Abstract
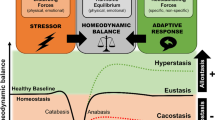
The human stress response has evolved to maintain homeostasis under conditions of real or perceived stress. This objective is achieved through autoregulatory neural and hormonal systems in close association with central and peripheral clocks. The hypothalamic–pituitary–adrenal axis is a key regulatory pathway in the maintenance of these homeostatic processes. The end product of this pathway — cortisol — is secreted in a pulsatile pattern, with changes in pulse amplitude creating a circadian pattern. During acute stress, cortisol levels rise and pulsatility is maintained. Although the initial rise in cortisol follows a large surge in adrenocorticotropic hormone levels, if long-term inflammatory stress occurs, adrenocorticotropic hormone levels return to near basal levels while cortisol levels remain raised as a result of increased adrenal sensitivity. In chronic stress, hypothalamic activation of the pituitary changes from corticotropin-releasing hormone-dominant to arginine vasopressin-dominant, and cortisol levels remain raised due at least in part to decreased cortisol metabolism. Acute elevations in cortisol levels are beneficial to promoting survival of the fittest as part of the fight-or-flight response. However, chronic exposure to stress results in reversal of the beneficial effects, with long-term cortisol exposure becoming maladaptive, which can lead to a broad range of problems including the metabolic syndrome, obesity, cancer, mental health disorders, cardiovascular disease and increased susceptibility to infections. Neuroimmunoendocrine modulation in disease states and glucocorticoid-based therapeutics are also discussed.
Key points
-
The hypothalamic–pituitary–adrenal (HPA) axis is a key system that synchronizes the stress response with circadian regulatory processes.
-
Regulation of the HPA axis is very dynamic with both ultradian and circadian oscillations.
-
Short-term and longer-term stress result in different regulatory mechanisms involving hypothalamic, pituitary and adrenal activity, as well as cortisol metabolism.
-
Chronic elevation and nonphysiological patterns of cortisol result in poor cognitive, metabolic and immune function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Szabo, S., Tache, Y. & Somogyi, A. The legacy of Hans Selye and the origins of stress research: a retrospective 75 years after his landmark brief “letter” to the editor of Nature. Stress 15, 472–478 (2012).
Levine, S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur. J. Pharmacol. 405, 149–160 (2000).
Brown, S. A. & Azzi, A. Peripheral circadian oscillators in mammals. Handb. Exp. Pharmacol. 2013, 45–66 (2013).
Roenneberg, T. & Merrow, M. The circadian clock and human health. Curr. Biol. 26, R432–R443 (2016).
Bass, J. & Lazar, M. A. Circadian time signatures of fitness and disease. Science 354, 994–999 (2016).
Turek, F. W. Circadian neural rhythms in mammals. Annu. Rev. Physiol. 47, 49–64 (1985).
Skene, D. J. et al. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl Acad. Sci. USA 115, 7825–7830 (2018).
Buhr, E. D. & Takahashi, J. S. Molecular components of the mammalian circadian clock. Handb. Exp. Pharmacol. 217, 3–27 (2013).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Baron, K. G. & Reid, K. J. Circadian misalignment and health. Int. Rev. Psychiatry 26, 139–154 (2014).
Potter, G. D. et al. Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr. Rev. 37, 584–608 (2016).
Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E. & Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111, 16219–16224 (2014).
Smarr, B. L. & Schirmer, A. E. 3.4 million real-world learning management system logins reveal the majority of students experience social jet lag correlated with decreased performance. Sci. Rep. 8, 4793 (2018).
Gardner, M. et al. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and cognitive capability at older ages: individual participant meta-analysis of five cohorts. Sci. Rep. 9, 4555 (2019).
Selye, H. Stress and the general adaptation syndrome. BMJ 1, 1383–1392 (1950).
Russell, G. M. & Lightman, S. L. Can side effects of steroid treatments be minimized by the temporal aspects of delivery method? Expert Opin. Drug Saf. 13, 1501–1513 (2014).
Sorrells, S. F. & Sapolsky, R. M. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 21, 259–272 (2007).
Busillo, J. M. & Cidlowski, J. A. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 24, 109–119 (2013).
McEwen, B. S. et al. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Rev. 23, 79–133 (1997).
Elenkov, I. J. & Chrousos, G. P. Stress system — organization, physiology and immunoregulation. Neuroimmunomodulation 13, 257–267 (2006).
Brinkmann, V. & Kristofic, C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO- and CD45RO+ subsets. J. Immunol. 155, 3322–3328 (1995).
Wiegers, G. J. & Reul, J. M. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol. Sci. 19, 317–321 (1998).
Abraham, I. M., Meerlo, P. & Luiten, P. G. Concentration dependent actions of glucocorticoids on neuronal viability and survival. Dose Response 4, 38–54 (2006).
Plaschke, K., Muller, D. & Hoyer, S. Effect of adrenalectomy and corticosterone substitution on glucose and glycogen metabolism in rat brain. J. Neural Transm. 103, 89–100 (1996).
Belanoff, J. K., Gross, K., Yager, A. & Schatzberg, A. F. Corticosteroids and cognition. J. Psychiatr. Res. 35, 127–145 (2001).
Roozendaal, B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 78, 578–595 (2002).
Brown, E. S. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann. NY Acad. Sci. 1179, 41–55 (2009).
de Kloet, E. R., Oitzl, M. S. & Joels, M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 22, 422–426 (1999).
Decani, S., Federighi, V., Baruzzi, E., Sardella, A. & Lodi, G. Iatrogenic Cushing’s syndrome and topical steroid therapy: case series and review of the literature. J. Dermatolog. Treat. 25, 495–500 (2014).
Kenna, H. A., Poon, A. W., de los Angeles, C. P. & Koran, L. M. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin. Neurosci. 65, 549–560 (2011).
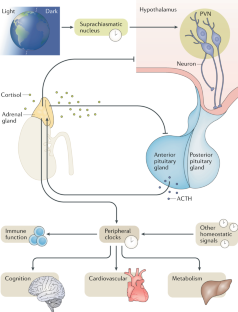
Buijs, R. M., Markman, M., Nunes-Cardoso, B., Hou, Y. X. & Shinn, S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J. Comp. Neurol. 335, 42–54 (1993).
Watts, A. G. & Swanson, L. W. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J. Comp. Neurol. 258, 230–252 (1987).
Jacobson, L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol. Metab. Clin. North Am. 34, 271–292 (2005).
Herman, J. P., Ostrander, M. M., Mueller, N. K. & Figueiredo, H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 1201–1213 (2005).
Dallman, M. F. et al. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann. NY Acad. Sci. 746, 22–31 (1994).
Jasper, M. S. & Engeland, W. C. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology 59, 97–109 (1994).
Buijs, R. M. et al. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 11, 1535–1544 (1999).
Kiessling, S., Sollars, P. J. & Pickard, G. E. Light stimulates the mouse adrenal through a retinohypothalamic pathway independent of an effect on the clock in the suprachiasmatic nucleus. PLOS ONE 9, e92959 (2014).
Husse, J., Leliavski, A., Tsang, A. H., Oster, H. & Eichele, G. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 28, 4950–4960 (2014).
Ishida, A. et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2, 297–307 (2005).
Oster, H. et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 4, 163–173 (2006).
Charmandari, E. et al. Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLOS ONE 6, e25612 (2011).
Bailey, S. L. & Heitkemper, M. M. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol. Int. 18, 249–261 (2001).
Donner, N. C., Montoya, C. D., Lukkes, J. L. & Lowry, C. A. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 37, 645–661 (2012).
Lightman, S. L. The neuroendocrinology of stress: a never ending story. J. Neuroendocrinol. 20, 880–884 (2008).
Nicolaides, N. C., Kyratzi, E., Lamprokostopoulou, A., Chrousos, G. P. & Charmandari, E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 22, 6–19 (2015).
Gibbison, B. et al. Dynamic pituitary-adrenal interactions in response to cardiac surgery. Crit. Care Med. 43, 791–800 (2015).
Spiga, F. et al. Dynamic responses of the adrenal steroidogenic regulatory network. Proc. Natl Acad. Sci. USA 114, E6466–E6474 (2017).
Ma, X. M., Levy, A. & Lightman, S. L. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology 138, 4351–4357 (1997).
Dallman, M. F. Stress update: adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends. Endocrinol. Metab. 4, 62–69 (1993).
Henley, D. E. et al. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J. Clin. Endocrinol. Metab. 94, 4234–4242 (2009).
Boonen, E. et al. Reduced cortisol metabolism during critical illness. N. Engl. J. Med. 368, 1477–1488 (2013).
Peeters, B. et al. Adrenocortical function during prolonged critical illness and beyond: a prospective observational study. Intensive Care Med. 44, 1720–1729 (2018).
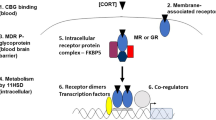
De Kloet, E. R., Vreugdenhil, E., Oitzl, M. S. & Joels, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301 (1998).
Reul, J. M. & de Kloet, E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117, 2505–2511 (1985).
Dallman, M. F. Fast glucocorticoid actions on brain: back to the future. Front. Neuroendocrinol. 26, 103–108 (2005).
Russell, G. M. et al. Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. J. Neurosci. 30, 6106–6115 (2010).
Lowenberg, M., Verhaar, A. P., van den Brink, G. R. & Hommes, D. W. Glucocorticoid signaling: a nongenomic mechanism for T cell immunosuppression. Trends Mol. Med. 13, 158–163 (2007).
Orchinik, M., Murray, T. F., Franklin, P. H. & Moore, F. L. Guanyl nucleotides modulate binding to steroid receptors in neuronal membranes. Proc. Natl Acad. Sci. USA 89, 3830–3834 (1992).
Orchinik, M., Murray, T. F. & Moore, F. L. A corticosteroid receptor in neuronal membranes. Science 252, 1848–1851 (1991).
Joels, M., Pasricha, N. & Karst, H. The interplay between rapid and slow corticosteroid actions in brain. Eur. J. Pharmacol. 719, 44–52 (2013).
Walker, J. J. et al. The origin of glucocorticoid hormone oscillations. PLOS Biol. 10, e1001341 (2012).
Patel, P. D. et al. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J. Psychiatr. Res. 34, 383–392 (2000).
Groeneweg, F. L., Karst, H., de Kloet, E. R. & Joels, M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 209, 153–167 (2011).
de Kloet, E. R., Fitzsimons, C. P., Datson, N. A., Meijer, O. C. & Vreugdenhil, E. Glucocorticoid signaling and stress-related limbic susceptibility pathway: about receptors, transcription machinery and microRNA. Brain Res. 1293, 129–141 (2009).
Russell, G. M., Kalafatakis, K. & Lightman, S. L. The importance of biological oscillators for HPA activity and tissue glucocorticoid response: coordinating stress and neurobehavioural adaptation. J. Neuroendocrinol. 27, 378–388 (2015).
Lewis, J. G. et al. Plasma variation of corticosteroid-binding globulin and sex hormone-binding globulin. Horm. Metab. Res. 38, 241–245 (2006).
Lewis, J. G., Bagley, C. J., Elder, P. A., Bachmann, A. W. & Torpy, D. J. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin. Chim. Acta 359, 189–194 (2005).
Hammond, G. L., Smith, C. L. & Underhill, D. A. Molecular studies of corticosteroid binding globulin structure, biosynthesis and function. J. Steroid Biochem. Mol. Biol. 40, 755–762 (1991).
Frairia, R. et al. Influence of naturally occurring and synthetic glucocorticoids on corticosteroid-binding globulin-steroid interaction in human peripheral plasma. Ann. NY Acad. Sci. 538, 287–303 (1988).
Cameron, A. et al. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J. Clin. Endocrinol. Metab. 95, 4689–4695 (2010).
Kyrou, I., Chrousos, G. P. & Tsigos, C. Stress, visceral obesity, and metabolic complications. Ann. NY Acad. Sci. 1083, 77–110 (2006).
Chapman, K., Holmes, M. & Seckl, J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 93, 1139–1206 (2013).
Seckl, J. R. 11beta-hydroxysteroid dehydrogenases: changing glucocorticoid action. Curr. Opin. Pharmacol. 4, 597–602 (2004).
Verma, M. et al. 11beta-hydroxysteroid dehydrogenase-1 deficiency alters brain energy metabolism in acute systemic inflammation. Brain Behav. Immun. 69, 223–234 (2018).
Follenius, M., Simon, C., Brandenberger, G. & Lenzi, P. Ultradian plasma corticotropin and cortisol rhythms: time-series analyses. J. Endocrinol. Invest. 10, 261–266 (1987).
Hartmann, A., Veldhuis, J. D., Deuschle, M., Standhardt, H. & Heuser, I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol. Aging 18, 285–289 (1997).
Rivest, R. W., Schulz, P., Lustenberger, S. & Sizonenko, P. C. Differences between circadian and ultradian organization of cortisol and melatonin rhythms during activity and rest. J. Clin. Endocrinol. Metab. 68, 721–729 (1989).
Waite, E. J. et al. Ultradian corticosterone secretion is maintained in the absence of circadian cues. Eur. J. Neurosci. 36, 3142–3150 (2012).
Ixart, G., Barbanel, G., Nouguier-Soule, J. & Assenmacher, I. A quantitative study of the pulsatile parameters of CRH-41 secretion in unanesthetized free-moving rats. Exp. Brain Res. 87, 153–158 (1991).
Spiga, F. et al. ACTH-dependent ultradian rhythm of corticosterone secretion. Endocrinology 152, 1448–1457 (2011).
Spiga, F., Liu, Y., Aguilera, G. & Lightman, S. L. Temporal effect of adrenocorticotrophic hormone on adrenal glucocorticoid steroidogenesis: involvement of the transducer of regulated cyclic AMP-response element-binding protein activity. J. Neuroendocrinol. 23, 136–142 (2011).
Lim, C. & Allada, R. Emerging roles for post-transcriptional regulation in circadian clocks. Nat. Neurosci. 16, 1544–1550 (2013).
Liston, C. et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat. Neurosci. 16, 698–705 (2013).
Lightman, S. L. & Conway-Campbell, B. L. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 11, 710–718 (2010).
Stavreva, D. A. et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat. Cell Biol. 11, 1093–1102 (2009).
Conway-Campbell, B. L., Pooley, J. R., Hager, G. L. & Lightman, S. L. Molecular dynamics of ultradian glucocorticoid receptor action. Mol. Cell. Endocrinol. 348, 383–393 (2012).
George, C. L., Lightman, S. L. & Biddie, S. C. Transcription factor interactions in genomic nuclear receptor function. Epigenomics 3, 471–485 (2011).
So, A. Y., Chaivorapol, C., Bolton, E. C., Li, H. & Yamamoto, K. R. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLOS Genet. 3, e94 (2007).
Zalachoras, I., Houtman, R. & Meijer, O. C. Understanding stress-effects in the brain via transcriptional signal transduction pathways. Neuroscience 242, 97–109 (2013).
Sarabdjitsingh, R. A. et al. Stress responsiveness varies over the ultradian glucocorticoid cycle in a brain-region-specific manner. Endocrinology 151, 5369–5379 (2010).
Sarabdjitsingh, R. A. et al. Ultradian corticosterone pulses balance glutaminergic transmission and synaptic plasticity. Proc. Natl Acad. Sci. USA 111, 14265–14270 (2014).
Kalafatakis, K. et al. Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proc. Natl Acad. Sci. USA 115, E4091–E4100 (2018).
Gjerstad, J. K., Lightman, S. L. & Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 21, 403–416 (2018).
Bornstein, S. R., Engeland, W. C., Ehrhart-Bornstein, M. & Herman, J. P. Dissociation of ACTH and glucocorticoids. Trends Endocrinol. Metab. 19, 175–180 (2008).
Silverman, M. N., Miller, A. H., Biron, C. A. & Pearce, B. D. Characterization of an interleukin-6- and adrenocorticotropin-dependent, immune-to-adrenal pathway during viral infection. Endocrinology 145, 3580–3589 (2004).
Franchimont, D. et al. Adrenal cortical activation in murine colitis. Gastroenterology 119, 1560–1568 (2000).
Viblanc, V. A. et al. An integrative appraisal of the hormonal and metabolic changes induced by acute stress using king penguins as a model. Gen. Comp. Endocrinol. 269, 1–10 (2018).
Cruz-Topete, D. & Cidlowski, J. A. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 22, 20–32 (2015).
Biddie, S. C., Conway-Campbell, B. L. & Lightman, S. L. Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology 51, 403–412 (2012).
Miller, G. E., Cohen, S. & Ritchey, A. K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 21, 531–541 (2002).
Oster, H. et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 38, 3–45 (2017).
Keller, M. et al. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl Acad. Sci. USA 106, 21407–21412 (2009).
Boivin, D. B. et al. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 102, 4143–4145 (2003).
Koo, J. W., Russo, S. J., Ferguson, D., Nestler, E. J. & Duman, R. S. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl Acad. Sci. USA 107, 2669–2674 (2010).
Pace, T. W., Hu, F. & Miller, A. H. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 21, 9–19 (2007).
Pace, T. W. et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 163, 1630–1633 (2006).
Cohen, S. et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl Acad. Sci. USA 109, 5995–5999 (2012).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999).
Hauner, H., Schmid, P. & Pfeiffer, E. F. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J. Clin. Endocrinol. Metab. 64, 832–835 (1987).
Dallman, M. F. et al. Glucocorticoids, chronic stress, and obesity. Prog. Brain Res. 153, 75–105 (2006).
Tsigos, C. et al. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J. Clin. Endocrinol. Metab. 82, 4167–4170 (1997).
McEwen, B. S. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism 55, S20–S23 (2006).
Zhu, B., Shi, C., Park, C. G., Zhao, X. & Reutrakul, S. Effects of sleep restriction on metabolism-related parameters in healthy adults: a comprehensive review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 45, 18–30 (2019).
Gavrila, A. et al. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J. Clin. Endocrinol. Metab. 88, 2838–2843 (2003).
Knutson, K. L. & Van Cauter, E. Associations between sleep loss and increased risk of obesity and diabetes. Ann. NY Acad. Sci. 1129, 287–304 (2008).
Adam, T. C. & Epel, E. S. Stress, eating and the reward system. Physiol. Behav. 91, 449–458 (2007).
Young, E. A., Carlson, N. E. & Brown, M. B. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology 25, 267–276 (2001).
Heuser, I., Yassouridis, A. & Holsboer, F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J. Psychiatr. Res. 28, 341–356 (1994).
Ising, M. et al. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 1085–1093 (2005).
Krishnan, V. & Nestler, E. J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Miller, A. H. Depression and immunity: a role for T cells? Brain Behav. Immun. 24, 1–8 (2010).
Koo, J. W. & Duman, R. S. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl Acad. Sci. USA 105, 751–756 (2008).
Horowitz, M. A., Zunszain, P. A., Anacker, C., Musaelyan, K. & Pariante, C. M. Glucocorticoids and inflammation: a double-headed sword in depression? How do neuroendocrine and inflammatory pathways interact during stress to contribute to the pathogenesis of depression? Mod. Trends Pharmacopsychiatry 28, 127–143 (2013).
Munhoz, C. D. et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J. Neurosci. 26, 3813–3820 (2006).
Pariante, C. M. Glucocorticoid receptor function in vitro in patients with major depression. Stress 7, 209–219 (2004).
Kenis, G. & Maes, M. Effects of antidepressants on the production of cytokines. Int. J. Neuropsychopharmacol. 5, 401–412 (2002).
Bjornsdottir, S. et al. Drug prescription patterns in patients with Addison’s disease: a Swedish population-based cohort study. J. Clin. Endocrinol. Metab. 98, 2009–2018 (2013).
Dunlop, D. Eighty-six cases of Addison’s disease. BMJ 2, 887–891 (1963).
Giordano, R. et al. Metabolic and cardiovascular profile in patients with Addison’s disease under conventional glucocorticoid replacement. J. Endocrinol. Invest. 32, 917–923 (2009).
Johannsson, G. et al. Adrenal insufficiency: review of clinical outcomes with current glucocorticoid replacement therapy. Clin. Endocrinol. 82, 2–11 (2015).
Lovas, K., Loge, J. H. & Husebye, E. S. Subjective health status in Norwegian patients with Addison’s disease. Clin. Endocrinol. 56, 581–588 (2002).
Feek, C. M. et al. Patterns of plasma cortisol and ACTH concentrations in patients with Addison’s disease treated with conventional corticosteroid replacement. Clin. Endocrinol. 14, 451–458 (1981).
Isidori, A. M. et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 6, 173–185 (2018).
Bancos, I. et al. Primary adrenal insufficiency is associated with impaired natural killer cell function: a potential link to increased mortality. Eur. J. Endocrinol. 176, 471–480 (2017).
Bjanesoy, T. E. et al. Altered DNA methylation profile in Norwegian patients with autoimmune Addison’s disease. Mol. Immunol. 59, 208–216 (2014).
Langenheim, J., Ventz, M., Hinz, A. & Quinkler, M. Modified-release prednisone decreases complaints and fatigue compared to standard prednisolone in patients with adrenal insufficiency. Horm. Metab. Res. 45, 96–101 (2013).
Mallappa, A. et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 100, 1137–1145 (2015).
Lovas, K. & Husebye, E. S. Continuous subcutaneous hydrocortisone infusion in Addison’s disease. Eur. J. Endocrinol. 157, 109–112 (2007).
Venneri, M. A. et al. Circadian rhythm of glucocorticoid administration entrains clock genes in immune cells: a DREAM trial ancillary study. J. Clin. Endocrinol. Metab. 103, 2998–3009 (2018).
Oksnes, M. et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of Addison’s disease: a randomized clinical trial. J. Clin. Endocrinol. Metab. 99, 1665–1674 (2014).
Riedel, M., Wiese, A., Schurmeyer, T. H. & Brabant, G. Quality of life in patients with Addison’s disease: effects of different cortisol replacement modes. Exp. Clin. Endocrinol. 101, 106–111 (1993).
van Staa, T. P. et al. Use of oral corticosteroids in the United Kingdom. QJM 93, 105–111 (2000).
Overman, R. A., Yeh, J. Y. & Deal, C. L. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 65, 294–298 (2013).
Curtis, J. R. et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 55, 420–426 (2006).
McDonough, A. K., Curtis, J. R. & Saag, K. G. The epidemiology of glucocorticoid-associated adverse events. Curr. Opin. Rheumatol. 20, 131–137 (2008).
Leung, D. Y. & Bloom, J. W. Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 111, 3–22 (2003).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Zeitgebers
-
Cues that entrain or synchronize the body’s 24-h cycle
- Ultradian rhythms
-
Biological rhythms that occur with a frequency of <24 h.
- Circadian clock
-
A biochemical oscillator with phases synchronized with solar time.
- Indirect projections
-
Neural pathways involving at least one relay.
- Hypophyseal portal system
-
The microcirculation that allows transport of hypothalamic hormones to the pituitary gland.
- Irradiance threshold
-
The threshold power of (solar) electromagnetic radiation needed to exert an effect.
- Stereotypic behaviours
-
Repetitive body movements that serve no biological function.
- Goal-directed behaviours
-
Behaviours engaged for a specific functional purpose.
- Circadian rhythm
-
Any biological process that displays an oscillation of approximately 24 h.
Rights and permissions
About this article
Cite this article
Russell, G., Lightman, S. The human stress response. Nat Rev Endocrinol 15, 525–534 (2019). https://doi.org/10.1038/s41574-019-0228-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0228-0
This article is cited by
-
Key HPI axis receptors facilitate light adaptive behavior in larval zebrafish
Scientific Reports (2024)
-
Evaluation of the benefits of neutral bicarbonate ionized water baths in an open-label, randomized, crossover trial
Scientific Reports (2024)
-
Sex-specific associations of serum cortisol with brain biomarkers of Alzheimer’s risk
Scientific Reports (2024)
-
Evaluation of indicators of acute emotional states in dogs
Scientific Reports (2024)
-
Early Childhood Education Teacher Workforce: Stress in Relation to Identity and Choices
Early Childhood Education Journal (2024)