Abstract
Despite considerable advances in the past few years, obesity and type 2 diabetes mellitus (T2DM) remain two major challenges for public health systems globally. In the past 9 years, genome-wide association studies (GWAS) have established a major role for genetic variation within the MTNR1B locus in regulating fasting plasma levels of glucose and in affecting the risk of T2DM. This discovery generated a major interest in the melatonergic system, in particular the melatonin MT2 receptor (which is encoded by MTNR1B). In this Review, we discuss the effect of melatonin and its receptors on glucose homeostasis, obesity and T2DM. Preclinical and clinical post-GWAS evidence of frequent and rare variants of the MTNR1B locus confirmed its importance in regulating glucose homeostasis and T2DM risk with minor effects on obesity. However, these studies did not solve the question of whether melatonin is beneficial or detrimental, an issue that will be discussed in the context of the peculiarities of the melatonergic system. Melatonin receptors might have therapeutic potential as they belong to the highly druggable G protein-coupled receptor superfamily. Clarifying the precise role of melatonin and its receptors on glucose homeostasis is urgent, as melatonin is widely used for other indications, either as a prescribed medication or as a supplement without medical prescription, in many countries in Europe and in the USA.
Key points
-
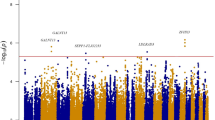
The rs10830963 single-nucleotide polymorphism (SNP) in the MTNR1B locus is associated with increased fasting plasma glucose levels and impaired insulin secretion, as well as increased risk of type 2 diabetes mellitus (T2DM) and gestational diabetes mellitus.
-
Obesity seems to not be associated with the rs10830963 SNP in adults but might have a role in fetal birth weight.
-
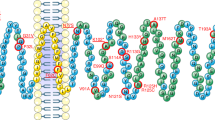
The phenotype of rs10830963 risk allele carriers includes increased MTNR1B mRNA expression, altered melatonin secretion and possibly further effects associated with the enhancer activity of the region surrounding the rs10830963 SNP.
-
Loss-of-function of rare MT2 receptor variants, in particular of melatonin-induced Gi1 and Gz and spontaneous β-arrestin 2 recruitment, is associated with increased risk of T2DM.
-
Lifestyle recommendations are emerging for rs10830963 risk allele carriers and further clinical evidence has to be gathered to evaluate the prescription of melatonin for patients with T2DM.
-
The wide use of melatonin by millions of people, both as a supplement and as a medicine, calls for a rapid assessment of the effect of melatonin on glucose homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jockers, R. et al. Update on melatonin receptors: IUPHAR review 20. Br. J. Pharmacol. 173, 2702–2725 (2016).
Bouatia-Naji, N. et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 41, 89–94 (2009).
Lyssenko, V. et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41, 82–88 (2009).
Prokopenko, I. et al. Variants in MTNR1B influence fasting glucose levels. Nat. Genet. 41, 77–81 (2009).
Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 175, 3190–3199 (2018).
Cipolla-Neto, J., Amaral, F. G., Afeche, S. C., Tan, D. X. & Reiter, R. J. Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56, 371–381 (2014).
Manchester, L. C. et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59, 403–419 (2015).
Bernard, M. et al. Melatonin synthesis pathway: circadian regulation of the genes encoding the key enzymes in the chicken pineal gland and retina. Reprod. Nutr. Dev. 39, 325–334 (1999).
Arendt, J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev. Reprod. 3, 13–22 (1998).
Tricoire, H., Locatelli, A., Chemineau, P. & Malpaux, B. Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology 143, 84–90 (2002).
Legros, C., Chesneau, D., Boutin, J. A., Barc, C. & Malpaux, B. Melatonin from cerebrospinal fluid but not from blood reaches sheep cerebral tissues under physiological conditions. J. Neuroendocrinol. 26, 151–163 (2014).
Pevet, P. & Challet, E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J. Physiol. Paris 105, 170–182 (2011).
Pevet, P., Klosen, P. & Felder-Schmittbuhl, M. P. The hormone melatonin: animal studies. Best Pract. Res. Clin. Endocrinol. Metab. 31, 547–559 (2017).
Zlotos, D. P., Jockers, R., Cecon, E., Rivara, S. & Witt-Enderby, P. A. MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential. J. Med. Chem. 57, 3161–3185 (2014).
Baba, K. et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci. Signal. 6, ra89 (2013).
Conti, A. et al. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 28, 193–202 (2000).
Markus, R. P., Fernandes, P. A., Kinker, G. S., da Silveira Cruz-Machado, S. & Marcola, M. Immune-pineal axis — acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 175, 3239–3250 (2017).
Yi, W. J. & Kim, T. S. Melatonin protects mice against stress-induced inflammation through enhancement of M2 macrophage polarization. Int. Immunopharmacol. 48, 146–158 (2017).
Bertrand, P. P., Polglaze, K. E., Bertrand, R. L., Sandow, S. L. & Pozo, M. J. Detection of melatonin production from the intestinal epithelium using electrochemical methods. Curr. Pharm. Des. 20, 4802–4806 (2014).
Chen, C. Q., Fichna, J., Bashashati, M., Li, Y. Y. & Storr, M. Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 17, 3888–3898 (2011).
Suofu, Y. et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl Acad. Sci. USA 114, E7997–E8006 (2017).
He, C. et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int. J. Mol. Sci. 17, 939 (2016).
Tan, D. X. et al. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 54, 127–138 (2013).
Dubocovich, M. L. et al. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 62, 343–380 (2010).
Bondi, C. D. et al. MT1 melatonin receptor internalization underlies melatonin-induced morphologic changes in Chinese hamster ovary cells and these processes are dependent on Gi proteins, MEK 1/2 and microtubule modulation. J. Pineal Res. 44, 288–298 (2008).
Hong, L. J. et al. Valproic acid influences MTNR1A intracellular trafficking and signaling in a beta-arrestin 2-dependent manner. Mol. Neurobiol. 53, 1237–1246 (2016).
Levoye, A. et al. The orphan GPR50 receptor specifically inhibits MT(1) melatonin receptor function through heterodimerization. EMBO J. 25, 3012–3023 (2006).
Benleulmi-Chaachoua, A. et al. Protein interactome mining defines melatonin MT1 receptors as integral component of presynaptic protein complexes of neurons. J. Pineal Res. 60, 95–108 (2016).
Guillaume, J. L. et al. The PDZ protein mupp1 promotes Gi coupling and signaling of the Mt1 melatonin receptor. J. Biol. Chem. 283, 16762–16771 (2008).
Maurice, P. et al. Molecular organization and dynamics of the melatonin MT receptor/RGS20/G(i) protein complex reveal asymmetry of receptor dimers for RGS and G(i) coupling. EMBO J. 29, 3646–3659 (2010).
Cecon, E., Oishi, A. & Jockers, R. Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol. 175, 3263–3280 (2017).
Oishi, A., Cecon, E. & Jockers, R. Melatonin receptor signaling: impact of receptor oligomerization on receptor function. Int. Rev. Cell. Mol. Biol. 338, 59–77 (2018).
Chen, L. et al. Melatonin receptor type 1 signals to extracellular signal-regulated kinase 1 and 2 via Gi and Gs dually coupled pathways in HEK-293 cells. Biochemistry 53, 2827–2839 (2014).
Chan, A. S. et al. Melatonin mt1 and MT2 receptors stimulate c-Jun N-terminal kinase via pertussis toxin-sensitive and -insensitive G proteins. Cell. Signal. 14, 249–257 (2002).
Sack, R. L., Brandes, R. W., Kendall, A. R. & Lewy, A. J. Entrainment of free-running circadian rhythms by melatonin in blind people. N. Engl. J. Med. 343, 1070–1077 (2000).
Isherwood, C. M., Van der Veen, D. R., Johnston, J. D. & Skene, D. J. Twenty-four-hour rhythmicity of circulating metabolites: effect of body mass and type 2 diabetes. FASEB J. 31, 5557–5567 (2017).
Qian, J. & Scheer, F. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol. Metab. 27, 282–293 (2016).
Bass, J. in A Time for Metabolism and Hormones (eds Sassone-Corsi, P. & Christen, Y.) 25–32 (Springer International Publishing, 2016).
von Gall, C., Weaver, D. R., Kock, M., Korf, H. W. & Stehle, J. H. Melatonin limits transcriptional impact of phosphoCREB in the mouse SCN via the Mel1a receptor. Neuroreport 11, 1803–1807 (2000).
Mc Arthur, A., Hunt, A. & Gillette, M. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinology 138, 627–634 (1997).
Hunt, A. E., AlGhoul, W. M., Gillette, M. U. & Dubocovich, M. L. Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Amer. J. Physiol. Cell Physiol. 280, C110–C118 (2001).
Nelson, C. S., Marino, J. L. & Allen, C. N. Melatonin receptors activate heteromeric G-protein coupled Kir3 channels. Neuroreport 7, 717–720 (1996).
Hablitz, L. M. et al. GIRK channels mediate the nonphotic effects of exogenous melatonin. J. Neurosci. 35, 14957–14965 (2015).
Pfeffer, M., Rauch, A., Korf, H. W. & von Gall, C. The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light-induced phase advances by acting upon MT2 receptors. Chronobiol. Int. 29, 415–429 (2012).
Nagy, A. D. et al. Melatonin adjusts the expression pattern of clock genes in the suprachiasmatic nucleus and induces antidepressant-like effect in a mouse model of seasonal affective disorder. Chronobiol. Int. 32, 447–457 (2015).
Kandalepas, P. C., Mitchell, J. W. & Gillette, M. U. Melatonin signal transduction pathways require E-box-mediated transcription of Per1 and Per2 to reset the SCN clock at dusk. PLOS ONE 11, e0157824 (2016).
Ha, E. et al. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J. Pineal Res. 41, 67–72 (2006).
Poon, A. M., Choy, E. H. & Pang, S. F. Modulation of blood glucose by melatonin: a direct action on melatonin receptors in mouse hepatocytes. Biol. Signals Recept. 10, 367–379 (2001).
Sauer, L. A., Dauchy, R. T. & Blask, D. E. Melatonin inhibits fatty acid transport in inguinal fat pads of hepatoma 7288CTC-bearing and normal Buffalo rats via receptor-mediated signal transduction. Life Sci. 68, 2835–2844 (2001).
Muhlbauer, E., Albrecht, E., Bazwinsky-Wutschke, I. & Peschke, E. Melatonin influences insulin secretion primarily via MT(1) receptors in rat insulinoma cells (INS-1) and mouse pancreatic islets. J. Pineal Res. 52, 446–459 (2012).
Zalatan, F., Krause, J. A. & Blask, D. E. Inhibition of isoproterenol-induced lipolysis in rat inguinal adipocytes in vitro by physiological melatonin via a receptor-mediated mechanism. Endocrinology 142, 3783–3790 (2001).
Stumpf, I., Muhlbauer, E. & Peschke, E. Involvement of the cGMP pathway in mediating the insulin-inhibitory effect of melatonin in pancreatic beta-cells. J. Pineal Res. 45, 318–327 (2008).
Kemp, D. M., Ubeda, M. & Habener, J. F. Identification and functional characterization of inelatonin Mel 1a receptors in pancreatic beta cells: potential role in incretin-mediated cell function by sensitization of cAMP signaling. Mol. Cell. Endocrinol. 191, 157–166 (2002).
Shieh, J. M., Wu, H. T., Cheng, K. C. & Cheng, J. T. Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKCzeta-Akt-GSK3beta pathway in hepatic cells. J. Pineal Res. 47, 339–344 (2009).
Owino, S. et al. Nocturnal activation of melatonin receptor type 1 signaling modulates diurnal insulin sensitivity via regulation of PI3K activity. J. Pineal Res. 64, e12462 (2018).
Tuomi, T. et al. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 23, 1067–1077 (2016).
Brydon, L., Petit, L., Delagrange, P., Strosberg, A. D. & Jockers, R. Functional expression ofmt2 (mel1b) melatonin receptors in human paz6 adipocytes. Endocrinology 142, 4264–4271 (2001).
Karamitri, A., Renault, N., Clement, N., Guillaume, J. L. & Jockers, R. Minireview: toward the establishment of a link between melatonin and glucose homeostasis: association of melatonin MT2 receptor variants with type 2 diabetes. Mol. Endocrinol. 27, 1217–1233 (2013).
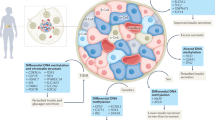
Peschke, E., Bahr, I. & Muhlbauer, E. Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon. Int. J. Mol. Sci. 14, 6981–7015 (2013).
Bahr, I., Muhlbauer, E., Schucht, H. & Peschke, E. Melatonin stimulates glucagon secretion in vitro and in vivo. J. Pineal Res. 50, 336–344 (2011).
Ramracheya, R. D. et al. Function and expression of melatonin receptors on human pancreatic islets. J. Pineal Res. 44, 273–279 (2008).
Costes, S., Boss, M., Thomas, A. P. & Matveyenko, A. V. Activation of melatonin signaling promotes beta-cell survival and function. Mol. Endocrinol. 29, 682–692 (2015).
Ruiz, L. et al. Proteasomal degradation of the histone acetyl transferase p300 contributes to beta-cell injury in a diabetes environment. Cell Death Dis. 9, 600 (2018).
Zibolka, J., Muhlbauer, E. & Peschke, E. Melatonin influences somatostatin secretion from human pancreatic delta-cells via MT1 and MT2 receptors. J. Pineal Res. 58, 198–209 (2015).
Zibolka, J., Bazwinsky-Wutschke, I., Muhlbauer, E. & Peschke, E. Distribution and density of melatonin receptors in human main pancreatic islet cell types. J. Pineal Res. 65, e12480 (2018).
Bartness, T. J., Powers, J. B., Hastings, M. H., Bittman, E. L. & Goldman, B. D. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J. Pineal Res. 15, 161–190 (1993).
Reiter, R. J. Photoperiod: its importance as an impeller of pineal and seasonal reproductive rhythms. Int. J. Biometeorol. 24, 57–63 (1980).
Peschke, E. & Muhlbauer, E. New evidence for a role of melatonin in glucose regulation. Best Pract. Res. Clin. Endocrinol. Metab. 24, 829–841 (2010).
Wade, G. N. & Bartness, T. J. Effects of photoperiod and gonadectomy on food intake, body weight, and body composition in Siberian hamsters. Am. J. Physiol. 246, R26–R30 (1984).
Le Gouic, S. et al. Characterization of a melatonin binding site in Siberian hamster brown adipose tissue. Eur. J. Pharmacol. 339, 271–278 (1997).
Prunet, M. B. et al. Evidence for a direct effect of melatonin on mitochondrial genome expression of Siberian hamster brown adipocytes. J. Pineal Res. 30, 108–115 (2001).
Lima, F. B. et al. The regulation of insulin action in isolated adipocytes. Role of the periodicity of food intake, time of day and melatonin. Braz. J. Med. Biol. Res. 27, 995–1000 (1994).
Contreras-Alcantara, S., Baba, K. & Tosini, G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity 18, 1861–1863 (2010).
Zhao, J. et al. Examination of all type 2 diabetes GWAS loci reveals HHEX-IDE as a locus influencing pediatric BMI. Diabetes 59, 751–755 (2010).
Bonnefond, A. et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat. Genet. 44, 297–301 (2012).
Yang, J. et al. Genetic association study with metabolic syndrome and metabolic-related traits in a cross-sectional sample and a 10-year longitudinal sample of chinese elderly population. PLOS ONE 9, e100548 (2014).
Goni, L. et al. Macronutrient-specific effect of the MTNR1B genotype on lipid levels in response to 2 year weight-loss diets. J. Lipid Res. 59, 155–161 (2018).
Goni, L. et al. A circadian rhythm-related MTNR1B genetic variant modulates the effect of weight-loss diets on changes in adiposity and body composition: the POUNDS LOST trial. Eur. J. Nutr. https://doi.org/10.1007/s00394-018-1660-y (2018).
Andersson, E. A. et al. MTNR1B G24E variant associates With BMI and fasting plasma glucose in the general population in studies of 22,142 Europeans. Diabetes 59, 1539–1548 (2010).
Karamitri, A. et al. Type 2 diabetes-associated variants of the MT2 melatonin receptor affect distinct modes of signaling. Sci. Signal. 11, eaan6622 (2018).
Beaumont, R. N. et al. Genome-wide association study of offspring birth weight in 86 577 women identifies five novel loci and highlights maternal genetic effects that are independent of fetal genetics. Hum. Mol. Genet. 27, 742–756 (2018).
Lane, J. M. et al. Impact of common diabetes risk variant in MTNR1B on sleep, circadian, and melatonin physiology. Diabetes 65, 1741–1751 (2016).
Okatani, Y. et al. Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 25, 129–134 (1998).
Reiter, R. J., Tan, D. X., Korkmaz, A. & Rosales-Corral, S. A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 20, 293–307 (2014).
Peter, I. et al. Association of type 2 diabetes susceptibility loci with one-year weight loss in the look AHEAD clinical trial. Obesity 20, 1675–1682 (2012).
Mirzaei, K. et al. Variants in glucose- and circadian rhythm-related genes affect the response of energy expenditure to weight-loss diets: the POUNDS LOST Trial. Am. J. Clin. Nutr. 99, 392–399 (2014).
Goni, L., Cuervo, M., Milagro, F. I. & Martinez, J. A. Gene-gene interplay and gene-diet interactions involving the MTNR1B rs10830963 variant with body weight loss. J. Nutrigenet. Nutrigenom. 7, 232–242 (2014).
Grotenfelt, N. E. et al. Interaction between rs10830963 polymorphism in MTNR1B and lifestyle intervention on occurrence of gestational diabetes. Diabetologia 59, 1655–1658 (2016).
Lima, F. B. et al. Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am. J. Physiol. 275, E934–E941 (1998).
Nogueira, T. C. et al. Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology 152, 1253–1263 (2011).
Oliveira, A. C. et al. Combined treatment with melatonin and insulin improves glycemic control, white adipose tissue metabolism and reproductive axis of diabetic male rats. Life Sci. 199, 158–166 (2018).
Champney, T. H., Brainard, G. C., Richardson, B. A. & Reiter, R. J. Experimentally-induced diabetes reduces nocturnal pineal melatonin content in the Syrian hamster. Comp. Biochem. Physiol. A 76, 199–201 (1983).
Peschke, E. et al. Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J. Pineal Res. 40, 135–143 (2006).
Sartori, C. et al. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology 150, 5311–5317 (2009).
McMullan, C. J., Schernhammer, E. S., Rimm, E. B., Hu, F. B. & Forman, J. P. Melatonin secretion and the incidence of type 2 diabetes. JAMA 309, 1388–1396 (2013).
Cagnacci, A. et al. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin. Endocrinol. 54, 339–346 (2001).
Rubio-Sastre, P., Scheer, F. A., Gomez-Abellan, P., Madrid, J. A. & Garaulet, M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 37, 1715–1719 (2014).
Garfinkel, D. et al. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab. Syndr. Obes. 4, 307–313 (2011).
Kadhim, H. M. et al. Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with metformin. J. Pineal Res. 41, 189–193 (2006).
Gonciarz, M. et al. Plasma insulin, leptin, adiponectin, resistin, ghrelin, and melatonin in nonalcoholic steatohepatitis patients treated with melatonin. J. Pineal Res. 54, 154–161 (2013).
Sparso, T. et al. G-Allele of intronic rs10830963 in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose-stimulated insulin release: studies involving 19,605 Europeans. Diabetes 58, 1450–1456 (2009).
Voight, B. F. et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 42, 579–589 (2010).
Dietrich, K. et al. Association and evolutionary studies of the melatonin receptor 1B gene (MTNR1B) in the self-contained population of Sorbs from Germany. Diabet. Med. 28, 1373–1380 (2011).
Marouli, E. et al. Evaluating the glucose raising effect of established loci via a genetic risk score. PLOS ONE 12, e0186669 (2017).
Sabatti, C. et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat. Genet. 41, 35–46 (2009).
Ohshige, T. et al. Association of new loci identified in European genome-wide association studies with susceptibility to type 2 diabetes in the Japanese. PLOS ONE 6, e26911 (2011).
Fujita, H. et al. Variations with modest effects have an important role in the genetic background of type 2 diabetes and diabetes-related traits. J. Hum. Genet. 57, 776–779 (2012).
Ramos, E. et al. Replication of genome-wide association studies (GWAS) loci for fasting plasma glucose in African-Americans. Diabetologia 54, 783–788 (2011).
Liu, C. T. et al. Transferability and fine-mapping of glucose and insulin quantitative trait loci across populations: CARe, the Candidate Gene Association Resource. Diabetologia 55, 2970–2984 (2012).
Palmer, N. D. et al. Genetic variants associated with quantitative glucose homeostasis traits translate to type 2 diabetes in mexican americans: the GUARDIAN (Genetics Underlying Diabetes in Hispanics) consortium. Diabetes 64, 1853–1866 (2015).
Rönn, T. et al. A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia 52, 830–833 (2009).
Liu, C. et al. MTNR1B rs10830963 is associated with fasting plasma glucose, HbA1C and impaired beta-cell function in Chinese Hans from Shanghai. BMC Med. Genet. 11, 59 (2010).
Hu, C. et al. Effects of GCK, GCKR, G6PC2 and MTNR1B variants on glucose metabolism and insulin secretion. PLOS ONE 5, e11761 (2010).
Kan, M. Y. et al. Two susceptible diabetogenic variants near/in MTNR1B are associated with fasting plasma glucose in a Han Chinese cohort. Diabet Med. 27, 598–602 (2010).
Takeuchi, F. et al. Common variants at the GCK, GCKR, G6PC2-ABCB11 and MTNR1B loci are associated with fasting glucose in two Asian populations. Diabetologia 53, 299–308 (2010).
Salman, M. et al. MTNR1B gene polymorphisms and susceptibility to type 2 diabetes: a pilot study in South Indians. Gene 566, 189–193 (2015).
Chambers, J. C. et al. Common genetic variation near melatonin receptor MTNR1B contributes to raised plasma glucose and increased risk of type 2 diabetes among Indian Asians and European Caucasians. Diabetes 58, 2703–2708 (2009).
Rees, S. D. et al. Effects of 16 genetic variants on fasting glucose and type 2 diabetes in South Asians: ADCY5 and GLIS3 variants may predispose to type 2 diabetes. PLOS ONE 6, e24710 (2011).
Kelliny, C. et al. Common genetic determinants of glucose homeostasis in healthy children: the European Youth Heart Study. Diabetes 58, 2939–2945 (2009).
Barker, A. et al. Association of genetic loci with glucose levels in childhood and adolescence: a meta-analysis of over 6,000 children. Diabetes 60, 1805–1812 (2011).
Song, J. Y. et al. Association of the rs10830963 polymorphism in MTNR1B with fasting glucose levels in Chinese children and adolescents. Obes. Facts 4, 197–203 (2011).
Langlois, C. et al. Evaluating the transferability of 15 European-derived fasting plasma glucose SNPs in Mexican children and adolescents. Sci. Rep. 6, 36202 (2016).
Holzapfel, C. et al. Association of a MTNR1B gene variant with fasting glucose and HOMA-B in children and adolescents with high BMI-SDS. Eur. J. Endocrinol. 164, 205–212 (2011).
Zheng, C. et al. A common variant in the MTNR1b gene is associated with increased risk of impaired fasting glucose (IFG) in youth with obesity. Obesity 23, 1022–1029 (2015).
Reinehr, T. et al. Relationship between MTNR1B (melatonin receptor 1B gene) polymorphism rs10830963 and glucose levels in overweight children and adolescents. Pediatr. Diabetes 12, 435–441 (2011).
Soranzo, N. et al. Common variants at 10 genomic loci influence hemoglobin A1(C) levels via glycemic and nonglycemic pathways. Diabetes 59, 3229–3239 (2010).
Stancáková, A. et al. Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes 58, 2129–2136 (2009).
Langenberg, C. et al. Common genetic variation in the melatonin receptor 1B gene (MTNR1B) is associated with decreased early-phase insulin response. Diabetologia 52, 1537–1542 (2009).
‘t Hart, L. M. et al. Combined risk allele score of eight type 2 diabetes genes is associated with reduced first-phase glucose-stimulated insulin secretion during hyperglycemic clamps. Diabetes 59, 287–292 (2010).
Jonsson, A. et al. Effects of common genetic variants associated with type 2 diabetes and glycemic traits on α- and β-cell function and insulin action in humans. Diabetes 62, 2978–2983 (2013).
Prokopenko, I. et al. A central role for GRB10 in regulation of islet function in man. PLOS Genet. 10, e1004235 (2014).
Wood, A. R. et al. A Genome-wide association study of IVGTT-based measures of first-phase insulin secretion refines the underlying physiology of type 2 diabetes variants. Diabetes 66, 2296–2309 (2017).
Walford, G. A. et al. Common genetic variants differentially influence the transition from clinically defined states of fasting glucose metabolism. Diabetologia 55, 331–339 (2012).
Vangipurapu, J. et al. Association of indices of liver and adipocyte insulin resistance with 19 confirmed susceptibility loci for type 2 diabetes in 6,733 non-diabetic Finnish men. Diabetologia 54, 563–571 (2011).
Ahlqvist, E. et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 6, 361–369 (2018).
Kwak, S. H. et al. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes 61, 531–541 (2012).
Vlassi, M. et al. The rs10830963 variant of melatonin receptor MTNR1B is associated with increased risk for gestational diabetes mellitus in a Greek population. Hormones 11, 70–76 (2012).
Huopio, H. et al. Association of risk variants for type 2 diabetes and hyperglycemia with gestational diabetes. Eur. J. Endocrinol. 169, 291–297 (2013).
Wang, Y. et al. Association of six single nucleotide polymorphisms with gestational diabetes mellitus in a Chinese population. PLOS ONE 6, e26953 (2011).
Rosta, K. et al. Association study with 77 SNPs confirms the robust role for the rs10830963/G of MTNR1B variant and identifies two novel associations in gestational diabetes mellitus development. PLOS ONE 12, e0169781 (2017).
Junior, J. P. et al. The MTNR1B gene polymorphism rs10830963 is associated with gestational diabetes in a Brazilian population. Gene 568, 114–115 (2015).
Wu, L., Cui, L., Tam, W. H., Ma, R. C. & Wang, C. C. Genetic variants associated with gestational diabetes mellitus: a meta-analysis and subgroup analysis. Sci. Rep. 6, 30539 (2016).
Robitaille, J. & Grant, A. M. The genetics of gestational diabetes mellitus: evidence for relationship with type 2 diabetes mellitus. Genet. Med. 10, 240–250 (2008).
Hinton, D. R. et al. Novel localization of a G protein, Gz-alpha, in neurons of brain and retina. J. Neurosci. 10, 2763–2770 (1990).
Slominski, R. M., Reiter, R. J., Schlabritz-Loutsevitch, N., Ostrom, R. S. & Slominski, A. T. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell Endocrinol. 351, 152–166 (2012).
Mulder, H. Melatonin signalling and type 2 diabetes risk: too little, too much or just right? Diabetologia 60, 826–829 (2017).
Bonnefond, A., Karamitri, A., Jockers, R. & Froguel, P. The difficult journey from genome-wide association studies to pathophysiology: the melatonin receptor 1B (MT2) paradigm. Cell Metab. 24, 345–347 (2016).
Bonnefond, A. & Froguel, P. Disentangling the role of melatonin and its receptor MTNR1B in type 2 diabetes: still a long way to go? Curr. Diab. Rep. 17, 122 (2017).
Bonnefond, A. & Froguel, P. The case for too little melatonin signalling in increased diabetes risk. Diabetologia 60, 823–825 (2017).
Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 62, e12377 (2017).
Ben-Dyke, R. Diurnal variation of oral glucose tolerance in volunteers and laboratory animals. Diabetologia 7, 156–159 (1971).
Barrett, P., Schuster, C., Mercer, J. & Morgan, P. J. Sensitization: a mechanism for melatonin action in the pars tuberalis. J. Neuroendocrinol. 15, 415–421 (2003).
Bach, A. G., Wolgast, S., Muhlbauer, E. & Peschke, E. Melatonin stimulates inositol-1,4,5-trisphosphate and Ca2+ release from INS1 insulinoma cells. J. Pineal Res. 39, 316–323 (2005).
Depner, C. M., Melanson, E. L., McHill, A. W. & Wright, K. P. Jr. Mistimed food intake and sleep alters 24-hour time-of-day patterns of the human plasma proteome. Proc. Natl Acad. Sci. USA 115, E5390–E5399 (2018).
Simsek, N. et al. Effects of melatonin on islet neogenesis and beta cell apoptosis in streptozotocin-induced diabetic rats: an immunohistochemical study. Domest. Anim. Endocrinol. 43, 47–57 (2012).
Kanter, M., Uysal, H., Karaca, T. & Sagmanligil, H. O. Depression of glucose levels and partial restoration of pancreatic beta-cell damage by melatonin in streptozotocin-induced diabetic rats. Arch. Toxicol. 80, 362–369 (2006).
de Lima, L. M., dos Reis, L. C. & de Lima, M. A. Influence of the pineal gland on the physiology, morphometry and morphology of pancreatic islets in rats. Braz. J. Biol. 61, 333–340 (2001).
Kimple, M. E. et al. Deletion of GalphaZ protein protects against diet-induced glucose intolerance via expansion of beta-cell mass. J. Biol. Chem. 287, 20344–20355 (2012).
Suofu, Y., Carlisle, D. L., Vilardaga, J. P. & Friedlander, R. M. Reply to Ahluwalia et al.: Contributions of melatonin receptors are tissue-dependent. Proc. Natl Acad. Sci. USA 115, E1944 (2018).
Savaskan, E. et al. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J. Pineal Res. 38, 10–16 (2005).
Dubocovich, M. L. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 8 (Suppl. 3), 34–42 (2007).
Fadista, J. et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc. Natl Acad. Sci. USA 111, 13924–13929 (2014).
van de Bunt, M. et al. Transcript expression data from human islets links regulatory signals from genome-wide association studies for type 2 diabetes and glycemic traits to their downstream effectors. PLOS Genet. 11, e1005694 (2015).
Segerstolpe, A. et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 24, 593–607 (2016).
Thomsen, S. K. et al. Systematic functional characterization of candidate causal genes for type 2 diabetes risk variants. Diabetes 65, 3805–3811 (2016).
Gerdin, M. J., Masana, M. I., Ren, D., Miller, R. J. & Dubocovich, M. L. Short-term exposure to melatonin differentially affects the functional sensitivity and trafficking of the hMT(1) and hMT(2) melatonin receptors. J. Pharmacol. Exp. Ther. 304, 931–939 (2003).
Solimena, M. et al. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia 61, 641–657 (2018).
Gaulton, K. J. et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat. Genet. 47, 1415–1425 (2015).
Fagerberg, L. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteomics 13, 397–406 (2014).
Lewy, A. J., Ahmed, S., Jackson, J. M. & Sack, R. L. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol. Int. 9, 380–392 (1992).
Burgess, H. J., Revell, V. L., Molina, T. A. & Eastman, C. I. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J. Clin. Endocrinol. Metab. 95, 3325–3331 (2010).
Gerdin, M. J. et al. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB J. 18, 1646–1656 (2004).
Witt-Enderby, P. A., Masana, M. I. & Dubocovich, M. L. Physiological exposure to melatonin supersensitizes the cyclic adenosine 3ʹ,5ʹ-monophosphate-dependent signal transduction cascade in Chinese hamster ovary cells expressing the human mt1 melatonin receptor. Endocrinology 139, 3064–3071 (1998).
Owino, S., Contreras-Alcantara, S., Baba, K. & Tosini, G. Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLOS ONE 11, e0148214 (2016).
Gbahou, F. et al. Design and validation of the first cell-impermeant melatonin receptor agonist. Br. J. Pharmacol. 174, 2409–2421 (2017).
Oishi, A. et al. Orphan GPR61, GPR62 and GPR135 receptors and the melatonin MT2 receptor reciprocally modulate their signaling functions. Sci. Rep. 7, 8990 (2017).
Kamal, M. et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J. Biol. Chem. 290, 11537–11546 (2015).
Santos, R. et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 16, 19–34 (2017).
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schioth, H. B. & Gloriam, D. E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 (2017).
Liu, J. et al. MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 56, 361–383 (2016).
Millan, M. J. et al. The melatonergic agonist and clinically active antidepressant, agomelatine, is a neutral antagonist at 5-HT2C receptors. Int. J. Neuropsychopharmacol. 14, 768–783 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02615002 (2018).
Neurim Pharmaceuticals. Piromelatine. Neurim Pharmaceuticals http://www.neurim.com/products/piromelatine (2018).
Clarke, T. C., Black, L. I., Stussman, B. J., Barnes, P. M. & Nahin, R. L. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat. Rep. 79, 1–16 (2015).
Black, L. I., Clarke, T. C., Barnes, P. M., Stussman, B. J. & Nahin, R. L. Use of complementary health approaches among children aged 4–17 years in the United States: National Health Interview Survey, 2007–2012. Natl Health Stat. Rep. 78, 1–19 (2015).
Syndicat National des Compléments Alimentaires. Du marché des compléments alimentaires en France. Synadiet.org http://www.synadiet.org/sites/default/files/page/files/chiffres_cles_2016_version_pdf.pdf (2016).
Hauser, A. S. et al. Pharmacogenomics of GPCR drug targets. Cell 172, 41–54 (2018).
Trades Union Congress. Number of people working night shifts up by more than 250,000 since 2011, new TUC analysis reveals. TUC.org.uk http://www.tuc.org.uk/news/number-people-working-night-shifts-more-250000-2011-new-tuc-analysis-reveals (2016).
Striegel-Moore, R. H. et al. Exploring the typology of night eating syndrome. Int. J. Eat. Disord. 41, 411–418 (2008).
Forrestel, A. C., Miedlich, S. U., Yurcheshen, M., Wittlin, S. D. & Sellix, M. T. Chronomedicine and type 2 diabetes: shining some light on melatonin. Diabetologia 60, 808–822 (2017).
Simonis-Bik, A. M. et al. Gene variants in the novel type 2 diabetes loci CDC123/CAMK1D, THADA, ADAMTS9, BCL11A, and MTNR1B affect different aspects of pancreatic beta-cell function. Diabetes 59, 293–301 (2010).
Florez, J. C. et al. Effects of genetic variants previously associated with fasting glucose and insulin in the Diabetes Prevention Program. PLOS ONE 7, e44424 (2012).
Garaulet, M. et al. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism 64, 1650–1657 (2015).
Lopez-Minguez, J., Saxena, R., Bandin, C., Scheer, F. A. & Garaulet, M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: a randomized, cross-over study. Clin. Nutr. 37, 1133–1140 (2017).
Eze, I. C. et al. Exposure to night-time traffic noise, melatonin-regulating gene variants and change in glycemia in adults. Int. J. Environ. Res. Public Health 14, 1492 (2017).
Stoschitzky, K. et al. Influence of beta-blockers on melatonin release. Eur. J. Clin. Pharmacol. 55, 111–115 (1999).
Ying, S. W. et al. Melatonin analogues as agonists and antagonists in the circadian system and other brain areas. Eur. J. Pharmacol. 296, 33–42 (1996).
Melke, J. et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatry 13, 90–98 (2008).
Chaste, P. et al. Genetic variations of the melatonin pathway in patients with attention-deficit and hyperactivity disorders. J. Pineal Res. 51, 394–399 (2011).
Chaste, P. et al. Identification of pathway-biased and deleterious melatonin receptor mutants in autism spectrum disorders and in the general population. PLOS ONE 5, e11495 (2010).
Reiter, R. J. et al. Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 23, 509 (2018).
Aschoff, J. Circadian rhythms in man. Science 148, 1427–1432 (1965).
Mayeuf-Louchart, A., Zecchin, M., Staels, B. & Duez, H. Circadian control of metabolism and pathological consequences of clock perturbations. Biochimie 143, 42–50 (2017).
Perelis, M. et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250 (2015).
Ruiter, M. et al. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes 52, 1709–1715 (2003).
Marcheva, B. et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 (2010).
Saini, C. et al. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obes. Metab. 18, 355–365 (2016).
West, A. C. & Bechtold, D. A. The cost of circadian desynchrony: evidence, insights and open questions. Bioessays 37, 777–788 (2015).
Simonneaux, V. Naughty melatonin: how mothers tick off their fetus. Endocrinology 152, 1734–1738 (2011).
Muhlbauer, E., Gross, E., Labucay, K., Wolgast, S. & Peschke, E. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur. J. Pharmacol. 606, 61–71 (2009).
de Farias Tda, S. et al. Pinealectomy interferes with the circadian clock genes expression in white adipose tissue. J. Pineal Res. 58, 251–261 (2015).
Sun, M. et al. Meta-analysis on shift work and risks of specific obesity types. Obes. Rev. 19, 28–40 (2018).
Anothaisintawee, T., Reutrakul, S., Van Cauter, E. & Thakkinstian, A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med. Rev. 30, 11–24 (2016).
Da Silva Xavier, G. The cells of the islets of Langerhans. J. Clin. Med. 7, E54 (2018).
Rorsman, P. & Ashcroft, F. M. Pancreatic beta-cell electrical activity and insulin secretion: of mice and men. Physiol. Rev. 98, 117–214 (2018).
Szewczyk-Golec, K. et al. Melatonin supplementation lowers oxidative stress and regulates adipokines in obese patients on a calorie-restricted diet. Oxid. Med. Cell. Longev. 2017, 8494107 (2017).
Chojnacki, C. et al. Effects of fluoxetine and melatonin on mood, sleep quality and body mass index in postmenopausal women. J. Physiol. Pharmacol. 66, 665–671 (2015).
Goyal, A. et al. Melatonin supplementation to treat the metabolic syndrome: a randomized controlled trial. Diabetol. Metab. Syndr. 6, 124 (2014).
Mesri Alamdari, N. et al. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm. Metab. Res. 47, 504–508 (2015).
Romo-Nava, F. et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 16, 410–421 (2014).
Cichoz-Lach, H., Celinski, K., Konturek, P. C., Konturek, S. J. & Slomka, M. The effects of L-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J. Physiol. Pharmacol. 61, 577–580 (2010).
Celinski, K. et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease — 14 months follow up. J. Physiol. Pharmacol. 65, 75–82 (2014).
Koziróg, M. et al. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 50, 261–266 (2011).
Borba, C. P. et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J. Clin. Psychopharmacol 31, 653–658 (2011).
Kedziora-Kornatowska, K. et al. Melatonin improves oxidative stress parameters measured in the blood of elderly type 2 diabetic patients. J. Pineal Res. 46, 333–337 (2009).
Cavallo, A., Daniels, S. R., Dolan, L. M., Bean, J. A. & Khoury, J. C. Blood pressure-lowering effect of melatonin in type 1 diabetes. J. Pineal Res. 36, 262–266 (2004).
Wakatsuki, A., Okatani, Y., Ikenoue, N., Kaneda, C. & Fukaya, T. Effects of short-term melatonin administration on lipoprotein metabolism in normolipidemic postmenopausal women. Maturitas 38, 171–177 (2001).
Tamura, H. et al. Melatonin treatment in peri- and postmenopausal women elevates serum high-density lipoprotein cholesterol levels without influencing total cholesterol levels. J. Pineal Res. 45, 101–105 (2008).
Amstrup, A. K. et al. Reduced fat mass and increased lean mass in response to 1 year of melatonin treatment in postmenopausal women: a randomized placebo-controlled trial. Clin. Endocrinol. 84, 342–347 (2016).
Tsunoda, T. et al. The effects of ramelteon on glucose metabolism and sleep quality in type 2 diabetic patients with insomnia: a pilot prospective randomized controlled trial. J. Clin. Med. Res. 8, 878–887 (2016).
Walecka-Kapica, E. et al. The effect of melatonin supplementation on the quality of sleep and weight status in postmenopausal women. Prz. Menopauzalny 13, 334–338 (2014).
Gonciarz, M. et al. The pilot study of 3-month course of melatonin treatment of patients with nonalcoholic steatohepatitis: effect on plasma levels of liver enzymes, lipids and melatonin. J. Physiol. Pharmacol. 61, 705–710 (2010).
Gonciarz, M. et al. The effects of long-term melatonin treatment on plasma liver enzymes levels and plasma concentrations of lipids and melatonin in patients with nonalcoholic steatohepatitis: a pilot study. J. Physiol. Pharmacol. 63, 35–40 (2012).
Kedziora-Kornatowska, K. et al. Antioxidative effects of melatonin administration in elderly primary essential hypertension patients. J. Pineal Res. 45, 312–317 (2008).
Mostafavi, A. et al. Melatonin decreases olanzapine induced metabolic side-effects in adolescents with bipolar disorder: a randomized double-blind placebo-controlled trial. Acta Med. Iran. 52, 734–739 (2014).
Cavallo, A., Daniels, S. R., Dolan, L. M., Khoury, J. C. & Bean, J. A. Blood pressure response to melatonin in type 1 diabetes. Pediatr. Diabetes 5, 26–31 (2004).
Rindone, J. P. & Achacoso, R. Effect of melatonin on serum lipids in patients with hypercholesterolemia: a pilot study. Am. J. Ther. 4, 409–411 (1997).
Scheer, F. A., Van Montfrans, G. A., van Someren, E. J., Mairuhu, G. & Buijs, R. M. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 43, 192–197 (2004).
Cagnacci, A. et al. Prolonged melatonin administration decreases nocturnal blood pressure in women. Am. J. Hypertens. 18, 1614–1618 (2005).
Acknowledgements
We thank Julie Dam and Erika Cecon (Institut Cochin, France) for their valuable expert advice during the preparation of the manuscript. The authors were supported by the Agence Nationale de la Recherche (ANR-2011-BSV1-012-01 “MLT2D” and ANR-2011-META “MELA-BETES”), the Fondation de la Recherche Médicale (Equipe FRM DEQ20130326503), Institut National de la Santé et de la Recherche Médicale (INSERM) and Centre National de la Recherche Scientifique (CNRS).
Reviewer information
Nature Reviews Endocrinology thanks J. Cipolla-Neto and other anonymous reviewers for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors contributed to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karamitri, A., Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol 15, 105–125 (2019). https://doi.org/10.1038/s41574-018-0130-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-018-0130-1
This article is cited by
-
The melatonin receptor genes are linked and associated with the risk of polycystic ovary syndrome
Journal of Ovarian Research (2024)
-
The potential of therapeutic strategies targeting mitochondrial biogenesis for the treatment of insulin resistance and type 2 diabetes mellitus
Archives of Pharmacal Research (2024)
-
Metabolomic and genetic architecture of gestational diabetes subtypes
Diabetologia (2024)
-
GMMAD: a comprehensive database of human gut microbial metabolite associations with diseases
BMC Genomics (2023)
-
Recurrent prescription of sleep medication among primary care patients with type 2 diabetes: an observational study of real-world registry data
BMC Primary Care (2023)