Abstract
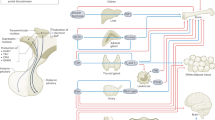
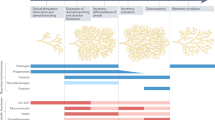
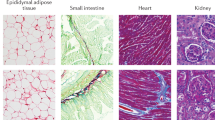
The principal role of prolactin in mammals is the regulation of lactation. Prolactin is a hormone that is mainly synthesized and secreted by lactotroph cells in the anterior pituitary gland. Prolactin signalling occurs via a unique transmembrane prolactin receptor (PRL-R). The structure of the PRL-R has now been elucidated and is similar to that of many biologically fundamental receptors of the class 1 haematopoietic cytokine receptor family such as the growth hormone receptor. The PRL-R is expressed in a wide array of tissues, and a growing number of biological processes continue to be attributed to prolactin. In this Review, we focus on the newly discovered roles of prolactin in human health and disease, particularly its involvement in metabolic homeostasis including body weight control, adipose tissue, skin and hair follicles, pancreas, bone, the adrenal response to stress, the control of lactotroph cell homeostasis and maternal behaviour. New data concerning the pathological states of hypoprolactinaemia and hyperprolactinaemia will also be presented and discussed.
Key points
Prolactin exerts its actions via a transmembrane prolactin receptor (PRL-R), the structure of which has now been elucidated.
Prolactin signalling is essential for the ontogenesis of pancreatic stem cells for the establishment of a functional β-cell reserve.
In addition to its role in increasing dopaminergic inhibitory tone, prolactin exerts autocrine and paracrine feedback on lactotroph cells.
Prolactin deficiency is rare and causes failure of lactation.
Hyperprolactinaemia can be caused by medications or pituitary disease, can have systemic causes or can be idiopathic; this condition frequently leads to hypogonadism and infertility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bole-Feysot, C., Goffin, V., Edery, M., Binart, N. & Kelly, P. A. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19, 225–268 (1998).
Bugge, K. et al. A combined computational and structural model of the full-length human prolactin receptor. Nat. Commun. 7, 11578 (2016).
Goffin, V., Binart, N., Touraine, P. & Kelly, P. A. Prolactin: the new biology of an old hormone. Annu. Rev. Physiol. 64, 47–67 (2002).
Ben-Jonathan, N., LaPensee, C. R. & LaPensee, E. W. What can we learn from rodents about prolactin in humans? Endocr. Rev. 29, 1–41 (2008).
Bernard, V., Young, J., Chanson, P. & Binart, N. New insights in prolactin: pathological implications. Nat. Rev. Endocrinol. 11, 265–275 (2015).
Halmi, N. S., Parsons, J. A., Erlandsen, S. L. & Duello, T. Prolactin and growth hormone cells in the human hypophysis: a study with immunoenzyme histochemistry and differential staining. Cell Tissue Res. 158, 497–507 (1975).
Shingo, T. et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299, 117–120 (2003).
Bridges, R. S. Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol. 36, 178–196 (2015).
Rizzoti, K., Akiyama, H. & Lovell-Badge, R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell 13, 419–432 (2013).
Hodson, D. J. et al. Existence of long-lasting experience-dependent plasticity in endocrine cell networks. Nat. Commun. 3, 605 (2012).
Gleiberman, A. S. et al. Genetic approaches identify adult pituitary stem cells. Proc. Natl Acad. Sci. USA 105, 6332–6337 (2008).
Karaca, Z., Tanriverdi, F., Unluhizarci, K. & Kelestimur, F. Pregnancy and pituitary disorders. Eur. J. Endocrinol. 162, 453–475 (2010).
Kline, J. B., Roehrs, H. & Clevenger, C. V. Functional characterization of the intermediate isoform of the human prolactin receptor. J. Biol. Chem. 274, 35461–35468 (1999).
Hu, Z. Z., Meng, J. & Dufau, M. L. Isolation and characterization of two novel forms of the human prolactin receptor generated by alternative splicing of a newly identified exon 11. J. Biol. Chem. 276, 41086–41094 (2001).
Trott, J. F., Hovey, R. C., Koduri, S. & Vonderhaar, B. K. Multiple new isoforms of the human prolactin receptor gene. Adv. Exp. Med. Biol. 554, 495–499 (2004).
Goffin, V., Shiverick, K. T., Kelly, P. A. & Martial, J. A. Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr. Rev. 17, 385–410 (1996).
Brooks, C. L. Molecular mechanisms of prolactin and its receptor. Endocr. Rev. 33, 504–525 (2012).
Qazi, A. M., Tsai-Morris, C.-H. & Dufau, M. L. Ligand-independent homo- and heterodimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol. Endocrinol. 20, 1912–1923 (2006).
Brooks, A. J. & Waters, M. J. The growth hormone receptor: mechanism of activation and clinical implications. Nat. Rev. Endocrinol. 6, 515–525 (2010).
Goffin, V., Martial, J. A. & Summers, N. L. Use of a model to understand prolactin and growth hormone specificities. Protein Eng. 8, 1215–1231 (1995).
Haxholm, G. W. et al. Intrinsically disordered cytoplasmic domains of two cytokine receptors mediate conserved interactions with membranes. Biochem. J. 468, 495–506 (2015).
Brooks, A. J. et al. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science 344, 1249783 (2014).
Freemark, M. et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143, 1378–1385 (2002).
Vasavada, R. C. et al. Growth factors and beta cell replication. Int. J. Biochem. Cell Biol. 38, 931–950 (2006).
Ben-Jonathan, N., Hugo, E. R., Brandebourg, T. D. & LaPensee, C. R. Focus on prolactin as a metabolic hormone. Trends Endocrinol. Metab. 17, 110–116 (2006).
Sauvé, D. & Woodside, B. Neuroanatomical specificity of prolactin-induced hyperphagia in virgin female rats. Brain Res. 868, 306–314 (2000).
Arumugam, R., Fleenor, D. & Freemark, M. Lactogenic and somatogenic hormones regulate the expression of neuropeptide Y and cocaine- and amphetamine-regulated transcript in rat insulinoma (INS-1) cells: interactions with glucose and glucocorticoids. Endocrinology 148, 258–267 (2007).
Perez Millan, M. I. et al. Selective disruption of dopamine D2 receptors in pituitary lactotropes increases body weight and adiposity in female mice. Endocrinology 155, 829–839 (2014).
Luque, G. M. et al. Chronic hyperprolactinemia evoked by disruption of lactotrope dopamine D2 receptors impacts on liver and adipocyte genes related to glucose and insulin balance. Am. J. Physiol. Endocrinol. Metab. 311, E974–E988 (2016).
Ling, C. et al. Prolactin (PRL) receptor gene expression in mouse adipose tissue: increases during lactation and in PRL-transgenic mice. Endocrinology 141, 3564–3572 (2000).
Auffret, J. et al. Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity. FASEB J. 26, 3728–3737 (2012).
Brelje, T. C. et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132, 879–887 (1993).
Auffret, J. et al. Defective prolactin signaling impairs pancreatic β-cell development during the perinatal period. Am. J. Physiol. Endocrinol. Metab. 305, E1309–E1318 (2013).
Benner, C. et al. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 15, 620 (2014).
Chen, H. et al. Augmented Stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human β-Cells. Diabetes 64, 3784–3797 (2015).
Nielsen, J. H. Beta cell adaptation in pregnancy: a tribute to Claes Hellerström. Ups. J. Med. Sci. 121, 151–154 (2016).
Huang, C. Wild-type offspring of heterozygous prolactin receptor-null female mice have maladaptive β-cell responses during pregnancy. J. Physiol. 591, 1325–1338 (2013).
Banerjee, R. R. et al. Gestational diabetes mellitus from inactivation of prolactin receptor and MafB in islet β-Cells. Diabetes 65, 2331–2341 (2016).
Langan, E. A., Foitzik-Lau, K., Goffin, V., Ramot, Y. & Paus, R. Prolactin: an emerging force along the cutaneous-endocrine axis. Trends Endocrinol. Metab. 21, 569–577 (2010).
Craven, A. J. et al. Prolactin signaling influences the timing mechanism of the hair follicle: analysis of hair growth cycles in prolactin receptor knockout mice. Endocrinology 142, 2533–2539 (2001).
Manzon, L. A. The role of prolactin in fish osmoregulation: a review. Gen. Comp. Endocrinol. 125, 291–310 (2002).
Foitzik, K. et al. Prolactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagen. Am. J. Pathol. 162, 1611–1621 (2003).
Littlejohn, M. D. et al. Functionally reciprocal mutations of the prolactin signalling pathway define hairy and slick cattle. Nat. Commun. 5, 5861 (2014).
Mills, D. E. & Robertshaw, D. Response of plasma prolactin to changes in ambient temperature and humidity in man. J. Clin. Endocrinol. Metab. 52, 279–283 (1981).
Porto-Neto, L. R. et al. Convergent evolution of slick coat in cattle through truncation mutations in the prolactin receptor. Front. Genet. 9, 57 (2018).
Giustina, A., Mazziotti, G. & Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 29, 535–559 (2008).
Clément-Lacroix, P. et al. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology 140, 96–105 (1999).
Macari, S. et al. Lactation induces increases in the RANK/RANKL/OPG system in maxillary bone. Bone 110, 160–169 (2018).
Klibanski, A. et al. Decreased bone density in hyperprolactinemic women. N. Engl. J. Med. 303, 1511–1514 (1980).
Schlechte, J. A., Sherman, B. & Martin, R. Bone density in amenorrheic women with and without hyperprolactinemia. J. Clin. Endocrinol. Metab. 56, 1120–1123 (1983).
Mazziotti, G. et al. High prevalence of radiological vertebral fractures in women with prolactin-secreting pituitary adenomas. Pituitary 14, 299–306 (2011).
Mazziotti, G. et al. Vertebral fractures in males with prolactinoma. Endocrine 39, 288–293 (2011).
Klibanski, A. & Greenspan, S. L. Increase in bone mass after treatment of hyperprolactinemic amenorrhea. N. Engl. J. Med. 315, 542–546 (1986).
Klibanski, A., Biller, B. M., Rosenthal, D. I., Schoenfeld, D. A. & Saxe, V. Effects of prolactin and estrogen deficiency in amenorrheic bone loss. J. Clin. Endocrinol. Metab. 67, 124–130 (1988).
Winter, E. M. & Appelman-Dijkstra, N. M. Parathyroid hormone-related protein-induced hypercalcemia of pregnancy successfully reversed by a dopamine agonist. J. Clin. Endocrinol. Metab. 102, 4417–4420 (2017).
Bridges, R. S., DiBiase, R., Loundes, D. D. & Doherty, P. C. Prolactin stimulation of maternal behavior in female rats. Science 227, 782–784 (1985).
Shyr, S. W., Crowley, W. R. & Grosvenor, C. E. Effect of neonatal prolactin deficiency on prepubertal tuberoinfundibular and tuberohypophyseal dopaminergic neuronal activity. Endocrinology 119, 1217–1221 (1986).
Grosvenor, C. E., Shyr, S. W. & Crowley, W. R. Effect of neonatal prolactin deficiency on prepubertal tuberoinfundibular and tuberohypophyseal dopaminergic neuronal activity. Endocrinol. Exp. 20, 223–228 (1986).
Lucas, B. K., Ormandy, C. J., Binart, N., Bridges, R. S. & Kelly, P. A. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 139, 4102–4107 (1998).
Bridges, R. S. et al. Endocrine communication between conceptus and mother: placental lactogen stimulation of maternal behavior. Neuroendocrinology 64, 57–64 (1996).
Freeman, M. E., Kanyicska, B., Lerant, A. & Nagy, G. Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 80, 1523–1631 (2000).
Brown, R. S. E. et al. Prolactin transport into mouse brain is independent of prolactin receptor. FASEB J. 30, 1002–1010 (2016).
Brown, R. S. E. et al. Prolactin action in the medial preoptic area is necessary for postpartum maternal nursing behavior. Proc. Natl Acad. Sci. USA 114, 10779–10784 (2017).
Melo, A. I. et al. Effects of prolactin deficiency during the early postnatal period on the development of maternal behavior in female rats: mother’s milk makes the difference. Horm. Behav. 56, 281–291 (2009).
Mann, P. E. & Bridges, R. S. Lactogenic hormone regulation of maternal behavior. Prog. Brain Res. 133, 251–262 (2001).
Salais-López, H., Lanuza, E., Agustín-Pavón, C. & Martínez-García, F. Tuning the brain for motherhood: prolactin-like central signalling in virgin, pregnant, and lactating female mice. Brain Struct. Funct. 222, 895–921 (2017).
Grattan, D. R. 60 YEARS OF NEUROENDOCRINOLOGY: the hypothalamo-prolactin axis. J. Endocrinol. 226, T101–122 (2015).
Armario, A., Marti, O., Molina, T., de Pablo, J. & Valdes, M. Acute stress markers in humans: response of plasma glucose, cortisol and prolactin to two examinations differing in the anxiety they provoke. Psychoneuroendocrinology 21, 17–24 (1996).
Kirk, S. E., Xie, T. Y., Steyn, F. J., Grattan, D. R. & Bunn, S. J. Restraint stress increases prolactin-mediated phosphorylation of signal transducer and activator of transcription 5 in the hypothalamus and adrenal cortex in the male mouse. J. Neuroendocrinol. https://doi.org/10.1111/jne.12477 (2017).
Sobrinho, L. G. Prolactin, psychological stress and environment in humans: adaptation and maladaptation. Pituitary 6, 35–39 (2003).
Carter, J. N. et al. Adrenocortical function in hyperprolactinemic women. J. Clin. Endocrinol. Metab. 45, 973–980 (1977).
Schiebinger, R. J., Chrousos, G. P., Cutler, G. B. & Loriaux, D. L. The effect of serum prolactin on plasma adrenal androgens and the production and metabolic clearance rate of dehydroepiandrosterone sulfate in normal and hyperprolactinemic subjects. J. Clin. Endocrinol. Metab. 62, 202–209 (1986).
Belisle, S. & Menard, J. Adrenal androgen production in hyperprolactinemic states. Fertil. Steril. 33, 396–400 (1980).
Parker, L. N., Chang, S. & Odell, W. D. Adrenal androgens in patients with chronic marked elevation of prolactin. Clin. Endocrinol. 8, 1–5 (1978).
Tritos, N. & Klibanski, A. in Yen & Jaffe’s Reproductive Endocrinology E-Book: Physiology, Pathophysiology, and Clinical Management (eds Strauss, J. F., Barbieri, R. L. & Gargiulo, A. R.) 8th edn 58–75 (Elsevier, 2018).
Grattan, D. R. & Kokay, I. C. Prolactin: a pleiotropic neuroendocrine hormone. J. Neuroendocrinol. 20, 752–763 (2008).
Kelly, M. A. et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19, 103–113 (1997).
Asa, S. L., Kelly, M. A., Grandy, D. K. & Low, M. J. Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology 140, 5348–5355 (1999).
Schuff, K. G. et al. Lack of prolactin receptor signaling in mice results in lactotroph proliferation and prolactinomas by dopamine-dependent and -independent mechanisms. J. Clin. Invest. 110, 973–981 (2002).
Bernard, V. et al. Natural and molecular history of prolactinoma: insights from a Prlr−/− mouse model. Oncotarget 9, 6144–6155 (2018).
Krause, D. S. & Van Etten, R. A. Tyrosine kinases as targets for cancer therapy. N. Engl. J. Med. 353, 172–187 (2005).
Liu, X. et al. ErbB receptor-driven prolactinomas respond to targeted lapatinib treatment in female transgenic mice. Endocrinology 156, 71–79 (2015).
Fielitz, K. et al. Characterization of pancreatic glucagon-producing tumors and pituitary gland tumors in transgenic mice overexpressing MYCN in hGFAP-positive cells. Oncotarget 7, 74415–74426 (2016).
Bernard, V. et al. Autocrine actions of prolactin contribute to the regulation of lactotroph function in vivo. FASEB J. 9, 4791–4797 (2018).
MohanKumar, P. S., MohanKumar, S. M., Quadri, S. K. & Voogt, J. L. Effects of chronic bromocriptine treatment on tyrosine hydroxylase (TH) mRNA expression, TH activity and median eminence dopamine concentrations in ageing rats. J. Neuroendocrinol. 13, 261–269 (2001).
Le Tissier, P. et al. An updated view of hypothalamic-vascular-pituitary unit function and plasticity. Nat. Rev. Endocrinol. 13, 257–267 (2017).
Nikolics, K., Mason, A. J., Szönyi, E., Ramachandran, J. & Seeburg, P. H. A prolactin-inhibiting factor within the precursor for human gonadotropin-releasing hormone. Nature 316, 511–517 (1985).
Bouligand, J. et al. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N. Engl. J. Med. 360, 2742–2748 (2009).
Cattanach, B. M., Iddon, C. A., Charlton, H. M., Chiappa, S. A. & Fink, G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269, 338–340 (1977).
Charlton, H. M. et al. Prolactin measurements in normal and hypogonadal (hpg) mice: developmental and experimental studies. Endocrinology 113, 545–548 (1983).
Bonomi, M. et al. A family with complete resistance to thyrotropin-releasing hormone. N. Engl. J. Med. 360, 731–734 (2009).
Yamada, M. et al. Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc. Natl Acad. Sci. USA 94, 10862–10867 (1997).
Hennighausen, L. & Robinson, G. W. Information networks in the mammary gland. Nat. Rev. Mol. Cell Biol. 6, 715–725 (2005).
Toledano, Y., Lubetsky, A. & Shimon, I. Acquired prolactin deficiency in patients with disorders of the hypothalamic-pituitary axis. J. Endocrinol. Invest. 30, 268–273 (2007).
Iwama, S., Welt, C. K., Romero, C. J., Radovick, S. & Caturegli, P. Isolated prolactin deficiency associated with serum autoantibodies against prolactin-secreting cells. J. Clin. Endocrinol. Metab. 98, 3920–3925 (2013).
Kauppila, A., Chatelain, P., Kirkinen, P., Kivinen, S. & Ruokonen, A. Isolated prolactin deficiency in a woman with puerperal alactogenesis. J. Clin. Endocrinol. Metab. 64, 309–312 (1987).
Powe, C. E. et al. Recombinant human prolactin for the treatment of lactation insufficiency. Clin. Endocrinol. 73, 645–653 (2010).
Ormandy, C. J. et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 11, 167–178 (1997).
Rasmussen, K. M., Hilson, J. A. & Kjolhede, C. L. Obesity may impair lactogenesis II. J. Nutr. 131, 3009S–3011S (2001).
Rasmussen, K. M., Hilson, J. A. & Kjolhede, C. L. Obesity as a risk factor for failure to initiate and sustain lactation. Adv. Exp. Med. Biol. 503, 217–222 (2002).
Rasmussen, K. M. & Kjolhede, C. L. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 113, e465–e471 (2004).
Garcia, A. H. et al. Maternal weight status, diet, and supplement use as determinants of breastfeeding and complementary feeding: a systematic review and meta-analysis. Nutr. Rev. 74, 490–516 (2016).
Pfäffle, R. & Klammt, J. Pituitary transcription factors in the aetiology of combined pituitary hormone deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 25, 43–60 (2011).
Nakamura, A. et al. Three novel IGSF1 mutations in four Japanese patients with X-linked congenital central hypothyroidism. J. Clin. Endocrinol. Metab. 98, E1682–E1691 (2013).
Voutetakis, A. et al. Ovulation induction and successful pregnancy outcome in two patients with Prop1 gene mutations. Fertil. Steril. 82, 454–457 (2004).
Carlson, H. E., Brickman, A. S. & Bottazzo, G. F. Prolactin deficiency in pseudohypoparathyroidism. N. Engl. J. Med. 296, 140–144 (1977).
Shapiro, M. S., Bernheim, J., Gutman, A., Arber, I. & Spitz, I. M. Multiple abnormalities of anterior pituitary hormone secretion in association with pseudohypoparathyroidism. J. Clin. Endocrinol. Metab. 51, 483–487 (1980).
Karaca, Z., Laway, B. A., Dokmetas, H. S., Atmaca, H. & Kelestimur, F. Sheehan syndrome. Nat. Rev. Dis. Primers 2, 16092 (2016).
Webster, J. A comparative review of the tolerability profiles of dopamine agonists in the treatment of hyperprolactinaemia and inhibition of lactation. Drug Saf. 14, 228–238 (1996).
Rains, C. P., Bryson, H. M. & Fitton, A. Cabergoline. A review of its pharmacological properties and therapeutic potential in the treatment of hyperprolactinaemia and inhibition of lactation. Drugs 49, 255–279 (1995).
Gallego, M. I. et al. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev. Biol. 229, 163–175 (2001).
Kobayashi, T., Usui, H., Tanaka, H. & Shozu, M. Variant prolactin receptor in agalactia and hyperprolactinemia. N. Engl. J. Med. 379, 2230–2236 (2018).
Vilar, L. et al. Diagnosis and management of hyperprolactinemia: results of a Brazilian multicenter study with 1234 patients. J. Endocrinol. Invest. 31, 436–444 (2008).
Soto-Pedre, E., Newey, P. J., Bevan, J. S. & Leese, G. P. Morbidity and mortality in patients with hyperprolactinaemia: the PROLEARS study. Endocr. Connect. 6, 580–588 (2017).
Therkelsen, K. E. et al. Association between prolactin and incidence of cardiovascular risk factors in the Framingham Heart Study. J. Am. Heart Assoc. 5, e002640 (2016).
Bouchard, P., Lagoguey, M., Brailly, S. & Schaison, G. Gonadotropin-releasing hormone pulsatile administration restores luteinizing hormone pulsatility and normal testosterone levels in males with hyperprolactinemia. J. Clin. Endocrinol. Metab. 60, 258–262 (1985).
Lecomte, P. et al. Pregnancy after intravenous pulsatile gonadotropin-releasing hormone in a hyperprolactinaemic woman resistant to treatment with dopamine agonists. Eur. J. Obstet. Gynecol. Reprod. Biol. 74, 219–221 (1997).
Sauder, S. E., Frager, M., Case, G. D., Kelch, R. P. & Marshall, J. C. Abnormal patterns of pulsatile luteinizing hormone secretion in women with hyperprolactinemia and amenorrhea: responses to bromocriptine. J. Clin. Endocrinol. Metab. 59, 941–948 (1984).
Li, Q., Rao, A., Pereira, A., Clarke, I. J. & Smith, J. T. Kisspeptin cells in the ovine arcuate nucleus express prolactin receptor but not melatonin receptor. J. Neuroendocrinol. 23, 871–882 (2011).
Smith, J. T. et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 152, 1001–1012 (2011).
Sonigo, C. et al. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J. Clin. Invest. 122, 3791–3795 (2012).
Millar, R. P. et al. Hypothalamic-pituitary-ovarian axis reactivation by kisspeptin-10 in hyperprolactinemic women with chronic amenorrhea. J. Endocr. Soc. 1, 1362–1371 (2017).
Abbara, A. et al. Interpretation of serum gonadotropin levels in hyperprolactinemia. Neuroendocrinology 107, 105–113 (2018).
Raappana, A., Koivukangas, J., Ebeling, T. & Pirilä, T. Incidence of pituitary adenomas in Northern Finland in 1992–2007. J. Clin. Endocrinol. Metab. 95, 4268–4275 (2010).
Santharam, S. et al. Prolactinomas diagnosed in the postmenopausal period: clinical phenotype and outcomes. Clin. Endocrinol. 87, 508–514 (2017).
Scoccia, B., Schneider, A. B., Marut, E. L. & Scommegna, A. Pathological hyperprolactinemia suppresses hot flashes in menopausal women. J. Clin. Endocrinol. Metab. 66, 868–871 (1988).
Rance, N. E., Dacks, P. A., Mittelman-Smith, M. A., Romanovsky, A. A. & Krajewski-Hall, S. J. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front. Neuroendocrinol. 34, 211–227 (2013).
Reviewer information
Nature Reviews Endocrinology thanks D. Grattan, and other anonymous reviewers, for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors provided a substantial contribution to the discussion of content, researching data for the article, writing the article and the review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bernard, V., Young, J. & Binart, N. Prolactin — a pleiotropic factor in health and disease. Nat Rev Endocrinol 15, 356–365 (2019). https://doi.org/10.1038/s41574-019-0194-6
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0194-6
This article is cited by
-
The relationship between plasma prolactin levels and clinical manifestations with neuromyelitis optica spectrum disorders
Neurological Sciences (2024)
-
Prolactin and oxytocin: potential targets for migraine treatment
The Journal of Headache and Pain (2023)
-
Splicing alterations in pancreatic ductal adenocarcinoma: a new molecular landscape with translational potential
Journal of Experimental & Clinical Cancer Research (2023)
-
Temperature modulates the osmosensitivity of tilapia prolactin cells
Scientific Reports (2023)
-
Hormonal regulation of mammary gland development and lactation
Nature Reviews Endocrinology (2023)