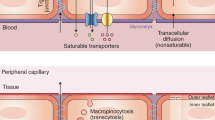
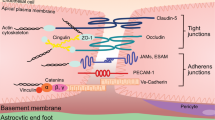
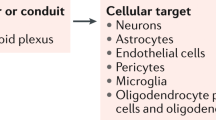
Abstract
The blood–brain barrier (BBB) was first noted for its ability to prevent the unregulated exchange of substances between the blood and the central nervous system (CNS). Over time, its characterization as an interface that enables regulated exchanges between the CNS and substances that are carried in the blood in a hormone-like fashion have emerged. Therefore, communication between the CNS, BBB and peripheral tissues has many endocrine-like properties. In this Review, I examine the various ways in which the BBB exhibits endocrine-related properties. The BBB is a target for hormones, such as leptin and insulin, that affect many of its functions. The BBB is also a secretory body, releasing substances either into the blood or the interstitial fluid of the brain. The BBB selectively allows classical and non-classical hormones entry to and exit from the CNS, thus allowing the CNS to be both an endocrine target and a secretory tissue. The BBB is affected by endocrine diseases such as diabetes mellitus and can cause or participate in endocrine diseases, including those related to thyroid hormones and obesity. The endocrine-like mechanisms of the BBB can extend the definition of endocrine disease to include neurodegenerative conditions, including Alzheimer disease, and of hormones to include cytokines, triglycerides and fatty acids.
Key points
-
The blood–brain barrier (BBB) acts as both a secretory and target endocrine tissue.
-
By regulating the transport of hormones into and out of the brain, the BBB provides a mechanism by which the central nervous system can act as an endocrine secretory and target tissue.
-
The BBB facilitates substances not typically thought of as hormones acting in an endocrine-like fashion, including triglycerides, short-chain fatty acids and lipopolysaccharide.
-
BBB function can be altered in endocrine diseases either because of adaptions to the disease condition or because it is a disease target.
-
BBB dysfunction or impairment can cause or promote the progression of endocrine or metabolic diseases.
-
The BBB is an important factor in the treatment of many diseases, often as a therapeutic target or as a barrier that must be negotiated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Neuwelt, E. et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 7, 84–96 (2008).
Davson, H. & Segal, M. B. (eds) in Physiology of the CSF and Blood–Brain Barriers 303–485 (CRC Press, Boca Raton, 1996)
Banks, W. A. in Efflux Transporters and the Blood-Brain Barrier (ed. Taylor, E. M.) 21–53 (Nova Science Publishers Inc.,2005).
Pan, W. & Kastin, A. J. Cytokine transport across the injured blood-spinal cord barrier. Curr. Pharm. Des. 14, 1620–1624 (2008).
Kastin, A. J. & Pan, W. Blood-brain barrier and feeding: Regulatory roles of saturable transport systems for ingestive peptides. Curr. Pharm. Des. 14, 1615–1619 (2008).
Banks, W. A. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 15, 275–292 (2016).
O’Donnell, M. E., Lam, T. I., Tran, L. Q., Foroutan, S. & Anderson, S. E. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permenent middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 26, 1234–1249 (2006).
Guo, U. X. et al. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Abeta1 = 40 brain-to-blood efflux and peripheral uptake transport. Neruoscience 322, 28–38 (2016).
Deane, R., Wu, Z. & Zlokovic, B. V. RAGE (yin) versus LRP (yang) balance regulates Alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke 35, 2628–2631 (2004).
Deane, R., Sagare, A. & Zlokovic, B. The role of the cell surface LRP and soluble LRP in blood-brain barrier A beta clearance in Alzheimer’s disease. Curr. Pharm. Des. 14, 1601–1605 (2008).
Fleegal-DeMotta, M. A., Dohgu, S. & Banks, W. A. Angiotensin II modulates BBB permeability via activation of the AT1 receptor in brain endothelial cells. J. Cereb. Blood Flow Metab. 29, 640–647 (2009).
Guillot, F. L. & Audus, K. L. Angiotensin peptide regulation of fluid-phase endocytosis in brain microvessel endothelial cell monolayers. J. Cereb. Blood Flow Metab. 10, 827–834 (1990).
Vaughan, C. J. & Delanty, N. Hypertensive emergencies. Lancet 356, 411–417 (2000).
Cangiano, C. et al. On the stimulation by insulin of tryptophan transport across the blood-brain barrier. Biochem. Int. 7, 617–627 (1983).
Daniel, P. M., Love, E. R., Moorhouse, S. R. & Pratt, O. E. The effect of insulin upon the influx of tryptophan into the brain of the rabbit. J. Physiol. 312, 551–562 (1981).
Walker, A. K., Wing, E. E., Banks, W. A. & Dantzer, R. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol. Psychiatry. https://doi.org/10.1038/s41380-018-0076-7 (2018).
Langston, J. W., Li, W., Harrison, L. & Aw, T. Y. Activation of promoter activity of the catalytic subunit of gamma-glutamylcysteine ligase (GCL) in brain endothelial cells by insulin requires antioxidant response element 4 and altered glycemic status: implication for GCL expression and GSH synthesis. Free Radic. Biol. Med. 51, 1749–1757 (2011).
Liu, H. et al. Insulin regulates P-glycoprotein in rat brain microvessel endothelial cells via an insulin receptor-mediated PKC/NF-kappaB pathway but not a PI3K/Akt pathway. Eur. J. Pharmacol. 602, 277–282 (2009).
Catalan, R. E., Martinez, A. M., Aragones, M. D., Miguel, B. G. & Robles, A. Insulin action on brain microvessels; effect on alkaline phosphatase. Biochem. Biophys. Res. Commun. 150, 583–590 (1988).
Kastin, A. J. & Akerstrom, V. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology 73, 237–242 (2001).
Ben-Shachar, D., Yehuda, S., Finberg, J. P., Spanier, I. & Youdim, M. B. Selective alteration in blood-brain barrier and insulin transport in iron-deficient rats. J. Neurochem. 50, 1434–1437 (1988).
May, A. A., Liu, M., Woods, S. C. & Begg, D. P. CCK increases the transport of insulin into the brain. Physiol. Behav. 165, 392–397 (2016).
Urayama, A. & Banks, W. A. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology 149, 3592–3597 (2008).
Kastin, A. J., Akerstrom, V. & Maness, L. M. Chronic loss of ovarian function decreases transport of leptin into mouse brain. Neurosci. Lett. 310, 69–71 (2001).
Chance, W. T., Balasubramaniam, A., Thomas, I. & Fischer, J. E. Amylin increases transport of tyrosine and tryptophan into the brain. Brain Res. 593, 20–24 (1992).
Banks, W. A. & Kastin, A. J. Modulation of the carrier-mediated transport of the Tyr-MIF-1 across the blood-brain barrier by essential amino acids. J. Pharmacol. Exp. Ther. 239, 668–672 (1986).
Urayama, A., Grubb, J. H., Banks, W. A. & Sly, W. S. Epinephrine enhances lysosomal enzyme delivery across the blood-brain barrier by up-regulation of the mannose 6-phosphate receptor. Proc. Natl Acad. Sci. USA 31, 12873–12878 (2007).
Urayama, A. et al. Alpha adrenergic induction of transport of lysosomal enzyme across the blood-brain barrier. PLOS ONE 10, e0142347 (2015).
Urayama, A., Grubb, J. H., Sly, W. S. & Banks, W. A. Pharmacologic manipulation of lysosomal enzyme transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 36, 476–476 (2016).
Jais, A. et al. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell 165, 882–895 (2016).
Fukuda, S. et al. Glucagon-like peptide-1 strengthens the barrier integrity in primary cultures of rat brain endothelial cells under basal and hyperglycemic conditions. J. Mol. Neurosci. 29, 211–219 (2016).
Dohgu, S. et al. Transforming growth factor-·1 upregulates the tight junction and p-glycoprotein of brain microvascular endothelial cells. Cell. Mol. Neurobiol. 24, 491–497 (2004).
Banks, W. A. et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53, 1253–1260 (2004).
McCarthy, R. C. & Kosman, D. J. Activation of C6 glioblastoma cell ceruloplasmin expression by neighboring human brain endothelia-derived interleukins in an in vitro blood-brain barrier model system. Cell Commun. Signal 12, 65 (2014).
Yu, C., Kastin, A. J., Tu, H., Waters, S. & Pan, W. TNF activates P-glycoprotein in cerebral microvascular endothelial cells. Cell Physiol. Biochem. 20, 853–858 (2007).
Reyes, T. M., Fabry, Z. & Coe, C. L. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Res. 851, 215–220 (1999).
Fabry, Z. et al. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J. Neuroimmunol. 47, 23–34 (1993).
Kis, B. et al. Cerebral endothelial cells are a major source of adrenomedullin. J. Neuroendocrinol. 14, 283–293 (2002).
Mandi, Y. et al. Nitric oxide production and MDR expression by human brain endothelial cells. Anticancer Res. 18, 3049–3052 (1998).
Verma, S., Nakaoke, R., Dohgu, S. & Banks, W. A. Release of cytokines by brain endothelial cells: a polarized response to lipopolysaccharide. Brain Behav. Immun. 20, 449–455 (2006).
McGuire, T. R. et al. Release of prostaglandin E-2 in bovine brain endothelial cells after exposure to three unique forms of the antifungal drug amphotericin-B: role of COX-2 in amphotericin-B induced fever. Life Sci. 72, 2581–2590 (2003).
Ebling, F. J. P. & Lewis, J. E. Tanycytes and hypothalamic control of energy metabolism. Glia 66, 1176–1184 (2018).
Ghersi-Egea, J. F. et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 35, 337–361 (2018).
Banks, W. A., Kovac, A. & Morofuji, Y. Neurovascular unit crosstalk: pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells. J. Cereb. Blood Flow Metab. 38, 1104–1118 (2018).
Dohgu, S., Fleegal-DeMotta, M. A. & Banks, W. A. Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by luminal microvessel IL-6 and GM-CSF. J. Neuroinflamm. 8, 167 (2011).
Engstrom, L. et al. Lipopolysaccharide-induced fever depends on prostaglandin E2 production specifically in brain endothelial cells. Endocrinology 153, 4849–4861 (2012).
Inoue, W. et al. Brain-specific endothelial induction of prostaglandin E(2) synthesis enzymes and its temporal relation to fever. Neurosci. Res. 44, 51–61 (2002).
McCarthy, R. C. & Kosman, D. J. Mechanisms and regulation of iron trafficking across the capillary endothelial cells of the blood-brain barrier. Front. Mol. Neurosci. 8, 31 (2015).
Miyajima, M. et al. Organic anion transporter 3 mediates the efflux transport of an amphipathic organic anion, dehydroepiandrosterone sulfate, across the blood-brain barrier in mice. Drug Metab. Dispos. 39, 814–819 (2011).
David, G. F. X. & Kumar, T. C. A. Transfer of steroidal hormones from blood to the cerebrospinal fluid in the rhesus monkey. Neuroendocrinology 14, 114–120 (1974).
Marynick, S. P., Haven, W. W., Ebert, M. H. & Loriaux, D. L. Studies on the transfer of steroid hormones across the blood-cerebrospinal fluid barrier in Rhesus monkey. Endocrinology 99, 400–405 (1976).
Ohtsuki, S. et al. Dominant expression of androgen receptors and their functional regulation of organic anion transporter 3 in rat brain capillary endothelial cells: comparison of gene expression between the blood-brain and -retinal barriers. J. Cell. Physiol. 204, 896–900 (2005).
Grube, M., Hagen, P. & Jedlitschky, G. Neurosteroid transport in the brain: role of ABC and SLC transporters. Front. Pharmacol. 9, 354 (2018).
Qaiser, M. Z. et al. Uptake and metabolism of sulphated steroids by the blood-brain barrier in the adult male rat. J. Neurochem. 142, 672–685 (2017).
Bernal, J., Guadano-Ferraz, A. & Morte, B. Thyroid hormone transporters — functions and clinical implications. Nat. Rev. Endocrinol. 11, 405–416 (2015).
Mayerl, S. et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J. Clin. Invest. 124, 1987–1999 (2014).
Roberts, L. M. et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149, 6251–6261 (2008).
Banks, W. A., Kastin, A. J. & Michals, E. A. Transport of thyroxine across the blood-brain barrier is directed primarily from brain to blood in the mouse. Life Sci. 37, 2407–2414 (1985).
Kodding, R., Fuhrmann, H. & van zur Muhlen, A. Investigations on iodothyronine deiodinase activity in the maturing brain. Endocrinology 118, 1347–1352 (1986).
Cserr, H. F. & Berman, B. J. Iodide and thiocyanate efflux from brain following injection into rat caudate nucleus. Am. J. Physiol. 4, F331–F337 (1978).
Davson, H. & Hollingsworth, J. R. Active transport of 131 I across the blood-brain barrier. J. Physiol. 233, 327–347 (1973).
Blasberg, R. G. Methotrexate, cytosine arabinoside, and BCNU concentration in brain after ventriculocisternal perfusion. Cancer Treat Rep. 61, 625–631 (1977).
Maness, L. M., Banks, W. A., Zadina, J. E. & Kastin, A. J. Periventricular penetration and disappearance of icv Tyr-MIF-1, DAMGO, tyrosine, and albumin. Peptides 17, 247–250 (1996).
Woods, S. C. & Porte, D. Jr. Relationship between plasma and cerebrospinal fluid insulin levels of dogs. Am. J. Physiol. 233, E331–E334 (1977).
Margolis, R. U. & Altszuler, N. Insulin in the cerebrospinal fluid. Nature 215, 1375–1376 (1967).
Greco, A. V., Ghirlanda, G., Fedeli, G. & Gambassi, G. Insulin in the cerebro spinal fluid of man. Eur. Neurol. 3, 303–307 (1970).
McRory, J. E. & Sherwood, N. M. Ancient divergence of insulin and insulin-like growth factor. DNA Cell Biol. 16, 939–949 (1997).
Bradbury, M. W. B., Segal, M. B. & Wilson, J. Transport of potassium at the blood-brain barrier. J. Physiol. 221, 617–632 (1972).
Maness, L. M., Kastin, A. J., Farrell, C. L. & Banks, W. A. Fate of leptin after intracerebroventricular injection into the mouse brain. Endocrinology 139, 4556–4562 (1998).
Brief, D. J. & Davis, J. D. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res. Bull. 12, 571–575 (1984).
McGowan, M. K., Andrews, K. M. & Grossman, S. P. Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol. Behav. 51, 753–766 (1992).
Banks, W. A. & Kastin, A. J. Physiological consequences of the passage of peptides across the blood-brain barrier. Rev. Neurosci. 4, 365–372 (1993).
Sandoval, D. A., Obici, S. & Seeley, R. J. Targeting the CNS to treat type 2 diabetes. Nat. Rev. Drug Discov. 8, 386–398 (2009).
Pocai, A. et al. Hypothalmic K(ATP) channels control hepatic glucose production. Nature 434, 1026–1031 (2005).
Biessels, G. J. & Reagan, L. P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 16, 660–671 (2015).
Banks, W. A. Insulin in the brain: There and back again. Pharmacol. Ther. 136, 82–93 (2012).
Nelson, T. J., Sun, M. K., Hongpaisan, J. & Alkon, D. L. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. Eur. J. Pharmacol. 585, 76–87 (2008).
Biessels, G. J., Bravenboer, B. & Gispen, H. W. Glucose, insulin and the brain: modulation of cognition and synaptic plasticity in health and disease: a preface. Eur. J. Pharmacol. 490, 1–4 (2004).
Ferrario, C. R. & Reagan, L. P. Insulin-mediated synaptic plasticity in the CNS: anatomical, functionaland temporal contexts. Neuropharmacology 136, 182–191 (2017).
Banks, W. A., Kastin, A. J., Huang, W., Jaspan, J. B. & Maness, L. M. Leptin enters the brain by a saturable system independent of insulin. Peptides 17, 305–311 (1996).
Banks, W. A., Tschop, M., Robinson, S. M. & Heiman, M. L. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J. Pharmacol. Exp. Ther. 302, 822–827 (2002).
Diano, S. et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 9, 381–388 (2006).
O’Malley, D. et al. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol. Cell Neurosci. 35, 559–572 (2007).
Garza, J. C., Guo, M., Zhang, W. & Lu, X. Y. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J. Biol. Chem. 283, 18238–18247 (2008).
Greco, S. J. et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 19, 1155–1167 (2010).
Cuevas, P., Carceller, F., Munoz-Willery, I. & Gimenez-Gallego, G. Intravenous fibroblast growth factor penetrates the blood-brain barrier and protects hippocampal neurons against ischemia-reperfusion injury. Surg. Neurol. 49, 77–83 (1998).
Cuevas, P. et al. Central nervous system distribution of fibroblast growth factor injected into the blood stream. Neurol. Res. 18, 267–272 (1996).
Hsuchou, H., Pan, W. & Kastin, A. J. Fibroblast growth factor 19 entry into brain. Fluids Barriers CNS 10, 32 (2013).
Wagner, J. P., Black, I. B. & DiCicco-Bloom, E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J. Neurosci. 19, 6006–6016 (1999).
Bookout, A. L. et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19, (1147–1152 (2013).
Lan, T. et al. FGF19, FGF21, and an FGFR1/β-klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26, 709–718 (2017).
Scarlett, J. M. et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat. Med. 22, 800–806 (2016).
Prevot, V. et al. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev. 39, 333–368 (2018).
Johanson, C. E., Duncan, J. A., Stopa, E. G. & Baird, A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm. Res. 22, 1011–1037 (2005).
de Lange, E. C. et al. Application of intracerebral microdialysis to study regional distribution kinetics of drug in rat brain. Br. J. Pharmacol. 116, 2538–2544 (1995).
Zlokovic, B. V. et al. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology 141, 1434–1441 (2000).
Nonaka, N., Hileman, S. M., Shioda, S., Vo, P. & Banks, W. A. Effects of lipopolysaccharide on leptin transport across the blood-brain barrier. Brain Res. 1016, 58–65 (2004).
Banks, W. A. & Farrell, C. L. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am. J. Physiol. 285, E10–E15 (2003).
Banks, W. A. et al. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int. J. Obes. (Lond.) 42, 391–397 (2018).
Romero, L. I., Kakucska, I., Lechan, R. M. & Reichlin, S. Interleukin-6 (IL-6) is secreted from the brain after intracerebroventricular injection of IL-1β in rats. Am. J. Physiol. 270, R518–R524 (1996).
Chen, G. & Reichlin, S. Clearance of [125 I]-tumor necrosis factor-alpha from the brain into the blood after intracerebroventricular injection into rats. Neuroimmunomodulation 5, 261–269 (1998).
Chen, G., McCuskey, R. S. & Reichlin, S. Blood interleukin-6 and tumor necrosis factor-alpha elevation after intracerebroventricular injection of Escherichia coli endotoxin in the rat is determined by two opposing factors: peripheral induction by LPS transferred from brain to blood and inhibition of peripheral response by a brain-mediated mechanism. Neuroimmunomodulation 8, 59–69 (2000).
Logsdon, A. F., Erickson, M. A., Rhea, E. M., Salameh, T. S. & Banks, W. A. Gut reactions: how the blood-brain barrier connects the microbiome and the brain. Exp. Biol. Med. (Maywood) 243, 159–165 (2018).
Erickson, M. A. & Banks, W. A. Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol. Rev. 70, 278–314 (2018).
Sampson, T. R. et al. Gut microbioata regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480 (2016).
Starr, J. M. et al. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry 74, 70–76 (2003).
Huber, J. D., VanGilder, R. L. & Houser, K. A. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am. J. Physiol. 291, H2660–H2668 (2006).
Abuhaiba, S. et al. Occipital blood-brain barrier permeability is an independent predictor of visual outcome in type 2 diabetes, irrespective of the retinal barrier: a logitudinal study. J. Neuroendocrinol. 30, e12566 (2018).
Huber, J. D. Diabetes, cognitive function, and the blood-brain barrier. Curr. Pharm. Des. 14, 1594–1600 (2008).
Takechi, R. et al. Blood-brain barrier dysfunction precedes cognitive decline and neurodegeneration in diabetic insulin resistant mouse model: an implication for causal link. Front. Aging Neurosci. 9, 399 (2017).
Hammes, H. P. et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51, 3107–3112 (2002).
Price, T. O., Eranki, V., Banks, W. A., Ercal, N. & Shah, G. N. Topiramate treatment protects blood-brain barrier pericytes from hyperglycemia-induced oxidative damage in diabetic mice. Endocrinology 153, 362–372 (2012).
Dore-Duffy, P. Pericytes: pluripotent cells of the blood brain barrier. Curr. Pharm. Des. 14, 1581–1593 (2008).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Zhang, Y. et al. Involvement of PUMA in pericyte migration induced by methamphetamine. Exp. Cell Res. 356, 28–39 (2017).
Sagare, A. P. et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 4, 2932 (2013).
Schulz, G. B. et al. Cerebral cavernous malformation-1 protein controls DLL4-Notch3 signalling between the endothelium and pericytes. Stroke 46, 1337–1343 (2015).
Kaiyala, K. J., Prigeon, R. L., Kahn, S. E., Woods, S. C. & Schwartz, M. W. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49, 1525–1533 (2000).
Mooradian, A. D. Blood-brain barrier choline transport is reduced in diabetic rats. Diabetes 36, 1094–1097 (1987).
Hong, H. et al. Downregulation of LRP1 at the blood-brain barrier in streptozotocin-induced diabetic mice. Neuropharmacology 56, 1054–1059 (2009).
Banks, W. A., DiPalma, C. R. & Farrell, C. L. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides 20, 1341–1345 (1999).
Ouyang, S. et al. Diet-induced obesity suppresses expression of many proteins at the blood-brain barrier. J. Cereb. Blood Flow Metab. 34, 43–51 (2014).
Mooradian, A. D. & Smith, T. L. The effect of experimentally induced diabetes mellitus on the lipid order and composition of rat cerebral microvessels. Neurosci. Lett. 145, 145–148 (1992).
Beard, R. S. Jr, Reynolds, J. J. & Bearden, S. E. Hyperhomocysteinemia increases permeability of the blood-brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood 118, 2007–2014 (2011).
Rhodehouse, B. C., Mayo, J. N., Beard, R. S. Jr, Chen, C. H. & Bearden, S. E. Opening of the blood-brain barrier before cerebral pathology in mild hyperhomocysteinemia. PLOS ONE 8, e63951 (2013).
Delange, F. The disorders induced by iodine deficiency. Thyroid 4, 107–128 (1994).
Nunez, B. et al. Cerebral cortex hyperthyroidism of newborn mct8-deficient mice transiently suppressed by lat2 inactivation. PLOS ONE 9, e96915 (2014).
Heni, M., Kullmann, S., Preissl, H., Fritsche, A. & Haring, H. U. Impaired insulin action in the human brain: causes and metabolic consequences. Nat. Rev. Endocrinol. 11, 701–711 (2015).
De Vivo, D. C. et al. Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N. Engl. J. Med. 325, 703–709 (1991).
Craft, S. et al. Cerebrosinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 50, 164–168 (1998).
Talbot, K. et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dyregulation, and cognitive decline. J. Clin. Invest. 122, 1316–1338 (2012).
Sartorius, T. et al. The brain response to peripheral insulin declines with age: a contribution of the blood-brain barrier? PLOS ONE 10, e0126804 (2015).
Pelleymounter, M. A. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543 (1995).
Banks, W. A., Clever, C. M. & Farrell, C. L. Partial saturation and regional variation in the blood to brain transport of leptin in normal weight mice. Am. J. Physiol. 278, E1158–E1165 (2000).
Licinio, J. et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc. Natl Acad. Sci. USA 101, 4531–4536 (2004).
Ingalls, A. M., Dickie, M. M. & Snell, G. D. Obese, a new mutation in the house mouse. J. Hered. 41, 317–318 (1950).
Bribiescas, R. G. Serum leptin levels and anthropometric correlates in Ache Amerindians of Eastern Paraguay. Am. J. Phys. Anthropol. 115, 297–303 (2001).
Halaas, J. L. et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl Acad. Sci. USA 94, 8878–8883 (1997).
van Heek, M. et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J. Clin. Invest. 99, 385–390 (1997).
El Haschimi, K., Pierroz, D. D., Hileman, S. M., Bjorbaek, C. & Flier, J. S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obestiy. J. Clin. Invest. 105, 1827–1832 (2000).
Rutenberg, G. W. et al. Body composition in baboons: evaluating a morphometric method. Am. J. Primatol. 12, 275–285 (1987).
Bribiescas, R. G. Serum leptin levels in Ache Amerindian females with normal adiposity are not significantly different from American anorexia nervosa patients. Am. J. Hum. Biol. 17, 207–210 (2005).
Kirchengast, S. Weight status of adult !Kung San and Kavango people from northern Namibia. Ann. Hum. Biol. 25, 541–551 (1996).
Schwartz, M. W. et al. Cerebrospinal fluid leptin levels: relationship to plasma levels and adiposity in humans. Nat. Med. 2, 589–593 (1996).
Caro, J. F. et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348, 159–161 (1996).
Mantzoros, C., Flier, J. S., Lesem, M. D., Brewerton, T. D. & Jimerson, D. C. Cerebrospinal fluid leptin in anorexia nervosa: Correlation with nutritional status and potential role in resistance to weight gain. J. Clin. Endocrinol. Metab. 82, 1845–1851 (1997).
Smith, Q. R., Momma, S., Aoyagi, M. & Rapoport, S. I. Kinetics of neutral amino acid transport across the blood-brain barrier. J. Neurochem. 49, 1651–1658 (1987).
Fernstrom, J. D. Branched-chain amino acids and brain function. J. Nutr. 135, 1439S–1546S (2005).
van Spronsen, F. J., de Groot, M. J., Hoeksma, M., Reijngoud, D.-J. & van Rijn, M. Large neutral amino acids in the treatment of PKU: from theory to practice. J. Inherit. Metab. Dis. 33, 671–676 (2010).
Banks, W. A., Burney, B. O. & Robinson, S. M. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides 29, 2061–2065 (2008).
Holscher, C. Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer’s and Parkinson’s disease models. Neuropharmacology 136, 251–259 (2018).
Gejl, M. et al. Blood-brain glucose transfer in Alzheimer’s disease: effect of GLP-1 analog treatment. Sci. Rep. 7, 17490 (2017).
Tamargo, I. A. et al. Novel GLP-1R/GIPR co-agonist “twincretin” is neuroprotective in cell and rodent models of mild traumatic brain injury. Exp. Neurol. 288, 176–186 (2017).
Deli, M. A., Abraham, C. R., Kataoka, Y. & Niwa, M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell. Mol. Neurobiol. 25, 59–127 (2005).
Wolburg, H., Noell, S., Mack, A., Wolburg-Buchholz, K. & Dallier-Becker, P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 335, 75–96 (2009).
Saunders, N. R., Dziegielewska, K. M., Mollgard, K. & Habgood, M. D. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. J. Physiol. 596, 5723–5756 (2018).
Cornford, E. M., Braun, L. D., Oldendorf, W. H. & Hill, M. A. Comparison of lipid-mediated blood-brain-barrier penetrability in neonates and adults. Am. J. Physiol. 243, C161–C168 (1982).
Dickson, P. W., Aldred, A. R., Marley, P. D., Bannister, D. & Schreiber, G. Rat choroid plexus specializes in the synthesis and the secretion of transthyretin (prealbumin). Regulation of tranthyretin synthesis in choroid plexus is indpendent from that in liver. J. Biol. Chem. 15, 3475–3478 (1986).
Zhao, F. et al. Effects of passage and cryopreservation on neurotophic factor secretion from choroid plexus epithelial cells. Biomed. Rep. 8, 535–539 (2018).
Broadwell, R. D. & Sofroniew, M. V. Serum proteins bypass the blood-brain barrier for extracellular entry to the central nervous system. Exp. Neurol. 120, 245–263 (1993).
Acknowledgements
Reviewer information
Nature Reviews Endocrinology thanks B. Levin, D. Begley and V. Prevot, and the other anonymous reviewers, for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Neurovascular unit
-
A multicellular and multicomponent network that is composed of neurons, glial cells, brain endothelial cells and extracellular matrix components. The neurovascular unit is key to neurovascular coupling and to the delivery of key nutrients and oxygen from the circulatory system into the brain.
- Plasma protein binding
-
The degree to which a substance binds to proteins within the blood.
- Choroid plexus
-
The choroid plexus, which consists of modified ependymal cells, produces the cerebrospinal fluid in the ventricles of the brain.
- Allan–Herndon–Dudley syndrome
-
A condition resulting from deficient thyroid hormone transport across the blood–brain barrier characterized by cognitive impairment, lack of speech and hypotonia.
Rights and permissions
About this article
Cite this article
Banks, W.A. The blood–brain barrier as an endocrine tissue. Nat Rev Endocrinol 15, 444–455 (2019). https://doi.org/10.1038/s41574-019-0213-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0213-7
This article is cited by
-
Blood–brain borders: a proposal to address limitations of historical blood–brain barrier terminology
Fluids and Barriers of the CNS (2024)
-
Metabolic control of puberty: 60 years in the footsteps of Kennedy and Mitra’s seminal work
Nature Reviews Endocrinology (2024)
-
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
Nature Reviews Gastroenterology & Hepatology (2024)
-
Blood-testis barrier: a review on regulators in maintaining cell junction integrity between Sertoli cells
Cell and Tissue Research (2024)
-
Bitter taste cells in the ventricular walls of the murine brain regulate glucose homeostasis
Nature Communications (2023)