Abstract
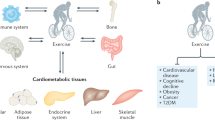
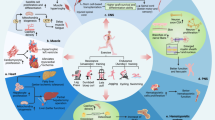
Osteoporosis, a condition of skeletal decline that undermines quality of life, is treated with pharmacological interventions that are associated with poor adherence and adverse effects. Complicating efforts to improve clinical outcomes, the incidence of obesity is increasing, predisposing the population to a range of musculoskeletal complications and metabolic disorders. Pharmacological management of obesity has yet to deliver notable reductions in weight and debilitating complications are rarely avoided. By contrast, exercise shows promise as a non-invasive and non-pharmacological method of regulating both osteoporosis and obesity. The principal components of exercise — mechanical signals — promote bone and muscle anabolism while limiting formation and expansion of fat mass. Mechanical regulation of bone and marrow fat might be achieved by regulating functions of differentiated cells in the skeletal tissue while biasing lineage selection of their common progenitors — mesenchymal stem cells. An inverse relationship between adipocyte versus osteoblast fate selection from stem cells is implicated in clinical conditions such as childhood obesity and increased marrow adiposity in type 2 diabetes mellitus, as well as contributing to skeletal frailty. Understanding how exercise-induced mechanical signals can be used to improve bone quality while decreasing fat mass and metabolic dysfunction should lead to new strategies to treat chronic diseases such as osteoporosis and obesity.
Key points
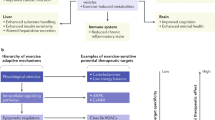
Ageing and inactivity each contribute towards a local and systemic environment conducive to poor bone quality, increased systemic adiposity, marrow adipogenesis and inflammation.
Mesenchymal stem cells and their lineage-differentiated progeny (for example, osteoblasts) are mechanosensitive, such that increased mechanical signals (such as exercise) stimulate muscle and bone anabolism.
Mechanical signals suppress obesity end points, including fat gain, adipocyte lipid acquisition, chronic inflammation and indices associated with type 2 diabetes mellitus.
Transduction of mechanical signals across the plasma membrane of stem cells into the nucleus activates signalling cascades and cytoskeletal adaptations to initiate osteogenic, chondrogenic and myogenic differentiation and inhibit adipocyte differentiation.
Mechanical signals, such as those induced through low-intensity vibration, need not be large in magnitude, or long in duration, to influence bone or fat phenotypes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ruff, C. B., Larsen, C. S. & Hayes, W. C. Structural changes in the femur with the transition to agriculture on the Georgia coast. Am. J. Phys. Anthropol. 64, 125–136 (1984).
Larsen, C. S. Biological changes in human-populations with agriculture. Annu. Rev. Anthropol. 24, 185–213 (1995).
Ruff, C. B. Gracilization of the modern human skeleton — the latent strength in our slender bones teaches lessons about human lives, current and past. Am. Sci. 94, 508–514 (2006).
Nowlan, N. C., Jepsen, K. J. & Morgan, E. F. Smaller, weaker, and less stiff bones evolve from changes in subsistence strategy. Osteoporos. Int. 22, 1967–1980 (2011).
Bilezikian, J. P. Osteoporosis in men. J. Clin. Endocrinol. Metab. 84, 3431–3434 (1999).
Hu, F. B., Li, T. Y., Colditz, G. A., Willett, W. C. & Manson, J. E. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 289, 1785–1791 (2003).
Manson, J. E., Skerrett, P. J., Greenland, P. & VanItallie, T. B. The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians. Arch. Intern. Med. 164, 249–258 (2004).
Watson, S. L. et al. High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J. Bone Miner. Res. 33, 211–220 (2018).
Wright, N. C. et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 29, 2520–2526 (2014).
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 285, 785–795 (2001).
Brown, M. Skeletal muscle and bone: effect of sex steroids and aging. Adv. Physiol. Educ. 32, 120–126 (2008).
Compston, J. E. Sex steroids and bone. Physiol. Rev. 81, 419–447 (2001).
Manolagas, S. C., O’Brien, C. A. & Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 9, 699–712 (2013).
Rosenberg, I. H. Sarcopenia: origins and clinical relevance. Clin. Geriatr. Med. 27, 337–339 (2011).
Black, D. M. & Rosen, C. J. Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 374, 254–262 (2016).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Shane, E. et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 29, 1–23 (2014).
Siris, E. S. et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin. Proc. 81, 1013–1022 (2006).
Cramer, J. A., Gold, D. T., Silverman, S. L. & Lewiecki, E. M. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos. Int. 18, 1023–1031 (2007).
Khosla, S. et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J. Bone Miner. Res. 32, 424–430 (2016).
Centers for Disease Control and Prevention. Childhood obesity facts. CDC http://www.cdc.gov/obesity/data/childhood.html (2018).
Ogden, C. L., Carroll, M. D., Kit, B. K. & Flegal, K. M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311, 806–814 (2014).
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Van Gaal, L. F., Mertens, I. L. & De Block, C. E. Mechanisms linking obesity with cardiovascular disease. Nature 444, 875–880 (2006).
Wearing, S. C., Hennig, E. M., Byrne, N. M., Steele, J. R. & Hills, A. P. The biomechanics of restricted movement in adult obesity. Obes. Rev. 7, 13–24 (2006).
Ko, S., Stenholm, S. & Ferrucci, L. Characteristic gait patterns in older adults with obesity—results from the Baltimore Longitudinal Study of Aging. J. Biomech. 43, 1104–1110 (2010).
Messier, S. P. Osteoarthritis of the knee and associated factors of age and obesity: effects on gait. Med. Sci. Sports Exerc. 26, 1446–1452 (1994).
Felson, D. T., Anderson, J. J., Naimark, A., Walker, A. M. & Meenan, R. F. Obesity and knee osteoarthritis. The Framingham Study. Ann. Intern. Med. 109, 18–24 (1988).
Hart, D. J. & Spector, T. D. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J. Rheumatol 20, 331–335 (1993).
Calle, E. E. & Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004).
Lashinger, L. M., Ford, N. A. & Hursting, S. D. Interacting inflammatory and growth factor signals underlie the obesity-cancer link. J. Nutr. 144, 109–113 (2014).
International Agency for Research on Cancer, Stewart, B. W. & Wild, C. P. World cancer report 2014. WHO https://www.who.int/cancer/publications/WRC_2014/en/ (2014).
Olson, O. C., Quail, D. F. & Joyce, J. A. Obesity and the tumor microenvironment. Science 358, 1130–1131 (2017).
Tilg, H. & Moschen, A. R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 6, 772–783 (2006).
Adler, B. J., Kaushansky, K. & Rubin, C. T. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat. Rev. Endocrinol. 10, 737–748 (2014).
Kennedy, D. E. & Knight, K. L. Bone marrow fat induces inflammation that inhibits B lymphopoiesis. J. Immunol. 196 (Suppl), 122.11 (2016).
Singh, L., Tyagi, S., Myers, D. & Duque, G. Good, bad, or ugly: the biological roles of bone marrow fat. Curr. Osteoporos. Rep. 16, 130–137 (2018).
National Osteoporosis Foundation. Exercise for your bone health. NOF https://cdn.nof.org/wp-content/uploads/2016/02/Exercise-for-Your-Bone-Health.pdf (2013).
Styner, M. et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 64, 39–46 (2014).
Bortz, W. M. 2nd. Disuse and aging. JAMA 248, 1203–1208 (1982).
Wolff, J. The Law Of Bone Remodeling (Springer, 1986).
Lang, T. et al. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J. Bone Miner. Res. 19, 1006–1012 (2004).
Jones, H. H., Priest, J. D., Hayes, W. C., Tichenor, C. C. & Nagel, D. A. Humeral hypertrophy in response to exercise. J. Bone Joint Surg. Am. 59, 204–208 (1977).
Heinonen, A. et al. Bone mineral density in female athletes representing sports with different loading characteristics of the skeleton. Bone 17, 197–203 (1995).
Vlachopoulos, D. et al. Longitudinal adaptations of bone mass, geometry, and metabolism in adolescent male athletes: the PRO-BONE study. J. Bone Miner. Res. 32, 2269–2277 (2017).
Gabel, L., Macdonald, H. M., Nettlefold, L. & McKay, H. A. Physical activity, sedentary time, and bone strength from childhood to early adulthood: a mixed longitudinal HR-pQCT study. J. Bone Miner. Res. 32, 1525–1536 (2017).
Leichter, I. et al. Gain in mass density of bone following strenuous physical activity. J. Orthop. Res. 7, 86–90 (1989).
McKay, H. A. et al. “Bounce at the Bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br. J. Sports Med. 39, 521–526 (2005).
Heinonen, A., Sievanen, H., Kannus, P., Oja, P. & Vuori, I. Effects of unilateral strength training and detraining on bone mineral mass and estimated mechanical characteristics of the upper limb bones in young women. J. Bone Miner. Res. 11, 490–501 (1996).
Rubin, C. T., Seeherman, H., Qin, Y. X. & Gross, T. S. The mechanical consequences of load bearing in the equine third metacarpal across speed and gait: the nonuniform distributions of normal strain, shear strain, and strain energy density. FASEB J. 27, 1887–1894 (2013).
Rubin, C. et al. Differentiation of the bone-tissue remodeling response to axial and torsional loading in the turkey ulna. J. Bone Joint Surg. Am. 78, 1523–1533 (1996).
Patel, V. S. et al. Incorporating refractory period in mechanical stimulation mitigates obesity-induced adipose tissue dysfunction in adult mice. Obesity 25, 1745–1753 (2017).
Wallace, B. A. & Cumming, R. G. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif. Tissue Int. 67, 10–18 (2000).
Warden, S. J. et al. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J. Bone Miner. Res. 20, 809–816 (2005).
Rubin, C. T. & Lanyon, L. E. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 37, 411–417 (1985).
Tjandrawinata, R. R., Vincent, V. L. & Hughes-Fulford, M. Vibrational force alters mRNA expression in osteoblasts. FASEB J. 11, 493–497 (1997).
Ko, K. S. & McCulloch, C. A. Intercellular mechanotransduction: cellular circuits that coordinate tissue responses to mechanical loading. Biochem. Biophys. Res. Commun. 285, 1077–1083 (2001).
Rubin, J., Rubin, C. & Jacobs, C. R. Molecular pathways mediating mechanical signaling in bone. Gene 367, 1–16 (2006).
Thompson, W. R. et al. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Sci. Rep. 5, 11049 (2015).
Uzer, G. et al. Cell mechanosensitivity to extremely low-magnitude signals is enabled by a LINCed nucleus. Stem Cells 33, 2063–2076 (2015).
Uzer, G., Fuchs, R. K., Rubin, J. & Thompson, W. R. Concise review: plasma and nuclear membranes convey mechanical information to regulate mesenchymal stem cell lineage. Stem Cells 34, 1455–1463 (2016).
Case, N. & Rubin, J. Beta-catenin—a supporting role in the skeleton. J. Cell. Biochem. 110, 545–553 (2010).
Sen, B. et al. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells 29, 1829–1836 (2011).
Sen, B. et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology 149, 6065–6075 (2008).
Samelson, E. J. et al. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: Framingham HR-pQCT study. J. Bone Miner. Res. 33, 54–62 (2018).
Murfee, W. L. et al. High-frequency, low-magnitude vibrations suppress the number of blood vessels per muscle fiber in mouse soleus muscle. J. Appl. Physiol. 98, 2376–2380 (2005).
Case, N. et al. Mechanical input restrains PPARgamma2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone 52, 454–464 (2013).
Luu, Y. K. et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone Miner. Res. 24, 50–61 (2009).
Styner, M., Sen, B., Xie, Z., Case, N. & Rubin, J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J. Cell. Biochem. 111, 1042–1050 (2010).
Sen, B. et al. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J. Biomech. 44, 593–599 (2011).
Globus, R. K., Bikle, D. D. & Morey-Holton, E. The temporal response of bone to unloading. Endocrinology 118, 733–742 (1986).
Bikle, D. D., Sakata, T. & Halloran, B. P. The impact of skeletal unloading on bone formation. Gravit. Space Biol. Bull. 16, 45–54 (2003).
Rubin, C., Xu, G. & Judex, S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 15, 2225–2229 (2001).
Rubin, C. et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J. Bone Miner. Res. 17, 349–357 (2002).
Judex, S. et al. Genetically linked site-specificity of disuse osteoporosis. J. Bone Miner. Res. 19, 607–613 (2004).
Squire, M., Brazin, A., Keng, Y. & Judex, S. Baseline bone morphometry and cellular activity modulate the degree of bone loss in the appendicular skeleton during disuse. Bone 42, 341–349 (2008).
Trudel, G. et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J. Appl. Physiol. 107, 540–548 (2009).
Rubin, J. et al. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J. Biol. Chem. 278, 34018–34025 (2003).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
Stattin, K., Michaelsson, K., Larsson, S. C., Wolk, A. & Byberg, L. Leisure-time physical activity and risk of fracture: a cohort study of 66,940 men and women. J. Bone Miner. Res. 32, 1599–1606 (2017).
Lindstrom, J. et al. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 26, 3230–3236 (2003).
Hu, F. B. et al. Adiposity as compared with physical activity in predicting mortality among women. N. Engl. J. Med. 351, 2694–2703 (2004).
Hinton, P. S., Nigh, P. & Thyfault, J. Effectiveness of resistance training or jumping-exercise to increase bone mineral density in men with low bone mass: a 12-month randomized, clinical trial. Bone 79, 203–212 (2015).
Dalsky, G. P. et al. Weight-bearing exercise training and lumbar bone-mineral content in postmenopausal women. Ann. Intern. Med. 108, 824–828 (1988).
Nilsson, M., Sundh, D., Mellstrom, D. & Lorentzon, M. Current physical activity is independently associated with cortical bone size and bone strength in elderly Swedish women. J. Bone Miner. Res. 32, 473–485 (2017).
Ensrud, K. E. & Crandall, C. J. Osteoporosis. Ann. Intern. Med. 167, ITC17–ITC32 (2017).
Ness, K. K. et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk. Lymphoma 56, 1004–1011 (2015).
Maratova, K. et al. Muscle functions and bone strength are impaired in adolescents with type 1 diabetes. Bone 106, 22–27 (2018).
Joyce, E. D. et al. Association of muscle strength and bone mineral density in adult survivors of childhood acute lymphoblastic leukemia. Arch. Phys. Med. Rehabil. 92, 873–879 (2011).
Ness, K. K. et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J. Clin. Oncol. 31, 4496–4503 (2013).
Huang, H. P. et al. Adherence to prescribed exercise time and intensity declines as the exercise program proceeds: findings from women under treatment for breast cancer. Support. Care Cancer 23, 2061–2071 (2015).
Hemmatian, H., Bakker, A. D., Klein-Nulend, J. & van Lenthe, G. H. Aging, osteocytes, and mechanotransduction. Curr. Osteoporos. Rep. 15, 401–411 (2017).
Bonewald, L. F. The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 (2011).
Joldersma, M., Klein-Nulend, J., Oleksik, A. M., Heyligers, I. C. & Burger, E. H. Estrogen enhances mechanical stress-induced prostaglandin production by bone cells from elderly women. Am. J. Physiol. Endocrinol. Metab. 280, E436–E442 (2001).
Joldersma, M., Burger, E. H., Semeins, C. M. & Klein-Nulend, J. Mechanical stress induces COX-2 mRNA expression in bone cells from elderly women. J. Biomech. 33, 53–61 (2000).
Sterck, J. G., Klein-Nulend, J., Lips, P. & Burger, E. H. Response of normal and osteoporotic human bone cells to mechanical stress in vitro. Am. J. Physiol. 274, E1113–E1120 (1998).
Rubin, C. T., Bain, S. D. & McLeod, K. J. Suppression of the osteogenic response in the aging skeleton. Calcif. Tissue Int. 50, 306–313 (1992).
Willie, B. M. et al. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone 55, 335–346 (2013).
Strube, P. et al. Sex-specific compromised bone healing in female rats might be associated with a decrease in mesenchymal stem cell quantity. Bone 45, 1065–1072 (2009).
Wiley, C. D. & Campisi, J. From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab. 23, 1013–1021 (2016).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23, 1072–1079 (2017).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Chiche, A. et al. Injury-induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell 20, 407–414 (2017).
Akunuru, S. & Geiger, H. Aging, clonality, and rejuvenation of hematopoietic stem cells. Trends Mol. Med. 22, 701–712 (2016).
Qin, Y. et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J. Biol. Chem. 292, 11021–11033 (2017).
Evans, W. J. & Campbell, W. W. Sarcopenia and age-related-changes in body-composition and functional-capacity. J. Nutr. 123, 465–468 (1993).
Lamberts, S. W., van den Beld, A. W. & van der Lely, A. J. The endocrinology of aging. Science 278, 419–424 (1997).
Hu, Z. Y. et al. MicroRNA-29 induces cellular senescence in aging muscle through multiple signaling pathways. Aging 6, 160–175 (2014).
Evans, W. J. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 91, 1123S–1127S (2010).
Morse, C. I., Thom, J. M., Reeves, N. D., Birch, K. M. & Narici, M. V. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J. Appl. Physiol. 99, 1050–1055 (2005).
[No authors listed.] Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People [Japanese]. Nihon Ronen Igakkai Zasshi 49, 788–805 (2012).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423 (2010).
Han, A., Bokshan, S. L., Marcaccio, S. E., DePasse, J. M. & Daniels, A. H. Diagnostic criteria and clinical outcomes in sarcopenia research: a literature review. J. Clin. Med. 7, 70 (2018).
Janssen, I., Shepard, D. S., Katzmarzyk, P. T. & Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 52, 80–85 (2004).
Cruz-Jentoft, A. J. et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43, 748–759 (2014).
Phillips, S. K., Rook, K. M., Siddle, N. C., Bruce, S. A. & Woledge, R. C. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin. Sci. 84, 95–98 (1993).
Huang, J. et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/beta-catenin pathway. JBMR Plus 1, 86–100 (2017).
Marks, A. R. Intracellular calcium-release channels: regulators of cell life and death. Am. J. Physiol. 272, H597–H605 (1997).
Wehrens, X. H. et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113, 829–840 (2003).
Bellinger, A. M. et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 15, 325–330 (2009).
Andersson, D. C. et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 14, 196–207 (2011).
Guise, T. A. et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 12, 6213S–6216S (2006).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Janssens, K. et al. Mutations in the gene encoding the latency-associated peptide of TGF-beta 1 cause Camurati-Engelmann disease. Nat. Genet. 26, 273–275 (2000).
Hauschka, P. V., Mavrakos, A. E., Iafrati, M. D., Doleman, S. E. & Klagsbrun, M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J. Biol. Chem. 261, 12665–12674 (1986).
Waning, D. L. et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat. Med. 21, 1262–1271 (2015).
Mundy, G. R., Yoneda, T. & Hiraga, T. Preclinical studies with zoledronic acid and other bisphosphonates: impact on the bone microenvironment. Semin. Oncol. 28, 35–44 (2001).
Waning, D. L. & Guise, T. A. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin. Cancer Res. 20, 3071–3077 (2014).
Tuttle, L. J., Sinacore, D. R., Cade, W. T. & Mueller, M. J. Lower physical activity is associated with higher intermuscular adipose tissue in people with type 2 diabetes and peripheral neuropathy. Phys. Ther. 91, 923–930 (2011).
Bittel, D. C. et al. Adipose tissue content, muscle performance and physical function in obese adults with type 2 diabetes mellitus and peripheral neuropathy. J. Diabetes Compl. 29, 250–257 (2015).
Papadakis, M. A. et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann. Intern. Med. 124, 708–716 (1996).
Brioche, T. et al. Growth hormone replacement therapy prevents sarcopenia by a dual mechanism: improvement of protein balance and of antioxidant defenses. J. Gerontol. A Biol. Sci. Med. Sci. 69, 1186–1198 (2014).
Jorgensen, J. O. et al. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet 1, 1221–1225 (1989).
Mikkelsen, U. R. et al. Skeletal muscle morphology and regulatory signalling in endurance-trained and sedentary individuals: the influence of ageing. Exp. Gerontol. 93, 54–67 (2017).
Rogers, M. A. & Evans, W. J. Changes in skeletal muscle with aging: effects of exercise training. Exerc. Sport Sci. Rev. 21, 65–102 (1993).
Ryan, A. S. Insulin resistance with aging: effects of diet and exercise. Sports Med. 30, 327–346 (2000).
Ivy, J. L. Role of exercise training in the prevention and treatment of insulin resistance and non-insulin-dependent diabetes mellitus. Sports Med. 24, 321–336 (1997).
Baar, K. & Esser, K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am. J. Physiol. 276, C120–C127 (1999).
Kubica, N., Bolster, D. R., Farrell, P. A., Kimball, S. R. & Jefferson, L. S. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J. Biol. Chem. 280, 7570–7580 (2005).
Ji, L. L., Gomez-Cabrera, M. C., Steinhafel, N. & Vina, J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 18, 1499–1506 (2004).
Senf, S. M., Dodd, S. L. & Judge, A. R. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am. J. Physiol. Cell Physiol. 298, C38–C45 (2010).
Kavazis, A. N., Smuder, A. J. & Powers, S. K. Effects of short-term endurance exercise training on acute doxorubicin-induced FoxO transcription in cardiac and skeletal muscle. J. Appl. Physiol. 117, 223–230 (2014).
Iyer, S. et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J. Clin. Invest. 123, 3409–3419 (2013).
Stitt, T. N. et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403 (2004).
Bodine, S. C. et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3, 1014–1019 (2001).
Salanova, M. et al. Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse. Redox Biol. 1, 514–526 (2013).
Salanova, M., Schiffl, G., Rittweger, J., Felsenberg, D. & Blottner, D. Ryanodine receptor type-1 (RyR1) expression and protein S-nitrosylation pattern in human soleus myofibres following bed rest and exercise countermeasure. Histochem. Cell Biol. 130, 105–118 (2008).
Frechette, D. M., Krishnamoorthy, D., Adler, B. J., Chan, M. E. & Rubin, C. T. Diminished satellite cells and elevated adipogenic gene expression in muscle as caused by ovariectomy are averted by low-magnitude mechanical signals. J. Appl. Physiol. 119, 27–36 (2015).
Frechette, D. M. et al. Mechanical signals protect stem cell lineage selection, preserving the bone and muscle phenotypes in obesity. Ann. NY Acad. Sci. 1409, 33–50 (2017).
Mera, P., Laue, K., Wei, J., Berger, J. M. & Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab. 5, 1042–1047 (2016).
Mera, P. et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 23, 1078–1092 (2016).
Febbraio, M. A., Hiscock, N., Sacchetti, M., Fischer, C. P. & Pedersen, B. K. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53, 1643–1648 (2004).
Karsenty, G. & Mera, P. Molecular bases of the crosstalk between bone and muscle. Bone 11, 43–49 (2017).
Messi, M. L. et al. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J. Gerontol. A Biol. Sci. Med. Sci. 71, 1273–1280 (2016).
Stewart, V. H., Saunders, D. H. & Greig, C. A. Responsiveness of muscle size and strength to physical training in very elderly people: a systematic review. Scand. J. Med. Sci. Sports 24, e1–e10 (2014).
Thompson, L. V. Effects of age and training on skeletal muscle physiology and performance. Phys. Ther. 74, 71–81 (1994).
Pedersen, B. K. & Saltin, B. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports 16 (Suppl. 1), 3–63 (2006).
Hedlund, P. B., Yanaihara, N. & Fuxe, K. Evidence for specific N-terminal galanin fragment binding sites in the rat brain. Eur. J. Pharmacol. 224, 203–205 (1992).
Kelly, O. J. & Gilman, J. C. Can unconventional exercise be helpful in the treatment, management and prevention of osteosarcopenic obesity? Curr. Aging Sci. 10, 106–121 (2017).
Tiedemann, A., O’Rourke, S., Sesto, R. & Sherrington, C. A. 12-week Iyengar yoga program improved balance and mobility in older community-dwelling people: a pilot randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 68, 1068–1075 (2013).
Courneya, K. S. et al. Predictors of adherence to different types and doses of supervised exercise during breast cancer chemotherapy. Int. J. Behav. Nutr. Phys. Act 11, 85 (2014).
Cox, C. L. et al. Modifying bone mineral density, physical function, and quality of life in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 65, e26929 (2018).
Schoenau, E., Neu, C. M., Beck, B., Manz, F. & Rauch, F. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J. Bone Miner. Res. 17, 1095–1101 (2002).
Hall, B. K. & Herring, S. W. Paralysis and growth of the musculoskeletal system in the embryonic chick. J. Morphol. 206, 45–56 (1990).
Sharir, A., Stern, T., Rot, C., Shahar, R. & Zelzer, E. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development 138, 3247–3259 (2011).
Hamrick, M. W., McGee-Lawrence, M. E. & Frechette, D. M. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front. Endocrinol. 7, 69 (2016).
Long, P., Liu, F., Piesco, N. P., Kapur, R. & Agarwal, S. Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone 30, 547–552 (2002).
Pagnotti, G. M. & Styner, M. Exercise regulation of marrow adipose tissue. Front. Endocrinol. 7, 94 (2016).
Cawthorn, W. P. & Scheller, E. L. Editorial: bone marrow adipose tissue: formation, function, and impact on health and disease. Front. Endocrinol. 8, 112 (2017).
Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115, 911–919; quiz 920 (2005).
Xue, Y. et al. Adipokines in psoriatic arthritis patients: the correlations with osteoclast precursors and bone erosions. PLOS ONE 7, e46740 (2012).
Patel, V. S., Ete Chan, M., Rubin, J. & Rubin, C. T. Marrow adiposity and hematopoiesis in aging and obesity: exercise as an intervention. Curr. Osteoporos. Rep. 16, 105–115 (2018).
Neumann, E., Junker, S., Schett, G., Frommer, K. & Muller-Ladner, U. Adipokines in bone disease. Nat. Rev. Rheumatol. 12, 296–302 (2016).
Kudo, O. et al. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 32, 1–7 (2003).
Cawthorn, W. P. et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 20, 368–375 (2014).
Basurto, L. et al. Adiponectin is associated with low bone mineral density in elderly men. Eur. J. Endocrinol. 160, 289–293 (2009).
Barbour, K. E. et al. The effects of adiponectin and leptin on changes in bone mineral density. Osteoporosis Int. 23, 1699–1710 (2012).
Liu, L. et al. Rosiglitazone inhibits bone regeneration and causes significant accumulation of fat at sites of new bone formation. Calcif. Tissue Int. 91, 139–148 (2012).
Styner, M. et al. Exercise regulation of marrow fat in the setting of PPARgamma agonist treatment in female C57BL/6 mice. Endocrinology 156, 2753–2761 (2015).
Grey, A. et al. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. Eur. J. Endocrinol. 166, 1087–1091 (2012).
Kannel, W. B., Gordon, T. & Castelli, W. P. Obesity, lipids, and glucose intolerance. The Framingham Study. Am. J. Clin. Nutr. 32, 1238–1245 (1979).
Adler, B. J., Green, D. E., Pagnotti, G. M., Chan, M. E. & Rubin, C. T. High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLOS ONE 9, e90639 (2014).
Ambrosi, T. H. et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20, 771–784 (2017).
Doucette, C. R. et al. A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J. Cell. Physiol. 230, 2032–2037 (2015).
Scheller, E. L. et al. Changes in skeletal integrity and marrow adiposity during high-fat diet and after weight loss. Front. Endocrinol. 7, 102 (2016).
Styner, M. et al. Exercise decreases marrow adipose tissue through β-oxidation in obese running mice. J. Bone Miner. Res. 32, 1692–1702 (2017).
Trottier, M. D., Naaz, A., Li, Y. & Fraker, P. J. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc. Natl Acad. Sci. USA 109, 7622–7629 (2012).
do Carmo, L. S. et al. A high-fat diet increases interleukin-3 and granulocyte colony-stimulating factor production by bone marrow cells and triggers bone marrow hyperplasia and neutrophilia in Wistar rats. Exp. Biol. Med. 238, 375–384 (2013).
van den Berg, S. M. et al. Diet-induced obesity in mice diminishes hematopoietic stem and progenitor cells in the bone marrow. FASEB J. 30, 1779–1788 (2016).
Singer, K. et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol. Metab. 3, 664–675 (2014).
Chan, M. E., Adler, B. J., Green, D. E. & Rubin, C. T. Bone structure and B cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals. FASEB J. 26, 4855–4863 (2012).
Nanji, A. A. & Freeman, J. B. Relationship between body weight and total leukocyte count in morbid obesity. Am. J. Clin. Pathol. 84, 346–347 (1985).
Womack, J. et al. Obesity and immune cell counts in women. Metabolism 56, 998–1004 (2007).
Ryder, E. et al. Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab. Syndr. 8, 197–204 (2014).
Pecht, T., Gutman-Tirosh, A., Bashan, N. & Rudich, A. Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes. Rev. 15, 322–337 (2014).
Xu, X. et al. Obesity is associated with more activated neutrophils in African American male youth. Int. J. Obes. 39, 26–32 (2015).
Ghigliotti, G. et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation 37, 1337–1353 (2014).
Nteeba, J., Ortinau, L. C., Perfield, J. W. 2nd & Keating, A. F. Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol. Reprod. Dev. 80, 948–958 (2013).
Caer, C. et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci. Rep. 7, 3000 (2017).
Rubin, C. T. et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc. Natl Acad. Sci. USA 104, 17879–17884 (2007).
Kahn, B. B. & Flier, J. S. Obesity and insulin resistance. J. Clin. Invest. 106, 473–481 (2000).
Qatanani, M. & Lazar, M. A. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Gene Dev. 21, 1443–1455 (2007).
Souza, P. P. & Lerner, U. H. The role of cytokines in inflammatory bone loss. Immunol. Invest. 42, 555–622 (2013).
Schett, G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Invest. 41, 1361–1366 (2011).
McLean, R. R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 7, 134–139 (2009).
Kimble, R. B. et al. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology 136, 3054–3061 (1995).
Rubin, C. T. & Lanyon, L. E. Regulation of bone formation by applied dynamic loads. J. Bone Joint Surg. Am. 66, 397–402 (1984).
O’Connor, J. A., Lanyon, L. E. & MacFie, H. The influence of strain rate on adaptive bone remodelling. J. Biomech. 15, 767–781 (1982).
Rath, B., Nam, J., Knobloch, T. J., Lannutti, J. J. & Agarwal, S. Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J. Biomech. 41, 1095–1103 (2008).
Poliachik, S. L., Threet, D., Srinivasan, S. & Gross, T. S. 32-wk-old C3H/HeJ mice actively respond to mechanical loading. Bone 42, 653–659 (2008).
Rubin, C., Turner, A. S., Bain, S., Mallinckrodt, C. & McLeod, K. Anabolism. Low mechanical signals strengthen long bones. Nature 412, 603–604 (2001).
Oxlund, B. S., Ortoft, G., Andreassen, T. T. & Oxlund, H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone 32, 69–77 (2003).
Tanaka, S. M. et al. Effects of broad frequency vibration on cultured osteoblasts. J. Biomech. 36, 73–80 (2003).
Garman, R., Gaudette, G., Donahue, L. R., Rubin, C. & Judex, S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J. Orthop. Res. 25, 732–740 (2007).
Wren, T. A. et al. Effect of high-frequency, low-magnitude vibration on bone and muscle in children with cerebral palsy. J. Pediatr. Orthop. 30, 732–738 (2010).
Pre, D. et al. The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. Bone 49, 295–303 (2011).
Robinson, T. L. et al. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J. Bone Miner. Res. 10, 26–35 (1995).
Verschueren, S. M. et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J. Bone Miner. Res. 19, 352–359 (2004).
Judex, S. & Rubin, C. T. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J. Musculoskelet. Neuronal Interact. 10, 3–11 (2010).
McGarry, J. G., Klein-Nulend, J., Mullender, M. G. & Prendergast, P. J. A comparison of strain and fluid shear stress in stimulating bone cell responses — a computational and experimental study. FASEB J. 19, 482–484 (2005).
Sikavitsas, V. I., Bancroft, G. N., Holtorf, H. L., Jansen, J. A. & Mikos, A. G. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc. Natl Acad. Sci. USA 100, 14683–14688 (2003).
Bancroft, G. N. et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc. Natl Acad. Sci. USA 99, 12600–12605 (2002).
Weinbaum, S., Cowin, S. C. & Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 27, 339–360 (1994).
Reich, K. M., Gay, C. V. & Frangos, J. A. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J. Cell. Physiol. 143, 100–104 (1990).
Qin, Y. X. & Hu, M. Intramedullary pressure induced by dynamic hydraulic pressure stimulation and its potential in treatment of osteopenia. Bone 48, S186 (2011).
Qin, Y. X. & Lam, H. Y. Intramedullary pressure and matrix strain induced by oscillatory skeletal muscle stimulation and its potential in adaptation. J. Biomech. 42, 140–145 (2009).
Zhang, P., Su, M., Liu, Y. L., Hsu, A. & Yokota, H. Knee loading dynamically alters intramedullary pressure in mouse femora. Bone 40, 538–543 (2007).
Chan, M. E., Uzer, G. & Rubin, C. T. The potential benefits and inherent risks of vibration as a non-drug therapy for the prevention and treatment of osteoporosis. Curr. Osteoporos. Rep. 11, 36–44 (2013).
Price, C., Zhou, X., Li, W. & Wang, L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J. Bone Miner. Res. 26, 277–285 (2011).
Coughlin, T. R. & Niebur, G. L. Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. J. Biomech. 45, 2222–2229 (2012).
Lim, K. T. et al. Physical stimulation-based osteogenesis: effect of secretion in vitro on fluid dynamic shear stress of human alveolar bone-derived mesenchymal stem cells. IEEE Trans. Nanobioscience 15, 881–890 (2016).
Williams, J. L., Iannotti, J. P., Ham, A., Bleuit, J. & Chen, J. H. Effects of fluid, shear-stress on bone-cells. Biorheology 31, 163–170 (1994).
Vainionpaa, A. et al. Intensity of exercise is associated with bone density change in premenopausal women. Osteoporosis Int. 17, 455–463 (2006).
Cabrita, G. J. M. et al. Hematopoietic stem cells: from the bone to the bioreactor. Trends Biotechnol. 21, 233–240 (2003).
Gordon, M. Stem cells handbook. Bone Marrow Transplant. 33, 1165 (2004).
Dickerson, D. A., Sander, E. A. & Nauman, E. A. Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects. Biomech. Model. Mechanobiol. 7, 191–202 (2008).
Bryant, J. D., David, T., Gaskell, P. H., King, S. & Lond, G. Rheology of bovine bone marrow. Proc. Inst. Mech. Eng. H 203, 71–75 (1989).
Blecha, L. D., Rakotomanana, L., Razafimahery, F., Terrier, A. & Pioletti, D. P. Mechanical interaction between cells and fluid for bone tissue engineering scaffold: modulation of the interfacial shear stress. J. Biomech. 43, 933–937 (2010).
Gimble, J. M., Robinson, C. E., Wu, X. & Kelly, K. A. The function of adipocytes in the bone marrow stroma: an update. Bone 19, 421–428 (1996).
Justesen, J. et al. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2, 165–171 (2001).
Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Thompson, W. R. et al. LARG GEF and ARHGAP18 orchestrate RhoA activity to control mesenchymal stem cell lineage. Bone 107, 172–180 (2018).
Sen, B. et al. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J. Bone Miner. Res. 29, 78–89 (2014).
Sen, B. et al. Intranuclear actin regulates osteogenesis. Stem Cells 33, 3065–3076 (2015).
Sen, B. et al. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J. Biol. Chem. 284, 34607–34617 (2009).
Lombardi, M. L. et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 286, 26743–26753 (2011).
Parsons, J. T., Horwitz, A. R. & Schwartz, M. A. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 (2010).
Burridge, K., Fath, K., Kelly, T., Nuckolls, G. & Turner, C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 4, 487–525 (1988).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010).
Turner, C. E., Glenney, J. R. Jr & Burridge, K. Paxillin: a new vinculin-binding protein present in focal adhesions. J. Cell Biol. 111, 1059–1068 (1990).
Pasapera, A. M., Schneider, I. C., Rericha, E., Schlaepfer, D. D. & Waterman, C. M. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 188, 877–890 (2010).
Machesky, L. M. & Insall, R. H. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347–1356 (1998).
Marchand, J. B., Kaiser, D. A., Pollard, T. D. & Higgs, H. N. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat. Cell Biol. 3, 76–82 (2001).
Mullins, R. D., Heuser, J. A. & Pollard, T. D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad. Sci. USA 95, 6181–6186 (1998).
Jaffe, A. B. & Hall, A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (2005).
Riddick, N., Ohtani, K. & Surks, H. K. Targeting by myosin phosphatase-RhoA interacting protein mediates RhoA/ROCK regulation of myosin phosphatase. J. Cell. Biochem. 103, 1158–1170 (2008).
Bhadriraju, K. et al. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp. Cell Res. 313, 3616–3623 (2007).
Thompson, W. R. et al. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells 31, 2528–2537 (2013).
Case, N. et al. Mechanical regulation of glycogen synthase kinase 3beta (GSK3beta) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein. J. Biol. Chem. 286, 39450–39456 (2011).
Stewart-Hutchinson, P. J., Hale, C. M., Wirtz, D. & Hodzic, D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp. Cell Res. 314, 1892–1905 (2008).
Neelam, S. et al. Direct force probe reveals the mechanics of nuclear homeostasis in the mammalian cell. Proc. Natl Acad. Sci. USA 112, 5720–5725 (2015).
Holaska, J. M., Kowalski, A. K. & Wilson, K. L. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLOS Biol. 2, E231 (2004).
Le, H. Q. et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 18, 864–875 (2016).
Sen, B. et al. Intranuclear actin structure modulates mesenchymal stem cell differentiation. Stem Cells 35, 1624–1635 (2017).
Kutscheidt, S. et al. FHOD1 interaction with nesprin-2G mediates TAN line formation and nuclear movement. Nat. Cell Biol. 16, 708–715 (2014).
Chancellor, T. J., Lee, J., Thodeti, C. K. & Lele, T. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 99, 115–123 (2010).
Chen, C. Y. et al. Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies. Cell 149, 565–577 (2012).
Thakar, K., May, C. K., Rogers, A. & Carroll, C. W. Opposing roles for distinct LINC complexes in regulation of the small GTPase RhoA. Mol. Biol. Cell 28, 182–191 (2017).
Uzer, G. et al. Sun-mediated mechanical LINC between nucleus and cytoskeleton regulates betacatenin nuclear access. J. Biomech. 74, 32–40 (2018).
Shiu, J. Y., Aires, L., Lin, Z. & Vogel, V. Nanopillar force measurements reveal actin-cap-mediated YAP mechanotransduction. Nat. Cell Biol. 20, 262–271 (2018).
Elosegui-Artola, A. et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410 (2017).
Koike, M. et al. beta-Catenin shows an overlapping sequence requirement but distinct molecular interactions for its bidirectional passage through nuclear pores. J. Biol. Chem. 279, 34038–34047 (2004).
Tolwinski, N. S. & Wieschaus, E. A nuclear function for armadillo/beta-catenin. PLOS Biol. 2, E95 (2004).
Neumann, S. et al. Nesprin-2 interacts with {alpha}-catenin and regulates Wnt signaling at the nuclear envelope. J. Biol. Chem. 285, 34932–34938 (2010).
Broers, J. L. et al. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum. Mol. Genet. 13, 2567–2580 (2004).
Stephens, A. D., Banigan, E. J., Adam, S. A., Goldman, R. D. & Marko, J. F. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol. Biol. Cell 28, 1984–1996 (2017).
Khatau, S. B. et al. The differential formation of the LINC-mediated perinuclear actin cap in pluripotent and somatic cells. PLOS ONE 7, e36689 (2012).
Vidal, C., Bermeo, S., Fatkin, D. & Duque, G. Role of the nuclear envelope in the pathogenesis of age-related bone loss and osteoporosis. Bonekey Rep. 1, 62 (2012).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004).
Bermeo, S., Vidal, C., Zhou, H. & Duque, G. Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/beta-catenin pathway. J. Cell. Biochem. 116, 2344–2353 (2015).
Constantinescu, D., Gray, H. L., Sammak, P. J., Schatten, G. P. & Csoka, A. B. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 24, 177–185 (2006).
Akter, R., Rivas, D., Geneau, G., Drissi, H. & Duque, G. Effect of lamin A/C knockdown on osteoblast differentiation and function. J. Bone Miner. Res. 24, 283–293 (2009).
Tong, J. et al. Lamin A/C deficiency is associated with fat infiltration of muscle and bone. Mech. Ageing Dev. 132, 552–559 (2011).
Li, W. et al. Decreased bone formation and osteopenia in lamin a/c-deficient mice. PLOS ONE 6, e19313 (2011).
Armstrong, V. J. et al. Estrogen receptor a is required for strain-related beta-catenin signaling in osteoblasts. J. Bone Miner. Res. 22, S95 (2007).
Bonewald, L. F. Mechanosensation and transduction in osteocytes. Bonekey Osteovision 3, 7–15 (2006).
Bradford, P. G., Gerace, K. V., Roland, R. L. & Chrzan, B. G. Estrogen regulation of apoptosis in osteoblasts. Physiol. Behav. 99, 181–185 (2010).
Jessop, H. L. et al. Mechanical strain and estrogen activate estrogen receptor alpha in bone cells. J. Bone Miner. Res. 16, 1045–1055 (2001).
Wehrle, E. et al. The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis. Model. Mech. 8, 93–104 (2015).
Guo, Y. et al. Mechanical strain promotes osteoblast ECM formation and improves its osteoinductive potential. Biomed. Eng. Online 11, 80 (2012).
Kamel, M. A. et al. Fluid flow shear stress and prostaglandin E2 activates beta-catenin signaling in MLO-Y4 osteocytic and 2T3 osteoblastic cells. J. Bone Miner. Res. 22, S375 (2007).
Srinivasan, S., Weimer, D. A., Agans, S. C., Bain, S. D. & Gross, T. S. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J. Bone Miner. Res. 17, 1613–1620 (2002).
Wright, L. E. et al. Single-limb irradiation induces local and systemic bone loss in a murine model. J. Bone Miner. Res. 30, 1268–1279 (2015).
Krishnamoorthy, D. et al. Marrow adipogenesis and bone loss that parallels estrogen deficiency is slowed by low-intensity mechanical signals. Osteoporos. Int. 27, 747–756 (2016).
Limonard, E. J. et al. Short-term effect of estrogen on human bone marrow fat. J. Bone Miner. Res. 30, 2058–2066 (2015).
Tuomilehto, J. et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344, 1343–1350 (2001).
Wallace, I. J. et al. Focal enhancement of the skeleton to exercise correlates with responsivity of bone marrow mesenchymal stem cells rather than peak external forces. J. Exp. Biol. 218, 3002–3009 (2015).
Bonewald, L. F. & Johnson, M. L. Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615 (2008).
Klein-Nulend, J., Semeins, C. M., Ajubi, N. E., Nijweide, P. J. & Burger, E. H. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts—correlation with prostaglandin upregulation. Biochem. Biophys. Res. Commun. 217, 640–648 (1995).
Qin, Y. X., Lin, W. & Rubin, C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann. Biomed. Eng. 30, 693–702 (2002).
Qin, Y. X., Kaplan, T., Saldanha, A. & Rubin, C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J. Biomech. 36, 1427–1437 (2003).
Rubin, C. T. & Lanyon, L. E. Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J. Orthop. Res. 5, 300–310 (1987).
Jarvinen, T. L. et al. Randomized controlled study of effects of sudden impact loading on rat femur. J. Bone Miner. Res. 13, 1475–1482 (1998).
Gross, T. S., Edwards, J. L., McLeod, K. J. & Rubin, C. T. Strain gradients correlate with sites of periosteal bone formation. J. Bone Miner. Res. 12, 982–988 (1997).
Lanyon, L. E. & Rubin, C. T. Static versus dynamic loads as an influence on bone remodelling. J. Biomech. 17, 897–905 (1984).
Reyes, M. L., Hernandez, M., Holmgren, L. J., Sanhueza, E. & Escobar, R. G. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. J. Bone Miner. Res. 26, 1759–1766 (2011).
Dumas, V. et al. Extracellular matrix produced by osteoblasts cultured under low-magnitude, high-frequency stimulation is favourable to osteogenic differentiation of mesenchymal stem cells. Calcif. Tissue Int. 87, 351–364 (2010).
Raso, V., Natale, V. M., Duarte, A. J., Greve, J. M. & Shephard, R. J. Immunological parameters in elderly women: correlations with aerobic power, muscle strength and mood state. Brain Behav. Immun. 26, 597–606 (2012).
Wenger, K. H. et al. Effect of whole-body vibration on bone properties in aging mice. Bone 47, 746–755 (2010).
Bacabac, R. G. et al. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J. 20, 858–864 (2006).
Sherk, V. D. et al. Acute bone marker responses to whole-body vibration and resistance exercise in young women. J. Clin. Densitom. 16, 104–109 (2013).
Pagnotti, G. M. et al. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone 51, 570–577 (2012).
Pagnotti, G. M. et al. Low intensity vibration mitigates tumor progression and protects bone quantity and quality in a murine model of myeloma. Bone 90, 69–79 (2016).
David, V. et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 148, 2553–2562 (2007).
Maddalozzo, G. F., Iwaniec, U. T., Turner, R. T., Rosen, C. J. & Widrick, J. J. Whole-body vibration slows the acquisition of fat in mature female rats. Int. J. Obes. 32, 1348–1354 (2008).
Huang, R. P., Rubin, C. T. & McLeod, K. J. Changes in postural muscle dynamics as a function of age. J. Gerontol. A Biol. Sci. Med. Sci. 54, B352–B357 (1999).
Adams, D. J. et al. Testing the daily stress stimulus theory of bone adaptation with natural and experimentally controlled strain histories. J. Biomech. 30, 671–678 (1997).
Fritton, S. P., McLeod, K. J. & Rubin, C. T. Quantifying the strain history of bone: spatial uniformity and self- similarity of low-magnitude strains. J. Biomech. 33, 317–325 (2000).
Rubin, C. et al. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone 30, 445–452 (2002).
Luu, Y. K. et al. Development of diet-induced fatty liver disease in the aging mouse is suppressed by brief daily exposure to low-magnitude mechanical signals. Int. J. Obes. 34, 401–405 (2010).
Rosen, C. J. et al. Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35, 1046–1058 (2004).
Mukherjee, S. et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J. Clin. Invest. 118, 491–504 (2008).
Luu, Y. K., Capilla, E., Pessin, J. E., Judex, S. & Rubin, C. T. in 2007 IEEE 33rd Annual Northeast Bioengineering Conference 203–204 (IEEE, Long Island, NY, 2007).
Klein-Nulend, J. et al. Donor age and mechanosensitivity of human bone cells. Osteoporosis Int. 13, 137–146 (2002).
Gilsanz, V. et al. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J. Bone Miner. Res. 21, 1464–1474 (2006).
McGee-Lawrence, M. E. et al. Whole-body vibration mimics the metabolic effects of exercise in male leptin receptor-deficient mice. Endocrinology 158, 1160–1171 (2017).
Robling, A. G., Burr, D. B. & Turner, C. H. Recovery periods restore mechanosensitivity to dynamically loaded bone. J. Exp. Biol. 204, 3389–3399 (2001).
Guo, S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J. Endocrinol. 220, T1–T23 (2014).
Ward, K. et al. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J. Bone Miner. Res. 19, 360–369 (2004).
Lam, T. P. et al. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos. Int. 24, 1623–1636 (2013).
Bianchi, M. et al. Effects of low-magnitude high-frequency vibration on bone density, bone resorption and muscular strength in ambulant children affected by Duchenne muscular dystrophy. J. Bone Miner. Res. 28 (Suppl. 1), LB–SU03 (2013).
Mogil, R. J. et al. Effect of low-magnitude, high-frequency mechanical stimulation on BMD among young childhood cancer survivors: a randomized clinical trial. JAMA Oncol. 2, 908–914 (2016).
Leonard, M. B. et al. Effect of low magnitude mechanical stimuli on bone density and structure in pediatric Crohn’s disease: a randomized placebo controlled trial. J. Bone Miner. Res. 31, 1177–1188 (2016).
DiVasta, A. D. et al. The ability of low-magnitude mechanical signals to normalize bone turnover in adolescents hospitalized for anorexia nervosa. Osteoporosis Int. 28, 1255–1263 (2017).
Fritton, J. C., Rubin, C. T., Qin, Y. X. & McLeod, K. J. Whole-body vibration in the skeleton: development of a resonance-based testing device. Ann. Biomed. Eng. 25, 831–839 (1997).
Muir, J., Kiel, D. P. & Rubin, C. T. Safety and severity of accelerations delivered from whole body vibration exercise devices to standing adults. J. Sci. Med. Sport 16, 526–531 (2013).
Kiel, D. P. et al. Insights from the conduct of a device trial in older persons: low magnitude mechanical stimulation for musculoskeletal health. Clin. Trials 7, 354–367 (2010).
Kiel, D. P. et al. Low-magnitude mechanical stimulation to improve bone density in persons of advanced age: a randomized, placebo-controlled trial. J. Bone Miner. Res. 30, 1319–1328 (2015).
Asselin, P., Spungen, A. M., Muir, J. W., Rubin, C. T. & Bauman, W. A. Transmission of low-intensity vibration through the axial skeleton of persons with spinal cord injury as a potential intervention for preservation of bone quantity and quality. J. Spinal Cord Med. 34, 52–59 (2011).
International Organization for Standardization. Evaluation of human exposure to whole-body vibration [ISO 2631-1:1985] (ISO, 1985).
Kiiski, J., Heinonen, A., Jarvinen, T. L., Kannus, P. & Sievanen, H. Transmission of vertical whole body vibration to the human body. J. Bone Miner. Res. 23, 1318–1325 (2008).
Ozcivici, E. et al. Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol. 6, 50–59 (2010).
Martinac, B. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci. 117, 2449–2460 (2004).
Kung, C., Martinac, B. & Sukharev, S. Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 64, 313–329 (2010).
Acknowledgements
Reviewer information
Nature Reviews Endocrinology thanks G. Duque, and other anonymous reviewers, for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors provided a substantial contribution to the discussion of the material. C.T.R., J.R., G.M.P., T.A.G., M.S. and G.U. contributed to all aspects of this Review. V.S.P., L.E.W. and K.K.N. researched data for the article, contributed to discussion of the content and wrote the article.
Corresponding author
Ethics declarations
Competing interests
C.T.R. is a founder of Marodyne Medical, Inc. and BTT Health and has several patents issued and pending related to the ability of mechanical signals to control musculoskeletal and metabolic disorders. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Loading
-
In terms of mechanical loading, a singular or compound series of static or dynamic (time-varying) forces applied to a system via gravity or direct application from an external body, causing tension, shear or compression.
- Unloading
-
A cell or body is considered mechanically unloaded if no static or dynamic strain is present, such as what might occur with bed rest or spaceflight (that is, microgravity).
- Ground-reaction forces
-
As applicable to biomechanics, ground-reaction forces consist of the normal forces exerted by the ground on the body making contact with it, particularly resulting from a heel strike during walking or running.
- Spectral content
-
Muscle contractive forces, specifically on bone, resonate within a discrete frequency range.
- Load sensation
-
Mechanical loads are ‘sensed’ by cells through transduction of external or internal forces across cytoskeletal proteins into the nucleus.
- Tissue senility
-
The ageing process is associated with the quiescence of regenerative cell populations residing in tissues throughout the body.
- Muscle-specific force
-
Quantification of the contractile forces generated by muscles can be normalized to muscle size ex vivo.
- Fluid shear
-
Fluidic forces applied tangentially across cell membranes or tissues.
- Dynamic shear forces
-
Physiological fluids exert a gradient of pulsatile flow across vessel walls, mineralized bone and cells housed in the bone marrow microenvironment.
- Tissue stiffness
-
In terms of bone, the stiffness of the tissue is correlated to its ability to resist deformation.
- Nuclear stiffness
-
Nuclear stiffness refers to its rigidity and is directly related to polymeric structural proteins (that is, microtubules, intermediate filaments and microfilaments) found across the cytoskeleton, of which actin proteins provide substantial reinforcement.
Rights and permissions
About this article
Cite this article
Pagnotti, G.M., Styner, M., Uzer, G. et al. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat Rev Endocrinol 15, 339–355 (2019). https://doi.org/10.1038/s41574-019-0170-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0170-1
This article is cited by
-
Insights and implications of sexual dimorphism in osteoporosis
Bone Research (2024)
-
Molecular and clinical effects of aromatase inhibitor therapy on skeletal muscle function in early-stage breast cancer
Scientific Reports (2024)
-
Rescuing SERCA2 pump deficiency improves bone mechano-responsiveness in type 2 diabetes by shaping osteocyte calcium dynamics
Nature Communications (2024)
-
LOX-1 regulation of H-type vascular endothelial cell regeneration in hyperglycemia
Acta Diabetologica (2024)
-
Higher prevalence of thyroid-specific autoantibodies (TPOAb and TgAb) is related to a higher prevalence of fractures in females: results from NHANES 2007–2010
Osteoporosis International (2024)