Abstract
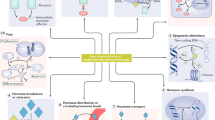
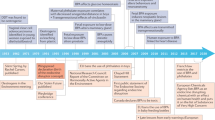
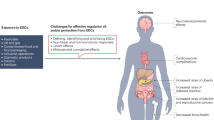
Five years ago, an ambitious collaboration, the Consortium Linking Academic and Regulatory Insights on Toxicity of BPA (CLARITY–BPA; henceforth CLARITY), was launched by three US agencies. The goal was to provide a definitive evaluation of bisphenol A (BPA) and explain disparities between traditional regulatory studies and findings from independent investigators. BPA or vehicle-treated rats from an FDA facility were used in a guideline study and animals and/or tissues were provided to academic researchers for analysis. An interim summary released in February 2018 by the FDA concluded that currently authorized uses of BPA continue to be safe. We disagree. In this Perspectives, we summarize the goals, design and problems of CLARITY. We conclude that, despite its flaws, CLARITY provides important insight and, taken together, the data provide compelling evidence that low-dose BPA exposure induces marked adverse effects. Indeed, the greatest number of effects were observed at doses 20,000 times lower than the current ‘safe’ dose of BPA for humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Geens, T. et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 50, 3725–3740 (2012).
Rochester, J. R. Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 42, 132–155 (2013).
Vandenberg, L. N. et al. Low dose effects of bisphenol A: an integrated review of in vitro, laboratory animal and epidemiology studies. Endocr. Disruptors 1, e25078 (2013).
Richter, C. et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 24, 199–224 (2007).
Gore, A. C. et al. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 36, E1–E150 (2015).
Vandenberg, L. N., Maffini, M. V., Sonnenschein, C., Rubin, B. S. & Soto, A. M. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 30, 75–95 (2009).
Birnbaum, L. S. et al. Consortium-based science: The NIEHS’s multipronged, collaborative approach to assessing the health effects of Bisphenol A. Environ. Health Perspect. 120, 1640–1644 (2012).
Myers, J. P. et al. Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A. Environ. Health Perspect. 117, 309–315 (2009).
Tyl, R. W. Basic exploratory research versus guideline-compliant studies used for hazard evaluation and risk assessment: bisphenol A as a case study. Environ. Health Perspect. 117, 1644–1651 (2009).
Stump, D. G. et al. Developmental neurotoxicity study of dietary bisphenol A in Sprague-Dawley rats. Toxicol. Sci. 115, 167–182 (2010).
Tyl, R. W. et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 104, 362–384 (2008).
Tyl, R. W. et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 68, 121–146 (2002).
Peretz, J. et al. Bisphenol A and reproductive health: update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 122, 775–786 (2014).
Myers, J. P., Zoeller, R. T. & vom Saal, F. S. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ. Health Perspect. 117, 1652–1655 (2009).
Schug, T. T. et al. Designing endocrine disruption out of the next generation of chemicals. Green Chem. 15, 181–198 (2013).
Gore, A. C., Heindel, J. J. & Zoeller, R. T. Endocrine disruption for endocrinologists (and others). Endocrinology 147, S1–S3 (2006).
Maffini, M. V. & Vandenberg, L. N. Closing the gap: improving additives safety evaluation to reflect human health concerns. Environ. Risk Assess. Remediat. 1, 26–33 (2017).
Bergman, A. et al. Science and policy on endocrine disrupters must not be mixed: a reply to a “common sense” intervention by toxicology journal editors. Environ. Health 12, 69 (2013).
Patel, S. et al. Bisphenol A exposure, ovarian follicle numbers, and female sex steroid hormone levels: results from a CLARITY-BPA study. Endocrinology 158, 1727–1738 (2017).
Li, J. et al. CLARITY-BPA: effects of chronic bisphenol A exposure on the immune system. Part 2 — characterization of lymphoproliferative and immune effector responses by splenic leukocytes. Toxicology 396–397, 54–67 (2018).
Li, J. et al. CLARITY-BPA: effects of chronic Bisphenol A exposure on the immune system. Part 1 — quantification of the relative number and proportion of leukocyte populations in the spleen and thymus. Toxicology 396–397, 46–53 (2018).
Dere, E. et al. Effects of continuous bisphenol A exposure from early gestation on 90day old rat testes function and sperm molecular profiles: a CLARITY-BPA consortium study. Toxicol. Appl. Pharmacol. 347, 1–9 (2018).
Arambula, S. E., Jima, D. & Patisaul, H. B. Prenatal bisphenol A (BPA) exposure alters the transcriptome of the neonate rat amygdala in a sex-specific manner: a CLARITY-BPA consortium study. Neurotoxicology 65, 207–220 (2018).
Gear, R., Kendziorski, J. A. & Belcher, S. M. Effects of bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: a CLARITY-BPA study. Toxicol. Lett. 275, 123–135 (2017).
Arambula, S. E., Fuchs, J., Cao, J. & Patisaul, H. B. Effects of perinatal bisphenol A exposure on the volume of sexually-dimorphic nuclei of juvenile rats: a CLARITY-BPA consortium study. Neurotoxicology 63, 33–42 (2017).
Johnson, S. A. et al. Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: a CLARITY-BPA study. Horm. Behav. 80, 139–148 (2016).
Arambula, S. E., Belcher, S. M., Planchart, A., Turner, S. D. & Patisaul, H. B. Impact of low dose oral exposure to bisphenol A (BPA) on the neonatal rat hypothalamic and hippocampal transcriptome: a CLARITY-BPA consortium study. Endocrinology 157, 3856–3872 (2016).
Rebuli, M. E. et al. Impact of low-dose oral exposure to bisphenol A (BPA) on juvenile and adult rat exploratory and anxiety behavior: a CLARITY-BPA consortium study. Toxicol. Sci. 148, 341–354 (2015).
National Toxicology Program. Draft NTP research report on the CLARITY-BPA core study: a perinatal and chronic extended-dose-range study of bisphenol A in rats. NIH.gov https://ntp.niehs.nih.gov/ntp/about_ntp/rrprp/2018/april/rr09peerdraft.pdf (2018).
US Food & Drug Administration. Statement from Stephen Ostroff M.D., Deputy Commissioner for Foods andVeterinary Medicine, on National Toxicology Program draft report on bisphenol A. FDA.gov https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm598100.htm (2018).
National Toxicology Program. NTP research report on the CLARITY-BPA core study: a perinatal and chronic extended-dose-range study of bisphenol A in rats. https://ntp.niehs.nih.gov/ntp/results/pubs/rr/reports/rr09_508.pdf (2018).
Delclos, K. B. Bisphenol A: toxicology and pharmacokinetic data to inform on-going safety assessements. FDA.gov https://www.fda.gov/ScienceResearch/AboutScienceResearchatFDA/ucm621121.htm (2018).
Heindel, J. J. et al. NIEHS/FDA CLARITY-BPA research program update. Reprod. Toxicol. 58, 33–44 (2015).
Vandenberg, L. N., Welshons, W. V., Vom Saal, F. S., Toutain, P. L. & Myers, J. P. Should oral gavage be abandoned in toxicity testing of endocrine disruptors? Environ. Health 13, 46 (2014).
vom Saal, F. S. et al. The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of bisphenol A. Birth Defects Res. A 73, 140–145 (2005).
Vandenberg, L. N. et al. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod. Toxicol. 38, 1–15 (2013).
vom Saal, F. S. et al. Flawed experimental design reveals the need for guidelines requiring appropriate positive controls in endocrine disruption research. Toxicol. Sci. 115, 612–613 (2010).
vom Saal, F. S. & Welshons, W. V. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ. Res. 100, 50–76 (2006).
Wetherill, Y. B. et al. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 24, 178–198 (2007).
Petitti, D. B. Clinical practice. Combination estrogen-progestin oral contraceptives. N. Engl. J. Med. 349, 1443–1450 (2003).
Schug, T. T. et al. A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program. Reprod. Toxicol. 40, 35–40 (2013).
Vandenberg, L. N., Hunt, P. A., Myers, J. P. & Vom Saal, F. S. Human exposures to bisphenol A: mismatches between data and assumptions. Rev. Environ. Health 28, 37–58 (2013).
Churchwell, M. I. et al. Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in sprague dawley rats. Toxicol. Sci. 139, 4–20 (2014).
Cao, J. et al. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol. Sci. 133, 157–173 (2013).
Tyl, R. W. In honor of the TeratologySociety’s 50th anniversary: the role of Teratology Society members in the development and evolution of in vivo developmental toxicity test guidelines. Birth Defects Res. C 90, 99–102 (2010).
Klimisch, H. J., Andreae, M. & Tillmann, U. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul. Toxicol. Pharmacol. 25, 1–5 (1997).
Vandenberg, L. N. et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455 (2012).
National Toxicology Program. Toxicology and carcinogenesis study of ethinyl estradiol in Sprague-Dawley rats (feed study). NIH.gov https://ntp.niehs.nih.gov/go/tr548abs (2010).
National Toxicology Program. Toxicology and carcinogenesis studies of genistein in Sprague-Dawley rats (feed study). NIH.gov https://ntp.niehs.nih.gov/go/tr545abs (2008).
Catanese, M. C. & Vandenberg, L. N. Developmental estrogen exposures and disruptions to maternal behavior and brain: effects of ethinyl estradiol, a common positive control. Horm. Behav. 101, 113–124 (2018).
Catanese, M. C. & Vandenberg, L. N. Low doses of 17α-ethinyl estradiol alter the maternal brain and induce stereotypies in CD-1 mice exposed during pregnancy and lactation. Reprod. Toxicol. 73, 20–29 (2017).
Shyu, C., Cavileer, T. D., Nagler, J. J. & Ytreberg, F. M. Computational estimation of rainbow trout estrogen receptor binding affinities for environmental estrogens. Toxicol. Appl. Pharmacol. 250, 322–326 (2011).
Beronius, A., Hanberg, A., Zilliacus, J. & Ruden, C. Bridging the gap between academic research and regulatory health risk assessment of endocrine disrupting chemicals. Curr. Opin. Pharmacol. 19, 99–104 (2014).
Cheong, A. et al. Gene expression and DNA methylation changes in the hypothalamus and hippocampus of adult rats developmentally exposed to bisphenol A or ethinyl estradiol: a CLARITY-BPA consortium study. Epigenetics 13, 704–720 (2018).
Prins, G. S. et al. Evaluation of bisphenol A (BPA) exposures on prostate stem cell homeostasis and prostate cancer risk in the NCTR-Sprague-Dawley rat: an NIEHS/FDA CLARITY-BPA consortium study. Environ. Health Perspect. 126, 117001 (2018).
Prins, G. S., Patisaul, H. B., Belcher, S. M. & Vandenberg, L. N. CLARITY-BPA academic laboratory studies identify consistent low-dose bisphenol A effects on multiple organ systems. Basic Clin. Pharmacol. Toxicol. https://doi.org/10.1111/bcpt.13125 (2018).
Woodruff, T. J. et al. Meeting report: moving upstream-evaluating adverse upstream end points for improved risk assessment and decision-making. Environ. Health Perspect. 116, 1568–1575 (2008).
Allard, P. & Colaiacovo, M. P. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc. Natl Acad. Sci. USA 107, 20405–20410 (2010).
Lundby, Z., Camacho, J. & Allard, P. Fast functional germline and epigenetic assays in the nematode. Caenorhabditis elegans. Methods Mol. Biol. 1473, 99–107 (2016).
Souder, J. P. & Gorelick, D. A. Assaying uptake of endocrine disruptor compounds in zebrafish embryos and larvae. Comp. Biochem. Physiol. C 208, 105–113 (2018).
Moreman, J. et al. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Technol. 51, 12796–12805 (2017).
Kinch, C. D., Ibhazehiebo, K., Jeong, J. H., Habibi, H. R. & Kurrasch, D. M. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc. Natl Acad. Sci. USA 112, 1475–1480 (2015).
Corrales, J. et al. Global assessment of bisphenol A in the environment: review and analysis of its occurrence and bioaccumulation. Dose Response 13, 1559325815598308 (2015).
Covaci, A. et al. Urinary BPA measurements in children and mothers from six European member states: overall results and determinants of exposure. Environ. Res. 141, 77–85 (2015).
Acknowledgements
The authors gratefully acknowledge C. Lawson for assistance with figures and tables. The authors are supported by the NIH National Institute of Environmental Health Sciences grants K22ES025811 (L.N.V.), R56 ES013527 (P.A.H.) and RO1 ES023254 and R56 ES020662 (A.C.G.). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders played no role in the writing of the report or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
L.N.V., P.A.H. and A.C.G. have received travel reimbursement from universities, governments, non-governmental organizations and industry to speak about endocrine-disrupting chemicals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vandenberg, L.N., Hunt, P.A. & Gore, A.C. Endocrine disruptors and the future of toxicology testing — lessons from CLARITY–BPA. Nat Rev Endocrinol 15, 366–374 (2019). https://doi.org/10.1038/s41574-019-0173-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0173-y
This article is cited by
-
Science, Values, and the New Demarcation Problem
Journal for General Philosophy of Science (2023)
-
The antioxidant effects of coenzyme Q10 on albino rat testicular toxicity and apoptosis triggered by bisphenol A
Environmental Science and Pollution Research (2023)
-
Characterisation and analysis of key studies used to restrict substances under REACH
Environmental Sciences Europe (2022)
-
Determination of bisphenol-A in plastic bottled water in markets of Zanjan, Iran
International Journal of Environmental Science and Technology (2022)
-
Effects of bisphenol a on hematological, serum biochemical, and histopathological biomarkers in bighead carp (Aristichthys nobilis) under long-term exposure
Environmental Science and Pollution Research (2022)