Wiersinga, W. M. Circadian changes in pulsatile TSH release in primary hypothyroidism. Clin. Endocrinol. (Oxf). 37, 504–510 (1992).
Roelfsema, F. et al. Thyrotropin secretion in mild and severe primary hypothyroidism is distinguished by amplified burst mass and basal secretion with increased spikiness and approximate entropy. J. Clin. Endocrinol. Metab. 95, 928–934 (2010).
Moon, S. H., Lee, B. J., Kim, S. J. & Kim, H. C. Relationship between thyroid stimulating hormone and night shift work. Ann. Occup. Environ. Med. 28, 53 (2016).
Magrini, A. et al. Shift work and autoimmune thyroid disorders. Int. J. Immunopathol. Pharmacol. 19, 31–36 (2006).
Adriaanse, R., Brabant, G., Endert, E. & Wiersinga, M. M. Pulsatile untreated thyrotropin pituitary release in patients disease. J. Clin. Endocrinol. Metab. 77, 205–209 (1993).
Rose, S. R. Cranial irradiation and central hypothyroidism. Trends Endocrinol. Metab. 12, 97–104 (2001).
Baenziger, J. U. & Green, E. D. Pituitary glycoprotein hormone oligosaccharides: Structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochim. Biophys. Acta 947, 287–306 (1988).
Ikegami, K. et al. Tissue-specific posttranslational modification allows functional targeting of thyrotropin. Cell Rep. 9, 801–809 (2014).
Persani, L., Ferretti, E., Borgato, S., Faglia, G. & Beck-Peccoz, P. Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J. Clin. Endocrinol. Metab. 85, 3631–3635 (2000).
Gesundheit, N., Magner, J. A., Chen, T. & Weintraub, B. D. Differential sulfation and sialylation of secreted mouse thyrotropin (TSH) subunits: Regulation by TSH releasing hormone. Endocrinology 119, 455–463 (1986).
Darzy, K. H. & Shalet, S. M. Circadian and stimulated thyrotropin secretion in cranially irradiated adult cancer survivors. J. Clin. Endocrinol. Metab. 90, 6490–6497 (2005).
Refetoff, S., Weiss, R. E. & Usala, S. J. The syndromes of resistance to thyroid hormone. Endocr. Rev. 14, 348–399 (1993).
Persani, L. et al. Evidence for the secretion of thyrotropin with enhanced bioactivity in syndromes of thyroid hormone resistance. J. Clin. Endocrinol. Metab. 78, 1034–1039 (1994).
Custro, N., Scafidi, V. & Notarbartolo, A. Pituitary resistance to thyroid hormone action with preserved circadian rhythm of thyrotropin in a postmenopausal woman. J. Endocrinol. Invest. 15, 121–126 (1992).
Moran, C. & Chatterjee, K. Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Pract. Res. Clin. Endocrinol. Metab. 29, 647–657 (2015).
DeGroot, L. J. Graves’ disease and the manifestations of thyrotoxicosis, in Endotext (Feingold, K. R. et al. (eds)) 1–77 (MDText.com, Inc., 2000).
Roelfsema, F., Pereira, A. M., Keenan, D. M., Veldhuis, J. D. & Romijn, J. A. Thyrotropin secretion by thyrotropinomas is characterized by increased pulse frequency, delayed and disorderliness. J. Clin. Endocrinol. Metab. 93, 4052–4057 (2008).
Schull, J. et al. Effects of thyroidectomy, parathyroidectomy and lithium on circadian wheelrunning in rats. Physiol. Behav. 42, 33–39 (1988).
McEachron, D. L., Lauchlan, C. L. & Midgley, D. E. Effects of thyroxine and thyroparathyroidectomy on circadian wheel running in rats. Pharmacol. Biochem. Behav. 46, 243–249 (1993).
Beasley, L. J. & Nelson, R. J. Thyroid gland influences the period of hamster circadian oscillations. Experientia 38, 870–871 (1982).
Dkhissi-Benyahya, O., Gronfier, C., De Vanssay, W., Flamant, F. & Cooper, H. M. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron 53, 677–687 (2007).
Gloss, B. et al. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology 142, 544–550 (2001).
Peliciari-Garcia, R. A., Bargi-Souza, P., Young, M. E. & Nunes, M. T. Repercussions of hypo and hyperthyroidism on the heart circadian clock. Chronobiol. Int. 35, 147–159 (2018).
Amir, S. & Robinson, B. Thyroidectomy alters the daily pattern of expression of the clock protein, PER2, in the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neurosci. Lett. 407, 254–257 (2006).
Noguchi, T., Ikeda, M., Ohmiya, Y. & Nakajima, Y. A dual-color luciferase assay system reveals circadian resetting of cultured fibroblasts by co-cultured adrenal glands. PLOS ONE 7, e37093 (2012).
Arendt, J. Managing jet lag: Some of the problems and possible new solutions. Sleep Med. Rev. 13, 249–256 (2009).
Gary, K. A. et al. Total sleep deprivation and the thyroid axis: Effects of sleep and walking activity. Aviat. Space Environ. Med. 67, 513–519 (1996).
Hirschfeld, U. et al. Progressive elevation of plasma thyrotropin during adaptation to simulated jet lag: effects of treatment with bright light or zolpidem. J. Clin. Endocrinol. Metab. 81, 3270–3276 (1996).
Polyzos, S. A. et al. Serum thyrotropin concentration as a biochemical predictor of thyroid malignancy in patients presenting with thyroid nodules. J. Cancer Res. Clin. Oncol. 134, 953–960 (2008).
Haymart, M. R. et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J. Clin. Endocrinol. Metab. 93, 809–814 (2008).
Boelaert, K. et al. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J. Clin. Endocrinol. Metab. 91, 4295–4301 (2006).
Pinkerton, L. E. et al. Melanoma, thyroid cancer, and gynecologic cancers in a cohort of female flight attendants. Am. J. Ind. Med. 61, 572–581 (2018).
Liu, G. S. et al. Thyroid cancer risk in airline cockpit and cabin crew: a meta-analysis. Cancers Head Neck 3, 7 (2018).
Kiessling, S. et al. Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biol. 15, 1–18 (2017).
Matsuo, T. et al. Control mechanism of the circadian clock for timing of cell division in vivo. Science 302, 255–260 (2003).
Gery, S. et al. The circadian gene Per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 22, 375–382 (2006).
Kowalska, E. et al. NONO couples the circadian clock to the cell cycle. Proc. Natl Acad. Sci. USA 110, 1592–1599 (2013).
Jiang, W. et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 371, 314–325 (2016).
Lee, S., Donehower, L. A., Herron, A. J., Moore, D. D. & Fu, L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLOS ONE 5, e10995 (2010).
Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C. C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 (2002).
Papagiannakopoulos, T. et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 24, 324–331 (2016).
Gallo, C. et al. The bHLH transcription factor DEC1 promotes thyroid cancer aggressiveness by the interplay with NOTCH1. Cell Death Dis. 9, 871 (2018).
Relógio, A. et al. Ras-mediated deregulation of the circadian clock in cancer. PLoS Genet. 10, e1004338 (2014).
Altman, B. J. et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 22, 1009–1019 (2015).
Durante, C. et al. The diagnosis and management of thyroid nodules. JAMA 319, 914–924 (2018).
Sherman, S. I. Thyroid carcinoma. Lancet 361, 501–511 (2003).
Kitahara, C. M. & Sosa, J. A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 12, 646–653 (2016).
Mannic, T. et al. Circadian clock characteristics are altered in human thyroid malignant nodules. J. Clin. Endocrinol. Metab. 98, 4446–4456 (2013).
Nakane, Y. & Yoshimura, T. Photoperiodic regulation of reproduction in vertebrates. Annu. Rev. Anim. Biosci. 7, 173–194 (2018).
Ottenweller, J. E., Tapp, W. N., Pitman, D. L. & Natelson, B. H. Adrenal, thyroid, and testicular hormone rhythms in male golden hamsters on long and short days. Am. J. Physiol. Regul. Integr. Comp. Physiol. 253, R321–R328 (1987).
Wong, C. C. et al. Influence of age, strain and season on diurnal periodicity of thyroid stimulating hormone, thyroxine, triiodothyronine and parathyroid hormone in the serum of male laboratory rats. Eur. J. Endocrinol. 102, 377–385 (1983).
Wirz-Justice, A. Seasonality in affective disorders. Gen. Comp. Endocrinol. 258, 244–249 (2018).
Dopico, X. C. et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 6, 1–13 (2015).
Maes, M. et al. Components of biological variation, including seasonality, in blood concentrations of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers. Clin. Endocrinol. 46, 587–598 (1997).
Smals, A. G. H., Ross, H. A. & Kloppenborg, P. W. C. Seasonal variation in serum T3 and T4 levels in man. J. Clin. Endocrinol. Metab. 44, 998–1001 (1977).
Bellastella, A. et al. Circannual rhythms of plasma growth hormone, thyrotropin and thyroid hormones in prepuberty. Clin. Endocrinol. 20, 531–537 (1984).
Gullo, D. et al. Seasonal variations in TSH serum levels in athyreotic patients under L-thyroxine replacement monotherapy. Clin. Endocrinol. 87, 207–215 (2017).
Buchinger, W., Semlitsch, G., Pongratz, R. & Rainer, B. H. F. Jahreszeitliche variationen im auftreten der hyperthyreose. Acta Med. Austriaca 27, 51–53 (2000).
Akslen, L. A. & Sothern, R. B. Seasonal variations in the presentation and growth of thyroid cancer. Br. J. Cancer 77, 1174–1179 (1998).
Nakao, N. et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452, 317–322 (2008).
Yoshimura, T. et al. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature 426, 178–181 (2003).
Yamamura, T., Hirunagi, K., Ebihara, S. & Yoshimura, T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology 145, 4264–4267 (2004).
Ono, H. et al. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc. Natl Acad. Sci. USA 105, 18238–18242 (2008).
Hanon, E. A. et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 18, 1147–1152 (2008).
Bockmann, J. et al. Thyrotropin expression in hypophyseal pars tuberalis-specific cells is 3,5,3′-triiodothyronine, thyrotropin-releasing hormone, and Pit-1 independent. Endocrinology 138, 1019–1028 (1997).
Arendt, J. Melatonin and the Mammalian Pineal Gland. (Chapman & Hall, 1995).
Yasuo, S., Yoshimura, T., Ebihara, S. & Korf, H. W. Melatonin transmits photoperiodic signals through the MT1 melatonin receptor. J. Neurosci. 29, 2885–2889 (2009).
Heldmaier, G., Ortmann, S. & Elvert, R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 141, 317–329 (2004).
Geiser, F. & Turbill, C. Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 96, 1235–1240 (2009).
Gautier, C. et al. Gene expression profiling during hibernation in the European hamster. Sci. Rep. 8, 1–17 (2018).
Antonica, F. et al. Generation of functional thyroid from embryonic stem cells. Nature 491, 66–71 (2012).
Tamai, T. K. et al. Identification of circadian clock modulators from existing drugs. EMBO Mol. Med. 10, e8724 (2018).
Oshima, T. et al. Cell-based screen identifies a new potent and highly selective CK2 inhibitor for modulation of circadian rhythms and cancer cell growth. Sci. Adv. 5, 1–16 (2019).
Sulli, G. et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018).
Biro, J. Specific binding of thyroid-stimulating hormone by human serum globulins. J. Endocrinol. 88, 339–349 (1980).
Spitz, I. M. et al. Increased high-molecular-weight thyrotropin with impaired biologic activity in a euthyroid man. N. Engl. J. Med. 304, 278–282 (1981).
DeCherney, G. S., Gesundheit, N., Gyves, P. W., Showalter, C. R. & Weintraub, B. D. Alterations in the sialylation and sulfation of secreted mouse thyrotropin in primary hypothyroidism. Biochem. Biophys. Res. Commun. 159, 755–762 (1989).
Loh, T. P. et al. Macro-thyrotropin: a case report and review of literature. J. Clin. Endocrinol. Metab. 97, 1823–1828 (2012).
Tamaki, H. et al. Novel thyrotropin (TSH) -TSH antibody complex in a woman and her neonates. Thyroid 5, 299–304 (1995).
Constant, R. B. & Weintraub, B. D. Differences in the metabolic clearance of pituitary and serum thyrotropin (TSH) derived from euthyroid and hypothyroid rats: Effects of chemical deglycosylation of pituitary TSH. Endocrinology 119, 2720–2727 (1986).
Asa, S. L., Kovacs, K. & Bilbao, J. M. The pars tuberalis of the human pituitary. Virchows Arch. A 399, 49–59 (1983).
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science KAKENHI Grants-in-Aid for Specially Promoted Research (26000013) and for Young Scientists (B) (17K15574), the Human Frontier Science Program (RGP0030/2015) and the National Institutes of Health (PO1 AG-11412 and R01 DK-15070). The Institute of Transformative Bio-Molecules is supported by the World Premier International Research Center Initiative, Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks S. Benvenga and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Ultradian rhythm
-
A recurrent cycle with a period shorter than 24 h.
Rights and permissions
About this article
Cite this article
Ikegami, K., Refetoff, S., Van Cauter, E. et al. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol 15, 590–600 (2019). https://doi.org/10.1038/s41574-019-0237-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0237-z
This article is cited by
-
Ginseng extracts improve circadian clock gene expression and reduce inflammation directly and indirectly through gut microbiota and PI3K signaling pathway
npj Biofilms and Microbiomes (2024)
-
Relationship between TSH and free thyroxine in outpatient cancer patient population
Endocrine (2023)
-
Assessment of the impact of shift work on thyroid disorders: a systematic review and meta-analysis
Sleep and Breathing (2023)
-
In silico analysis decodes transthyretin (TTR) binding and thyroid disrupting effects of per- and polyfluoroalkyl substances (PFAS)
Archives of Toxicology (2023)
-
One seasonal clock fits all?
Journal of Comparative Physiology A (2023)