Abstract
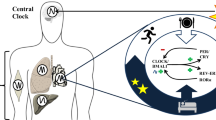
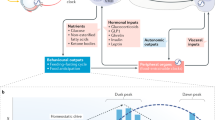
Insulin resistance is a main determinant in the development of type 2 diabetes mellitus and a major cause of morbidity and mortality. The circadian timing system consists of a central brain clock in the hypothalamic suprachiasmatic nucleus and various peripheral tissue clocks. The circadian timing system is responsible for the coordination of many daily processes, including the daily rhythm in human glucose metabolism. The central clock regulates food intake, energy expenditure and whole-body insulin sensitivity, and these actions are further fine-tuned by local peripheral clocks. For instance, the peripheral clock in the gut regulates glucose absorption, peripheral clocks in muscle, adipose tissue and liver regulate local insulin sensitivity, and the peripheral clock in the pancreas regulates insulin secretion. Misalignment between different components of the circadian timing system and daily rhythms of sleep–wake behaviour or food intake as a result of genetic, environmental or behavioural factors might be an important contributor to the development of insulin resistance. Specifically, clock gene mutations, exposure to artificial light–dark cycles, disturbed sleep, shift work and social jet lag are factors that might contribute to circadian disruption. Here, we review the physiological links between circadian clocks, glucose metabolism and insulin sensitivity, and present current evidence for a relationship between circadian disruption and insulin resistance. We conclude by proposing several strategies that aim to use chronobiological knowledge to improve human metabolic health.
Key points
-
The circadian timing system consists of a central brain clock in the hypothalamic suprachiasmatic nucleus and peripheral clocks in tissues, including the liver, muscle, adipose tissue and pancreas.
-
Misalignment between different components of the circadian timing system and daily rhythms of sleep–wake behaviour and food intake might contribute to the development of insulin resistance.
-
Strategies to improve metabolic health by circadian synchrony include modulating light exposure, modulating rhythmic behaviour and chronotherapy.
-
Circadian molecules are a promising new treatment option for insulin resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
World Health Organization. Global Report on Diabetes (WHO, 2016).
American Diabetes Association. Standards of medical care in diabetes-2018. Diabetes Care 41, S1–S159 (2018).2018 Current guideline of the American Diabetes Association for the diagnosis and treatment of diabetes mellitus.
Ouyang, Y., Andersson, C. R., Kondo, T., Golden, S. S. & Johnson, C. H. Resonating circadian clocks enhance fitness in cyanobacteria. Proc. Natl Acad. Sci. USA 95, 8660–8664 (1998).
Woelfle, M. A., Ouyang, Y., Phanvijhitsiri, K. & Johnson, C. H. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr. Biol. 14, 1481–1486 (2004).
Dibner, C., Schibler, U. & Albrecht, U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549 (2010).
Stenvers, D. J., Jonkers, C. F., Fliers, E., Bisschop, P. H. & Kalsbeek, A. Nutrition and the circadian timing system. Progress Brain Res. 199, 359–376 (2012).
Lowrey, P. L. & Takahashi, J. S. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genom. Hum. Genet. 5, 407–441 (2004).
Reppert, S. M. & Weaver, D. R. Coordination of circadian timing in mammals. Nature 418, 935–941 (2002).
Roberts, H. J. Afternoon glucose tolerance testing: a key to the pathogenesis, early diagnosis and prognosis of diabetogenic hyperinsulinism. J. Am. Geriatr. Soc. 12, 423–472 (1964).
Van Cauter, E., Polonsky, K. S. & Scheen, A. J. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr. Rev. 18, 716–738 (1997).Seminal review on the diurnal rhythms of human glucose metabolism.
Gibson, T. & Jarrett, R. J. Diurnal variation in insulin sensitivity. Lancet 2, 947–948 (1972).
Boden, G., Ruiz, J., Urbain, J. L. & Chen, X. Evidence for a circadian rhythm of insulin secretion. Am. J. Physiol. 271, E246–E252 (1996).
Saad, A. et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61, 2691–2700 (2012).
Morris, C. J. et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl Acad. Sci. USA 112, E2225–E2234 (2015).
Stephan, F. K. & Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl Acad. Sci. USA 69, 1583–1586 (1972).
Czeisler, C. A., Weitzman, E., Moore-Ede, M. C., Zimmerman, J. C. & Knauer, R. S. Human sleep: its duration and organization depend on its circadian phase. Science 210, 1264–1267 (1980).
Saper, C. B., Scammell, T. E. & Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263 (2005).
Strubbe, J. H. & van Dijk, G. The temporal organization of ingestive behaviour and its interaction with regulation of energy balance. Neurosci. Biobehav. Rev. 26, 485–498 (2002).
Scheer, F. A., Morris, C. J. & Shea, S. A. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 21, 421–423 (2013).First study showing the control of human appetite by the endogenous circadian timing system.
Herrera-Moro Chao, D. et al. The suprachiasmatic nucleus modulates the sensitivity of arcuate nucleus to hypoglycemia in the male rat. Endocrinology 157, 3439–3451 (2016).
Blancas-Velazquez, A., Mendoza, J., Garcia, A. N. & la Fleur, S. E. Diet-induced obesity and circadian disruption of feeding behavior. Front. Neurosci. 11, 23 (2017).
Buckley, T. M. & Schatzberg, A. F. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J. Clin. Endocrinol. Metab. 90, 3106–3114 (2005).
van Raalte, D. H. & Diamant, M. Steroid diabetes: from mechanism to treatment? Neth. J. Med. 72, 62–72 (2014).
Ramracheya, R. D. et al. Function and expression of melatonin receptors on human pancreatic islets. J. Pineal Res. 44, 273–279 (2008).
Tuomi, T. et al. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 23, 1067–1077 (2016).
Claustrat, B., Brun, J. & Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 9, 11–24 (2005).
Moller, N. & Jorgensen, J. O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 30, 152–177 (2009).
Morris, C. J., Aeschbach, D. & Scheer, F. A. Circadian system, sleep and endocrinology. Mol. Cell. Endocrinol. 349, 91–104 (2012).
Willoughby, J. O. & Martin, J. B. The suprachiasmatic nucleus synchronizes growth hormone secretory rhythms with the light-dark cycle. Brain Res. 151, 413–417 (1978).
La Fleur, S. E., Kalsbeek, A., Wortel, J., Fekkes, M. L. & Buijs, R. M. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes 50, 1237–1243 (2001).
Coomans, C. P. et al. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 62, 1102–1108 (2013).
Morris, C. J., Purvis, T. E., Mistretta, J. & Scheer, F. A. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J. Clin. Endocrinol. Metab. 101, 1066–1074 (2016).
Morris, C. J. et al. The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity 23, 2053–2058 (2015).
Krauchi, K. & Wirz-Justice, A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am. J. Physiol. 267, R819–R829 (1994).
Moran-Ramos, S. et al. The suprachiasmatic nucleus drives day-night variations in postprandial triglyceride uptake into skeletal muscle and brown adipose tissue. Exp. Physiol. 102, 1584–1595 (2017).
Amir, S., Shizgal, P. & Rompre, P. P. Glutamate injection into the suprachiasmatic nucleus stimulates brown fat thermogenesis in the rat. Brain Res. 498, 140–144 (1989).
Bamshad, M., Song, C. K. & Bartness, T. J. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 276, R1569–R1578 (1999).
Hussain, M. M. & Pan, X. Circadian regulation of macronutrient absorption. J. Biol. Rhythms 30, 459–469 (2015).
Scheving, L. A. Biological clocks and the digestive system. Gastroenterology 119, 536–549 (2000).
Hoogerwerf, W. A. Role of clock genes in gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G549–G555 (2010).
Iwashina, I., Mochizuki, K., Inamochi, Y. & Goda, T. Clock genes regulate the feeding schedule-dependent diurnal rhythm changes in hexose transporter gene expressions through the binding of BMAL1 to the promoter/enhancer and transcribed regions. J. Nutr. Biochem. 22, 334–343 (2011).
Nishida, T., Saito, M. & Suda, M. Parallel between circadian rhythms of intestinal disaccharidases and food intake of rats under constant lighting conditions. Gastroenterology 74, 224–227 (1978).
Saito, M., Kato, H. & Suda, M. Circadian rhythm of intestinal disaccharidases of rats fed with adiurnal periodicity. Am. J. Physiol. 238, G97–G101 (1980).
Hansen, J. et al. Synchronized human skeletal myotubes of lean, obese and type 2 diabetic patients maintain circadian oscillation of clock genes. Sci. Rep. 6, 35047 (2016).
Perrin, L. et al. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol. Metab. 4, 834–845 (2015).
Guo, H., Brewer, J. M., Lehman, M. N. & Bittman, E. L. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J. Neurosci. 26, 6406–6412 (2006).
Guo, H., Brewer, J. M., Champhekar, A., Harris, R. B. & Bittman, E. L. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc. Natl Acad. Sci. USA 102, 3111–3116 (2005).
Yamanaka, Y., Honma, S. & Honma, K. Scheduled exposures to a novel environment with a running-wheel differentially accelerate re-entrainment of mice peripheral clocks to new light-dark cycles. Genes Cells 13, 497–507 (2008).
Wolff, G. & Esser, K. A. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med. Sci. Sports Exercise 44, 1663–1670 (2012).
Reznick, J. et al. Altered feeding differentially regulates circadian rhythms and energy metabolism in liver and muscle of rats. Biochim. Biophys. Acta 1832, 228–238 (2013).
Opperhuizen, A. L. et al. Feeding during the resting phase causes profound changes in physiology and desynchronization between liver and muscle rhythms of rats. Eur. J. Neurosci. 44, 2795–2806 (2016).
Feneberg, R. & Lemmer, B. Circadian rhythm of glucose uptake in cultures of skeletal muscle cells and adipocytes in Wistar-Kyoto, Wistar, Goto-Kakizaki, and spontaneously hypertensive rats. Chronobiol. Int. 21, 521–538 (2004).
Dyar, K. A. et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 3, 29–41 (2014).
Liu, J. et al. CLOCK and BMAL1 regulate muscle insulin sensitivity via SIRT1 in male mice. Endocrinology 157, 2259–2269 (2016).
Hong, S. et al. Dissociation of muscle insulin sensitivity from exercise endurance in mice by HDAC3 depletion. Nat. Med. 23, 223–234 (2017).
Verrillo, A. et al. Differential roles of splanchnic and peripheral tissues in determining diurnal fluctuation of glucose tolerance. Am. J. Physiol. 257, E459–E465 (1989).
van Moorsel, D. et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol. Metab. 5, 635–645 (2016).
Otway, D. T., Frost, G. & Johnston, J. D. Circadian rhythmicity in murine pre-adipocyte and adipocyte cells. Chronobiol. Int. 26, 1340–1354 (2009).
Ramanathan, C. et al. Cell type-specific functions of period genes revealed by novel adipocyte and hepatocyte circadian clock models. PLOS Genet. 10, e1004244 (2014).
Huang, T. S. et al. Induction of circadian rhythm in cultured human mesenchymal stem cells by serum shock and cAMP analogs in vitro. Chronobiol. Int. 26, 242–257 (2009).
Gomez-Santos, C. et al. Circadian rhythm of clock genes in human adipose explants. Obesity 17, 1481–1485 (2009).
Kolbe, I. et al. The SCN clock governs circadian transcription rhythms in murine epididymal white adipose tissue. J. Biol. Rhythms 31, 577–587 (2016).
Wehrens, S. M. T. et al. Meal timing regulates the human circadian system. Curr. Biol. 27, 1768–1775 (2017).Study demonstrating the effect of meal timing on adipose tissue clock gene expression in humans.
Su, Y., Foppen, E., Zhang, Z., Fliers, E. & Kalsbeek, A. Effects of 6-meals-a-day feeding and 6-meals-a-day feeding combined with adrenalectomy on daily gene expression rhythms in rat epididymal white adipose tissue. Genes Cells 21, 6–24 (2016).
Loboda, A. et al. Diurnal variation of the human adipose transcriptome and the link to metabolic disease. BMC. Med. Genom. 2, 7 (2009).
Carrasco-Benso, M. P. et al. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J. 30, 3117–3123 (2016).First study showing a circadian rhythm in insulin signalling in cultured white adipose tissue explants from humans.
Gliniak, C. M., Brown, J. M. & Noy, N. The retinol-binding protein receptor STRA6 regulates diurnal insulin responses. J. Biol. Chem. 292, 15080–15093 (2017).
Delezie, J. et al. The nuclear receptor REV-ERBalpha is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 26, 3321–3335 (2012).
Shostak, A., Meyer-Kovac, J. & Oster, H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 62, 2195–2203 (2013).
van der Veen, D. R., Shao, J., Chapman, S., Leevy, W. M. & Duffield, G. E. A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET-FDG imaging. Obesity 20, 1527–1529 (2012).
Lee, P. et al. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab. 23, 602–609 (2016).
Sakamoto, K. et al. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J. Biol. Chem. 273, 27039–27042 (1998).
Akhtar, R. A. et al. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 12, 540–550 (2002).
Stokkan, K. A., Yamazaki, S., Tei, H., Sakaki, Y. & Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 (2001).
Panda, S. et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 (2002).
Kornmann, B., Schaad, O., Bujard, H., Takahashi, J. S. & Schibler, U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLOS Biol. 5, e34 (2007).
Robles, M. S., Cox, J. & Mann, M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLOS Genet. 10, e1004047 (2014).
Mauvoisin, D. et al. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl Acad. Sci. USA 111, 167–172 (2014).
Gooley, J. J. & Chua, E. C. Diurnal regulation of lipid metabolism and applications of circadian lipidomics. J. Genet. Genomics 41, 231–250 (2014).
Abbondante, S., Eckel-Mahan, K. L., Ceglia, N. J., Baldi, P. & Sassone-Corsi, P. Comparative circadian metabolomics reveal differential effects of nutritional challenge in the serum and liver. J. Biol. Chem. 291, 2812–2828 (2016).
Krishnaiah, S. Y. et al. Clock regulation of metabolites reveals coupling between transcription and metabolism. Cell Metab. 25, 1206 (2017).
Lamia, K. A., Storch, K. F. & Weitz, C. J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl Acad. Sci. USA 105, 15172–15177 (2008).Key study showing that the hepatic clock is essential to maintain euglycemia during the fasting period in mice.
Rudic, R. D. et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLOS Biol. 2, e377 (2004).
Lamia, K. A. et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480, 552–556 (2011).
Zhang, E. E. et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 16, 1152–1156 (2010).
Jang, H. et al. SREBP1c-CRY1 signalling represses hepatic glucose production by promoting FOXO1 degradation during refeeding. Nat. Commun. 7, 12180 (2016).
Tong, X. et al. DDB1-mediated CRY1 degradation promotes FOXO1-driven gluconeogenesis in liver. Diabetes 66, 2571–2582 (2017).
Macauley, M., Smith, F. E., Thelwall, P. E., Hollingsworth, K. G. & Taylor, R. Diurnal variation in skeletal muscle and liver glycogen in humans with normal health and Type 2 diabetes. Clin. Sci. 128, 707–713 (2015).
Peek, C. B. et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 25, 86–92 (2017).
Neufeld-Cohen, A. et al. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc. Natl Acad. Sci. USA 113, E1673–E1682 (2016).
Jacobi, D. et al. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 22, 709–720 (2015).
Vieira, E., Burris, T. P. & Quesada, I. Clock genes, pancreatic function, and diabetes. Trends Mol. Med. 20, 685–693 (2014).
Peschke, E. & Peschke, D. Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia 41, 1085–1092 (1998).
Marcheva, B. et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 (2010).Pivotal study showing that specific ablation of the pancreatic clock disrupts insulin secretion in mice.
Qian, J., Block, G. D., Colwell, C. S. & Matveyenko, A. V. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes 62, 3469–3478 (2013).
Pulimeno, P. et al. Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia 56, 497–507 (2013).
Saini, C. et al. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obes. Metab. 18, 355–365 (2016).Study demonstrating that CLOCK inhibition with small interfering RNA disrupts insulin secretion in cultured human pancreatic islet cells.
Buijs, R. M., Chun, S. J., Niijima, A., Romijn, H. J. & Nagai, K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 431, 405–423 (2001).
Perelis, M. et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250 (2015).
Sadacca, L. A., Lamia, K. A., deLemos, A. S., Blum, B. & Weitz, C. J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 54, 120–124 (2011).
Lee, J. et al. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in β-cells. Islets 3, 381–388 (2011).
Defronzo, R. A. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009).
Shulman, G. I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 106, 171–176 (2000).
Shulman, G. I. et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 322, 223–228 (1990).
Reaven, G. M., Chen, Y. D., Donner, C. C., Fraze, E. & Hollenbeck, C. B. How insulin resistant are patients with noninsulin-dependent diabetes mellitus? J. Clin. Endocrinol. Metab. 61, 32–36 (1985).
Winocour, P. H. et al. A randomized cross-over study of the effects of proinsulin on lipid metabolism in type 2 diabetes. Diabet. Med. 8, 22–27 (1991).
Meek, S. E., Nair, K. S. & Jensen, M. D. Insulin regulation of regional free fatty acid metabolism. Diabetes 48, 10–14 (1999).
Unger, R. H. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144, 5159–5165 (2003).
Jarrett, R. J. & Keen, H. Diurnal variation of oral glucose tolerance: a possible pointer to the evolution of diabetes mellitus. Br. Med. J. 2, 341–344 (1969).First study showing an altered daily rhythm in glucose tolerance in patients with type 2 diabetes mellitus.
Turek, F. W. et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 (2005).First study to show metabolic disease in a clock gene knockout mouse.
Arble, D. M., Bass, J., Laposky, A. D., Vitaterna, M. H. & Turek, F. W. Circadian timing of food intake contributes to weight gain. Obes.(Silver. Spring) 17, 2100–2102 (2009).
Scheer, F. A., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458 (2009).First study demonstrating that humans develop reduced glucose tolerance when subjected to conditions of circadian misalignment.
Bass, J. & Takahashi, J. S. Circadian integration of metabolism and energetics. Science 330, 1349–1354 (2010).
Lee, J. et al. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced beta-cell failure in mice. Mol. Cell. Biol. 33, 2327–2338 (2013).
Harfmann, B. D. et al. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 6, 12 (2016).
Paschos, G. K. et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 18, 1768–1777 (2012).
Oishi, K. et al. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 580, 127–130 (2006).
Zani, F. et al. PER2 promotes glucose storage to liver glycogen during feeding and acute fasting by inducing Gys2 PTG and G L expression. Mol. Metab. 2, 292–305 (2013).
Grimaldi, B. et al. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 12, 509–520 (2010).
Stenvers, D. J. et al. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci. Rep. 6, 35662 (2016).
Shamsi, N. A. et al. Metabolic consequences of timed feeding in mice. Physiol. Behav. 128, 188–201 (2014).
Sherman, H. et al. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 26, 3493–3502 (2012).
Kalsbeek, A., la Fleur, S. & Fliers, E. Circadian control of glucose metabolism. Mol. Metab. 3, 372–383 (2014).
Eckel-Mahan, K. & Sassone-Corsi, P. Metabolism and the circadian clock converge. Physiol. Rev. 93, 107–135 (2013).
Fonken, L. K. & Nelson, R. J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 35, 648–670 (2014).
Woon, P. Y. et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl Acad. Sci. USA 104, 14412–14417 (2007).First study showing an association between a clock gene single nucleotide polymorphism and type 2 diabetes mellitus.
Scott, E. M., Carter, A. M. & Grant, P. J. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int. J. Obes. 32, 658–662 (2008).
Sookoian, S. et al. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am. J. Clin. Nutr. 87, 1606–1615 (2008).
Dupuis, J. et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 42, 105–116 (2010).
Barker, A. et al. Association of genetic Loci with glucose levels in childhood and adolescence: a meta-analysis of over 6,000 children. Diabetes 60, 1805–1812 (2011).
Ruano, E. G., Canivell, S. & Vieira, E. REV-ERB ALPHA polymorphism is associated with obesity in the Spanish obese male population. PLOS ONE 9, e104065 (2014).
Dashti, H. S. et al. Gene-environment interactions of circadian-related genes for cardiometabolic traits. Diabetes Care 38, 1456–1466 (2015).
Dashti, H. S. et al. CRY1 circadian gene variant interacts with carbohydrate intake for insulin resistance in two independent populations: Mediterranean and North American. Chronobiol. Int. 31, 660–667 (2014).
Garcia-Rios, A. et al. Beneficial effect of CLOCK gene polymorphism rs1801260 in combination with low-fat diet on insulin metabolism in the patients with metabolic syndrome. Chronobiol. Int. 31, 401–408 (2014).
Garaulet, M. et al. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 90, 1466–1475 (2009).
Loria-Kohen, V. et al. Polymorphism in the CLOCK gene may influence the effect of fat intake reduction on weight loss. Nutrition 32, 453–460 (2016).
Corella, D. et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial. Cardiovasc. Diabetol. 15, 4 (2016).
Ando, H. et al. Clock gene expression in peripheral leucocytes of patients with type 2 diabetes. Diabetologia 52, 329–335 (2009).
Otway, D. T. et al. Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes 60, 1577–1581 (2011).First study comparing in vivo clock gene expression rhythms between healthy participants, participants with obesity and patients with type 2 diabetes mellitus.
Wright, K. P. Jr. et al. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 23, 1554–1558 (2013).
Fonken, L. K. et al. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18664–18669 (2010).
Obayashi, K. et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J. Clin. Endocrinol. Metab. 98, 337–344 (2013).
McFadden, E., Jones, M. E., Schoemaker, M. J., Ashworth, A. & Swerdlow, A. J. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the Breakthrough Generations study. Am. J. Epidemiol. 180, 245–250 (2014).
Obayashi, K., Saeki, K., Iwamoto, J., Ikada, Y. & Kurumatani, N. Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol. Int. 31, 394–400 (2014).
Cheung, I. N. et al. Morning and evening blue-enriched light exposure alters metabolic function in normal weight adults. PLOS ONE 11, e0155601 (2016).
Albreiki, M. S., Middleton, B. & Hampton, S. M. A single night light exposure acutely alters hormonal and metabolic responses in healthy participants. Endocr. Connect. 6, 100–110 (2017).
Versteeg, R. I. et al. Acute effects of morning light on plasma glucose and triglycerides in healthy men and men with type 2 diabetes. J. Biol. Rhythms 32, 130–142 (2017).
Opperhuizen, A. L. et al. Light at night acutely impairs glucose tolerance in a time-, intensity- and wavelength-dependent manner in rats. Diabetologia 60, 1333–1343 (2017).
Lewy, A. J. & Sack, R. L. The dim light melatonin onset as a marker for circadian phase position. Chronobiol. Int. 6, 93–102 (1989).
Rubio-Sastre, P., Scheer, F. A., Gomez-Abellan, P., Madrid, J. A. & Garaulet, M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 37, 1715–1719 (2014).
Cagnacci, A. et al. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin. Endocrinol. (Oxf.) 54, 339–346 (2001).
Garaulet, M. et al. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism 64, 1650–1657 (2015).
Bonnefond, A. & Froguel, P. The case for too little melatonin signalling in increased diabetes risk. Diabetologia 60, 823–825 (2017).
McMullan, C. J., Schernhammer, E. S., Rimm, E. B., Hu, F. B. & Forman, J. P. Melatonin secretion and the incidence of type 2 diabetes. JAMA 309, 1388–1396 (2013).
Bonnefond, A. et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat. Genet. 44, 297–301 (2012).
Shan, Z. et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 38, 529–537 (2015).Meta-analysis demonstrating an association between sleep duration and the incidence of type 2 diabetes mellitus.
Stamatakis, K. A. & Punjabi, N. M. Long sleep duration: a risk to health or a marker of risk? Sleep Med. Rev. 11, 337–339 (2007).
Cappuccio, F. P., D’Elia, L., Strazzullo, P. & Miller, M. A. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33, 414–420 (2010).
Bosy-Westphal, A. et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes. Facts 1, 266–273 (2008).
Nedeltcheva, A. V. et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr. 89, 126–133 (2009).
Reutrakul, S. & Mokhlesi, B. Obstructive sleep apnea and diabetes: a state of the art review. Chest 152, 1070–1086 (2017).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999).Seminal experimental study showing the detrimental effect of sleep debt on glucose metabolism.
Donga, E. et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J. Clin. Endocrinol. Metab. 95, 2963–2968 (2010).
Broussard, J. L., Ehrmann, D. A., Van Cauter, E., Tasali, E. & Brady, M. J. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann. Intern. Med. 157, 549–557 (2012).
Buxton, O. M. et al. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 59, 2126–2133 (2010).
Cedernaes, J. et al. A single night of partial sleep loss impairs fasting insulin sensitivity but does not affect cephalic phase insulin release in young men. J. Sleep Res. 25, 5–10 (2016).
Rao, M. N. et al. Subchronic sleep restriction causes tissue-specific insulin resistance. J. Clin. Endocrinol. Metab. 100, 1664–1671 (2015).
van Leeuwen, W. M. et al. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int. J. Endocrinol. 2010, 108641 (2010).
Robertson, M. D., Russell-Jones, D., Umpleby, A. M. & Dijk, D. J. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism 62, 204–211 (2013).
St-Onge, M. P., O’Keeffe, M., Roberts, A. L., RoyChoudhury, A. & Laferrere, B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep 35, 1503–1510 (2012).
Tasali, E., Leproult, R., Ehrmann, D. A. & Van Cauter, E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl Acad. Sci. USA 105, 1044–1049 (2008).
Stamatakis, K. A. & Punjabi, N. M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 137, 95–101 (2010).
Herzog, N. et al. Selective slow wave sleep but not rapid eye movement sleep suppression impairs morning glucose tolerance in healthy men. Psychoneuroendocrinology 38, 2075–2082 (2013).
Lee, S. W. H., Ng, K. Y. & Chin, W. K. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Med. Rev. 31, 91–101 (2017). Systematic review showing the relationship between sleep and glycaemic control in patients with type 2 diabetes mellitus.
Merikanto, I. et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol. Int. 30, 470–477 (2013).
Yu, J. H. et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J. Clin. Endocrinol. Metab. 100, 1494–1502 (2015).
Vetter, C. et al. Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care 38, 1707–1713 (2015). Study demonstrating the association between social jet lag and the risk of type 2 diabetes mellitus.
Parsons, M. J. et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study.Int. J. Obes. 39, 842–848 (2015).
Koopman, A. D. M. et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the New Hoorn study. J. Biol. Rhythms 32, 359–368 (2017).
Reutrakul, S. et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care 36, 2523–2529 (2013).
Osonoi, Y. et al. Morningness-eveningness questionnaire score and metabolic parameters in patients with type 2 diabetes mellitus. Chronobiol. Int. 31, 1017–1023 (2014).
Gan, Y. et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occupat. Environ. Med. 72, 72–78 (2015). Meta-analysis showing the association between shift work and diabetes mellitus.
Vetter, C. et al. Night shift work, genetic risk, and type 2 diabetes in the UK biobank. Diabetes Care 41, 762–769 (2018).
Leproult, R., Holmback, U. & Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63, 1860–1869 (2014).
Qian, J., Dalla Man, C., Morris, C. J., Cobelli, C. & Scheer, F. A. Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes. Metab. 20, 2481–2485 (2018).
Wefers, J. et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl Acad. Sci. USA 115, 7789–7794 (2018).
Opperhuizen, A. L., van Kerkhof, L. W., Proper, K. I., Rodenburg, W. & Kalsbeek, A. Rodent models to study the metabolic effects of shiftwork in humans. Front. Pharmacol. 6, 50 (2015).
Thaiss, C. A. et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 (2014).
Riemersma-van der Lek, R. F. et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA 299, 2642–2655 (2008).
Hu, K. et al. Progression of dementia assessed by temporal correlations of physical activity: results from a 3.5-Year, longitudinal randomized controlled trial. Sci. Rep. 6, 27742 (2016).
Sander, B., Markvart, J., Kessel, L., Argyraki, A. & Johnsen, K. Can sleep quality and wellbeing be improved by changing the indoor lighting in the homes of healthy, elderly citizens? Chronobiol. Int. 32, 1049–1060 (2015).
Chang, A.-M., Aeschbach, D., Duffy, J. F. & Czeisler, C. A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl Acad. Sci. 112, 1232–1237 (2015).
Tan, X., van Egmond, L., Chapman, C. D., Cedernaes, J. & Benedict, C. Aiding sleep in type 2 diabetes: therapeutic considerations. Lancet Diabetes Endocrinol. 6, 60–68 (2018).
Young, T. Increasing sleep duration for a healthier (and less obese?) population tomorrow. Sleep 31, 593–594 (2008).
Leproult, R., Deliens, G., Gilson, M. & Peigneux, P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep 38, 707–715 (2015).
Cizza, G., Piaggi, P., Rother, K. I. & Csako, G. & Sleep Extension Study, G. Hawthorne effect with transient behavioral and biochemical changes in a randomized controlled sleep extension trial of chronically short-sleeping obese adults: implications for the design and interpretation of clinical studies. PLOS ONE 9, e104176 (2014).
Riemann, D. et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 26, 675–700 (2017).
Chen, L. et al. Continuous positive airway pressure and diabetes risk in sleep apnea patients: a systemic review and meta-analysis. Eur. J. Intern. Med. 39, 39–50 (2017).Meta-analysis investigating a potential relation between obstructive sleep apnoea treatment and the risk of developing type 2 diabetes mellitus.
Zhu, B., Ma, C., Chaiard, J. & Shi, C. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath. 22, 287–295 (2017).
Martinez-Ceron, E. et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am. J. Respir. Crit. Care Med. 194, 476–485 (2016).
Mokhlesi, B., Grimaldi, D., Beccuti, G. & Van Cauter, E. Effect of one week of CPAP treatment of obstructive sleep apnoea on 24-hour profiles of glucose, insulin and counter-regulatory hormones in type 2 diabetes. Diabetes Obes. Metab. 19, 452–456 (2017).
Colberg, S. R. et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 39, 2065–2079 (2016).
Buxton, O. M., Lee, C. W., L’Hermite-Baleriaux, M., Turek, F. W. & Van Cauter, E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R714–R724 (2003).
Kredlow, M. A., Capozzoli, M. C., Hearon, B. A., Calkins, A. W. & Otto, M. W. The effects of physical activity on sleep: a meta-analytic review. J. Behav. Med. 38, 427–449 (2015).
Mattson, M. P. et al. Meal frequency and timing in health and disease. Proc. Natl Acad. Sci. USA 111, 16647–16653 (2014).
St-Onge, M. P. et al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation 135, e96–e121 (2017).Statement from the American Heart Association on the effects of meal timing and frequency for metabolic health.
Jakubowicz, D., Barnea, M., Wainstein, J. & Froy, O. High caloric intake at breakfast versus dinner differentially influences weight loss of overweight and obese women. Obesity 21, 2504–2512 (2013).
Farshchi, H. R., Taylor, M. A. & Macdonald, I. A. Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. Am. J. Clin. Nutr. 81, 16–24 (2005).
Farshchi, H. R., Taylor, M. A. & Macdonald, I. A. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am. J. Clin. Nutr. 81, 388–396 (2005).
Chowdhury, E. A. et al. The causal role of breakfast in energy balance and health: a randomized controlled trial in obese adults. Am. J. Clin. Nutr. 103, 747–756 (2016).
Betts, J. A. et al. The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am. J. Clin. Nutr. 100, 539–547 (2014).
Versteeg, R. I. et al. Meal timing effects on insulin sensitivity and intrahepatic triglycerides during weight loss. Int. J. Obes. 42, 156–162 (2017).
Gill, S. & Panda, S. A. Smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 22, 789–798 (2015).Study demonstrating chaotic diurnal eating patterns in humans, as well as the potential clinical benefits of time-restricted feeding.
Sutton, E. F. et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221 (2018).
Holman, R. R., Sourij, H. & Califf, R. M. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet 383, 2008–2017 (2014).
Um, J. H. et al. Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J. Biol. Chem. 282, 20794–20798 (2007).
Barnea, M. et al. Metformin affects the circadian clock and metabolic rhythms in a tissue-specific manner. Biochim. Biophys. Acta 1822, 1796–1806 (2012).
Henriksson, E. et al. The liver circadian clock modulates biochemical and physiological responses to metformin. J. Biol. Rhythms 32, 345–358 (2017).
Baker, I. A. & Jarrett, R. J. Diurnal variation in the blood-sugar and plasma-insulin response to tolbutamide. Lancet 2, 945–947 (1972).
Webb, I. C., Lehman, M. N. & Coolen, L. M. Diurnal and circadian regulation of reward-related neurophysiology and behavior. Physiol. Behav. 143, 58–69 (2015).
Ter Horst, K. W. et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci. Transl Med. 10, eaar3752 (2018).
Liang, W. et al. Efficacy and safety of bromocriptine-QR in type 2 diabetes: a systematic review and meta-analysis. Horm. Metab. Res. 47, 805–812 (2015).
Garfinkel, D. et al. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab. Syndr. Obes. 4, 307–313 (2011).
Holleman, F. & Gale, E. A. Nice insulins, pity about the evidence. Diabetologia 50, 1783–1790 (2007).
Wallia, A. & Molitch, M. E. Insulin therapy for type 2 diabetes mellitus. JAMA 311, 2315–2325 (2014).
Stenvers, D. J., DeVries, J. H. & la Fleur, S. E. What’s the time? Does the artificial pancreas need to know? Diabetes 62, 2173–2174 (2013).
Thabit, H. et al. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol. 5, 117–124 (2016).
Johannsson, G. et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J. Clin. Endocrinol. Metab. 97, 473–481 (2012).
Quinkler, M., Miodini Nilsen, R., Zopf, K., Ventz, M. & Oksnes, M. Modified-release hydrocortisone decreases BMI and HbA1c in patients with primary and secondary adrenal insufficiency. Eur. J. Endocrinol. 172, 619–626 (2015).
Giordano, R. et al. Improvement of anthropometric and metabolic parameters, and quality of life following treatment with dual-release hydrocortisone in patients with Addison’s disease. Endocr 51, 360–368 (2016).
Zhang, E. E. et al. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell 139, 199–210 (2009).
Chen, Z. et al. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc. Natl Acad. Sci. USA 109, 101–106 (2012).
Chen, Z., Yoo, S. H. & Takahashi, J. S. Development and therapeutic potential of small-molecule modulators of circadian systems. Annu. Rev. Pharmacol. Toxicol. 58, 231–252 (2018).
Solt, L. A. et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68 (2012).Study demonstrating beneficial metabolic effects of targeting the molecular clock with REV-ERB agonists in mice.
He, B. et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 23, 610–621 (2016).Study demonstrating that nobiletin improves metabolic health in mouse models of the metabolic syndrome by strengthening the molecular clock.
Humphries, P. S. et al. Carbazole-containing sulfonamides and sulfamides: discovery of cryptochrome modulators as antidiabetic agents. Bioorg. Med. Chem. Lett. 26, 757–760 (2016).
Humphries, P. S. et al. Carbazole-containing amides and ureas: discovery of cryptochrome modulators as antihyperglycemic agents. Bioorg. Med. Chem. Lett. 28, 293–297 (2017).
Laing, E. E. et al. Blood transcriptome based biomarkers for human circadian phase. eLife 6, e20214 (2017).
Woller, A., Duez, H., Staels, B. & Lefranc, M. A. Mathematical model of the liver circadian clock linking feeding and fasting cycles to clock function. Cell Rep. 17, 1087–1097 (2016).
Samuel, V. T. & Shulman, G. I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 126, 12–22 (2016).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27, 1487–1495 (2004).
Sperling, L. S. et al. The CardioMetabolic Health Alliance: working toward a new care model for the metabolic syndrome. J. Am. College Cardiol. 66, 1050–1067 (2015).
Author information
Authors and Affiliations
Contributions
D.J.S. researched data for article, all authors provided a substantial contribution to discussion of content and wrote the article, F.A.J.L.S., P.S., S.E.L.F. and A.K. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
F.A.J.L.S. received speaker fees from Bayer Healthcare, Kellogg Company, Philips, Pfizer, Sentara Healthcare and Vanda Pharmaceuticals. F.A.J.L.S. was supported in part by NIH grants R01DK099512, R01HL118601, R01DK102696, R01DK105072 and R01HL140574. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Period
-
The time difference between two consecutive peaks or troughs, or any other fixed point in the rhythm. In the case of daily or circadian rhythms, this period is exactly or approximately 24 h, respectively. The period of the rhythm in constant conditions is called the free-running period and is denoted by the Greek letter τ.
- Amplitude
-
On a line graph, the amplitude is half the distance between the peak and trough of a daily or circadian rhythm.
Rights and permissions
About this article
Cite this article
Stenvers, D.J., Scheer, F.A.J.L., Schrauwen, P. et al. Circadian clocks and insulin resistance. Nat Rev Endocrinol 15, 75–89 (2019). https://doi.org/10.1038/s41574-018-0122-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-018-0122-1