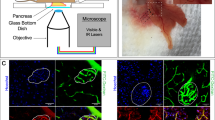
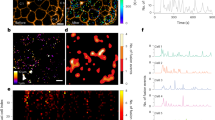
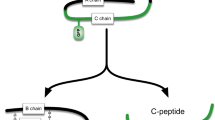
Abstract
Following stimulation, pancreatic β-cells must orchestrate a plethora of signalling events to ensure the appropriate release of insulin and maintenance of normal glucose homeostasis. Failure at any point in this cascade leads to impaired insulin secretion, elevated blood levels of glucose and eventually type 2 diabetes mellitus. Likewise, β-cell replacement or regeneration strategies for the treatment of both type 1 and type 2 diabetes mellitus might fail if the correct cell signalling phenotype cannot be faithfully recreated. However, current understanding of β-cell function is complicated because of the highly dynamic nature of their intracellular and intercellular signalling as well as insulin release itself. β-Cells must precisely integrate multiple signals stemming from multiple cues, often with differing intensities, frequencies and cellular and subcellular localizations, before converging these signals onto insulin exocytosis. In this respect, optical approaches with high resolution in space and time are extremely useful for properly deciphering the complexity of β-cell signalling. An increased understanding of β-cell signalling might identify new mechanisms underlying insulin release, with relevance for future drug therapy and de novo stem cell engineering of functional islets.
Key points
-
β-Cells are capable of integrating signals that vary over space and time.
-
The conventional view of β-cells does not fully consider the dynamic nature of β-cell stimulus–secretion coupling and insulin secretion.
-
The mechanisms by which β-cells dynamically integrate multiple signals or cues might be implicated in metabolic disease.
-
Optical approaches possess the necessary spatial and temporal resolution to unravel the complexity of β-cell signalling.
-
Restoring normal β-cell signalling dynamics might allow improved treatment of type 2 diabetes mellitus (T2DM).
-
β-Cell engineering, reprogramming or regeneration studies should consider the normal signalling phenotype to provide optimal type 1 diabetes mellitus and T2DM therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Halban, P. A. et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 37, 1751–1758 (2014).
Eizirik, D. L., Colli, M. L. & Ortis, F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 5, 219–226 (2009).
World Health Organization in Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors (eds Ezzati, M., Lopez, A. D., Rodgers, A. A., Murray, C. J. L.) (WHO, Geneva, 2004).
Porte, D. & Kahn, S. E. Beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes 50, S160–S163 (2001).
Prentki, M. & Nolan, C. J. Islet beta cell failure in type 2 diabetes. J. Clin. Invest. 116, 1802–1812 (2006).
Simmons, K. M., Gottlieb, P. A. & Michels, A. W. Immune intervention and preservation of pancreatic beta cell function in type 1 diabetes. Curr. Diab. Rep. 16, 97 (2016).
Chaudhury, A. et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front. Endocrinol. 8, 6 (2017).
Li, W. et al. Long-term persistence and development of induced pancreatic beta cells generated by lineage conversion of acinar cells. Nat. Biotechnol. 32, 1223–1230 (2014).
Chera, S. et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514, 503–507 (2014).
Thorel, F. et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464, 1149–1154 (2010).
Rezania, A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133 (2014).
Vegas, A. J. et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 22, 306–311 (2016).
Rutter, G. A., Pullen, T. J., Hodson, D. J. & Martinez-Sanchez, A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 466, 203–218 (2015).
Rorsman, P. & Ashcroft, F. M. Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol. Rev. 98, 117–214 (2018).
Merrins, M. J. et al. Phase analysis of metabolic oscillations and membrane potential in pancreatic islet β-cells. Biophys. J. 110, 691–699 (2016).
Watts, M. et al. Calcium and metabolic oscillations in pancreatic islets: who’s driving the bus? SIAM J. Appl. Dyn. Syst. 13, 683–703 (2014).
Jacobson, D. A. et al. Calcium-activated and voltage-gated potassium channels of the pancreatic islet impart distinct and complementary roles during secretagogue induced electrical responses. J. Physiol. 588, 3525–3537 (2010).
Gilon, P., Ravier, M. A., Jonas, J. C. & Henquin, J. C. Control mechanisms of the oscillations of insulin secretion in vitro and in vivo. Diabetes 51, S144–S151 (2002).
Henquin, J. C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49, 1751–1760 (2000).
Kalwat, M. A. & Cobb, M. H. Mechanisms of the amplifying pathway of insulin secretion in the β cell. Pharmacol. Ther. 179, 17–30 (2017).
Dyachok, O., Isakov, Y., Sagetorp, J. & Tengholm, A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 439, 349–352 (2006). The article presents the first study that revealed the dynamics of cAMP in β-cells and provides an early glimpse of the signalling complexity inherent to these cells.
Wuttke, A., Idevall-Hagren, O. & Tengholm, A. P2Y1 receptor-dependent diacylglycerol signaling microdomains in beta cells promote insulin secretion. FASEB J. 27, 1610–1620 (2013).
Seino, S. & Shibasaki, T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 85, 1303–1342 (2005).
Seino, S., Takahashi, H., Fujimoto, W. & Shibasaki, T. Roles of cAMP signalling in insulin granule exocytosis. Diabetes Obes. Metab. 11 (Suppl. 4), 180–188 (2009).
Biden Trevor, J. et al. The diverse roles of protein kinase C in pancreatic β-cell function. Biochem. Soc. Trans. 36, 916–919 (2008).
Tian, G., Sol, E. R., Xu, Y., Shuai, H. & Tengholm, A. Impaired cAMP generation contributes to defective glucose-stimulated insulin secretion after long-term exposure to palmitate. Diabetes 64, 904–915 (2015).
Chen, C. et al. Alterations in β-cell calcium dynamics and efficacy outweigh islet mass adaptation in compensation of insulin resistance and prediabetes onset. Diabetes 65, 2676–2685 (2016).
Hoppa, M. B. et al. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca2+ channels from secretory granules. Cell Metab. 10, 455–465 (2009).
Gandasi, N. R. et al. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. J. Clin. Invest. 127, 2353–2364 (2017). Super-resolution imaging reveals the clustering of VDCCs in microdomains with insulin granules, adding a hitherto unknown element to the regulation of Ca 2+ fluxes in β-cells.
Rutter, G. A. et al. Local and regional control of calcium dynamics in the pancreatic islet. Diabetes Obes. Metab. 19, 30–41 (2017).
Spiegel, S., Foster, D. & Kolesnick, R. Signal transduction through lipid second messengers. Curr. Opin. Cell Biol. 8, 159–167 (1996).
O’Neill, C. M. et al. Circulating levels of IL-1B + IL-6 cause ER stress and dysfunction in islets from prediabetic male mice. Endocrinology 154, 3077–3088 (2013).
Hara, T. et al. Calcium efflux from the endoplasmic reticulum leads to beta-cell death. Endocrinology 155, 758–768 (2014).
Kuchenov, D. et al. High-content imaging platform for profiling intracellular signaling network activity in living cells. Cell Chem. Biol. 23, 1550–1559 (2016). This paper presents a novel imaging platform for screening the effects of ligands or drugs on multiple pathways.
Benninger, R. K. & Piston, D. W. Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics. Trends Endocrinol. Metab. 25, 399–406 (2014).
Zhang, Q. et al. Cell coupling in mouse pancreatic beta-cells measured in intact islets of Langerhans. Philos. Trans. A Math. Phys. Eng. Sci. 366, 3503–3523 (2008).
Benninger, R. K., Zhang, M., Head, W. S., Satin, L. S. & Piston, D. W. Gap junction coupling and calcium waves in the pancreatic islet. Biophys. J. 95, 5048–5061 (2008).
Nunemaker, C. S. et al. Glucose metabolism, islet architecture, and genetic homogeneity in imprinting of [Ca2+]i and insulin rhythms in mouse islets. PLOS ONE 4, e8428 (2009).
Tian, G., Sandler, S., Gylfe, E. & Tengholm, A. Glucose- and hormone-induced cAMP oscillations in alpha- and beta-cells within intact pancreatic islets. Diabetes 60, 1535–1543 (2011).
Westacott, M. J. et al. Age-dependent decline in the coordinated [Ca2+] and insulin secretory dynamics in human pancreatic islets. Diabetes 66, 2436–2445 (2017).
Martin, F. & Soria, B. Glucose-induced [Ca2+]i oscillations in single human pancreatic islets. Cell Calcium 20, 409–414 (1996).
Cabrera, O. et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl Acad. Sci. USA 103, 2334–2339 (2006). This early study points to critical differences in islet architecture and β-cell Ca 2+ signalling in rodent versus porcine versus human islets.
Low, J. T. et al. Glucose principally regulates insulin secretion in mouse islets by controlling the numbers of granule fusion events per cell. Diabetologia 56, 2629–2637 (2013).
Almaca, J. et al. Spatial and temporal coordination of insulin granule exocytosis in intact human pancreatic islets. Diabetologia 58, 2810–2818 (2015).
Hodson, D. J. et al. Lipotoxicity disrupts incretin-regulated human beta cell connectivity. J. Clin. Invest. 123, 4182–4194 (2013).
Head, W. S. et al. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes 61, 1700–1707 (2012). This landmark study links β-cell-specific loss of connexin 36 gap junction channels with defective Ca 2+ signals and loss of pulsatile insulin secretion in mice.
Hauke, S., Keutler, K., Phapale, P., Yushchenko, D. A. & Schultz, C. Endogenous fatty acids are essential signaling factors of pancreatic β-cells and insulin secretion. Diabetes 67, 1986–1998 (2018).
Braun, M., Ramracheya, R. & Rorsman, P. Autocrine regulation of insulin secretion. Diabetes Obes. Metab. 14 (Suppl. 3), 143–151 (2012).
Caicedo, A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin. Cell Dev. Biol. 24, 11–21 (2013).
van der Meulen, T. et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med. 21, 769–776 (2015).
Salomon, D. & Meda, P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp. Cell Res. 162, 507–520 (1986).
Maedler, K. et al. Restructuring of pancreatic islets and insulin secretion in a postnatal critical window. PLOS ONE 1, e35 (2006).
Hiriart, M. & Ramirez-Medeles, M. C. Functional subpopulations of individual pancreatic B-cells in culture. Endocrinology 128, 3193–3198 (1991).
Van Schravendijk, C. F., Kiekens, R. & Pipeleers, D. G. Pancreatic beta cell heterogeneity in glucose-induced insulin secretion. J. Biol. Chem. 267, 21344–21348 (1992).
Benninger, R. K. P. & Hodson, D. J. New understanding of β-cell heterogeneity and in situ islet function. Diabetes 67, 537–547 (2018).
Dorrell, C. et al. Human islets contain four distinct subtypes of β cells. Nat. Commun. 7, 11756 (2016).
Wang Yue, J. et al. Single-cell mass cytometry analysis of the human endocrine pancreas. Cell Metab. 24, 616–626 (2016).
Bader, E. et al. Identification of proliferative and mature beta-cells in the islets of Langerhans. Nature 535, 430–434 (2016).
van der Meulen, T. et al. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab. 25, 911–926 (2017).
Singh, S. P. et al. Different developmental histories of beta-cells generate functional and proliferative heterogeneity during islet growth. Nat. Commun. 8, 664 (2017).
Ling, Z. et al. Intercellular differences in interleukin 1beta-induced suppression of insulin synthesis and stimulation of noninsulin protein synthesis by rat pancreatic beta-cells. Endocrinology 139, 1540–1545 (1998).
Rui, J. et al. β cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metab. 25, 727–738 (2017).
Janjuha, S. et al. Age-related islet inflammation marks the proliferative decline of pancreatic beta-cells in zebrafish. eLife 7, e32965 (2018).
Dufer, M. Determination of beta-cell function: ion channel function in beta cells. Methods Mol. Biol. 933, 203–217 (2012).
Dunlop, J., Bowlby, M., Peri, R., Vasilyev, D. & Arias, R. High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology. Nat. Rev. Drug Discov. 7, 358–368 (2008).
Trammell, S. A. & Brenner, C. Targeted, LCMS-based metabolomics for quantitative measurement of NAD+ metabolites. Comput. Struct. Biotechnol. J. 4, e201301012 (2013).
Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 12, 668–679 (2016).
Murray, A. J. Pharmacological PKA inhibition: all may not be what it seems. Sci. Signal. 1, re4 (2008).
Paredes, R. M., Etzler, J. C., Watts, L. T., Zheng, W. & Lechleiter, J. D. Chemical calcium indicators. Methods 46, 143–151 (2008).
Smith, N. A. et al. Fluorescent Ca2+ indicators directly inhibit the Na, K-ATPase and disrupt cellular functions. Sci. Signal. 11, eaal2039 (2018).
Düfer, M. et al. Activation of the Na+/K+-ATPase by insulin and glucose as a putative negative feedback mechanism in pancreatic beta-cells. Pflugers Arch. 457, 1351–1360 (2008).
Miller, E. W. et al. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc. Natl Acad. Sci. USA 109, 2114–2119 (2012).
Chen, L. B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell Biol. 4, 155–181 (1988).
Dolensek, J., Stozer, A., Skelin Klemen, M., Miller, E. W. & Slak Rupnik, M. The relationship between membrane potential and calcium dynamics in glucose-stimulated beta cell syncytium in acute mouse pancreas tissue slices. PLOS ONE 8, e82374 (2013).
Li, L. et al. Defects in β-cell Ca2+ dynamics in age-induced diabetes. Diabetes 63, 4100–4114 (2014).
Lu, H., Koshkin, V., Allister, E. M., Gyulkhandanyan, A. V. & Wheeler, M. B. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes 59, 448–459 (2010).
Tour, O. et al. Calcium Green FlAsH as a genetically targeted small-molecule calcium indicator. Nat. Chem. Biol. 3, 423–431 (2007).
Zhao, Y. et al. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 (2011).
Akerboom, J. et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6, 2 (2013).
Johnston Natalie, R. et al. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab. 24, 389–401 (2016). This study uses all-optical approaches to interrogate the role of β-cell subpopulations in the intact islet, revealing disproportionate control of insulin release.
Fosque, B. F. et al. Labeling of active neural circuits in vivo with designed calcium integrators. Science 347, 755–760 (2015).
Whitaker, M. Genetically encoded probes for measurement of intracellular calcium. Methods Cell Biol. 99, 153–182 (2010).
Akemann, W., Mutoh, H., Perron, A., Rossier, J. & Knopfel, T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat. Methods 7, 643–649 (2010).
Berg, J., Hung, Y. P. & Yellen, G. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat. Methods 6, 161–166 (2009).
Zhao, Y. et al. SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents. Cell Metab. 21, 777–789 (2015).
Cambronne, X. A. et al. Biosensor reveals multiple sources for mitochondrial NAD+. Science 352, 1474–1477 (2016).
Cameron, W. D. et al. Apollo-NADP+: a spectrally tunable family of genetically encoded sensors for NADP+. Nat. Methods 13, 352–358 (2016).
Kitaguchi, T., Oya, M., Wada, Y., Tsuboi, T. & Miyawaki, A. Extracellular calcium influx activates adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells. Biochem. J. 450, 365–373 (2013).
Anderson, K. I. et al. Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity. PLOS ONE 10, e0122513 (2015).
Brun, M. A. et al. A semisynthetic fluorescent sensor protein for glutamate. J. Am. Chem. Soc. 134, 7676–7678 (2012).
Li, J., Shuai, H. Y., Gylfe, E. & Tengholm, A. Oscillations of sub-membrane ATP in glucose-stimulated beta cells depend on negative feedback from Ca2+. Diabetologia 56, 1577–1586 (2013).
Tarasov, A. I. et al. Frequency-dependent mitochondrial Ca2+ accumulation regulates ATP synthesis in pancreatic beta cells. Pflugers Arch. 465, 543–554 (2012).
Hodson, D. J. et al. Incretin-modulated beta cell energetics in intact islets of Langerhans. Mol. Endocrinol. 28, 860–871 (2014).
Harvey, C. D. et al. A genetically encoded fluorescent sensor of ERK activity. Proc. Natl Acad. Sci. USA 105, 19264–19269 (2008).
Pratt, E. P., Salyer, A. E., Guerra, M. L. & Hockerman, G. H. Ca2+ influx through L-type Ca2+ channels and Ca2+-induced Ca2+ release regulate cAMP accumulation and Epac1-dependent ERK 1/2 activation in INS-1 cells. Mol. Cell Endocrinol. 419, 60–71 (2016).
Leduc, M. et al. ERK1 is dispensable for mouse pancreatic beta cell function but is necessary for glucose-induced full activation of MSK1 and CREB. Diabetologia 60, 1999–2010 (2017).
Ni, Q. et al. Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat. Chem. Biol. 7, 34–40 (2011).
Violin, J. D., Zhang, J., Tsien, R. Y. & Newton, A. C. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J. Cell Biol. 161, 899–909 (2003).
Codazzi, F., Teruel, M. N. & Meyer, T. Control of astrocyte Ca2+ oscillations and waves by oscillating translocation and activation of protein kinase C. Curr. Biol. 11, 1089–1097 (2001).
Shigeto, M. et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J. Clin. Invest. 125, 4714–4728 (2015). GLP1 activates a TRPM4 and TRPM5 pathway; previously, the incretin was assumed to be active only in the nanomolar range, activating cAMP–PKA.
Stauffer, T. P., Ahn, S. & Meyer, T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr. Biol. 8, 343–346 (1998).
Xie, B. et al. Plasma membrane phosphatidylinositol 4,5-bisphosphate regulates Ca2+-influx and insulin secretion from pancreatic β cells. Cell Chem. Biol. 23, 816–826 (2016).
Rost, B. R., Schneider-Warme, F., Schmitz, D. & Hegemann, P. Optogenetic tools for subcellular applications in neuroscience. Neuron 96, 572–603 (2017).
Shibasaki, T. et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc. Natl Acad. Sci. USA 104, 19333–19338 (2007).
Takahashi, N., Kishimoto, T., Nemoto, T., Kadowaki, T. & Kasai, H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science 297, 1349–1352 (2002).This article presents the first study to show the orientation of insulin granule fusion in intact islets.
Low, J. T. et al. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia 57, 1655–1663 (2014).
Do, O. H., Low, J. T., Gaisano, H. Y. & Thorn, P. The secretory deficit in islets from db/db mice is mainly due to a loss of responding beta cells. Diabetologia 57, 1400–1409 (2014).
Li, D. et al. Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR). Proc. Natl Acad. Sci. USA 108, 21063–21068 (2011).
Pancholi, J. et al. Biologically targeted probes for Zn2+: a diversity oriented modular “click-SNAr-click” approach. Chem. Sci. 5, 3528–3535 (2014).
Rivera-Fuentes, P. et al. A far-red emitting probe for unambiguous detection of mobile zinc in acidic vesicles and deep tissue. Chem. Sci. 6, 1944–1948 (2015).
Qian, W.-J., Gee, K. R. & Kennedy, R. T. Imaging of Zn2+ release from pancreatic β-cells at the level of single exocytotic events. Anal. Chem. 75, 3468–3475 (2003).
Li, D., Liu, L. & Li, W. H. Genetic targeting of a small fluorescent zinc indicator to cell surface for monitoring zinc secretion. ACS Chem. Biol. 10, 1054–1063 (2015).
Li, D., Huang, Z., Chen, S., Hu, Z. & Li, W. H. GLP-1 receptor mediated targeting of a fluorescent Zn2+ sensor to beta cell surface for imaging insulin/Zn2+ release. Bioconjug. Chem. 26, 1443–1450 (2015).
Tsuboi, T. & Rutter, G. A. Multiple forms of “kiss-and-run” exocytosis revealed by evanescent wave microscopy. Curr. Biol. 13, 563–567 (2003). This paper presents an early study of β-cell granule dynamics and fate using three reporter constructs targeted to the vesicle membrane and cargo.
Martineau, M. et al. Semisynthetic fluorescent pH sensors for imaging exocytosis and endocytosis. Nat. Commun. 8, 1412 (2017).
Schifferer, M., Yushchenko, D. A., Stein, F., Bolbat, A. & Schultz, C. A. Ratiometric sensor for imaging insulin secretion in single β cells. Cell Chem. Biol. 24, 525–531 (2017).
Broichhagen, J., Frank, J. A. & Trauner, D. A. Roadmap to success in photopharmacology. Acc. Chem. Res. 48, 1947–1960 (2015).
Broichhagen, J. et al. Optical control of insulin release using a photoswitchable sulfonylurea. Nat. Commun. 5, 5116 (2014).
Broichhagen, J. et al. Allosteric optical control of a class B G-protein-coupled receptor. Angew. Chem. Int. Ed. 55, 5865–5868 (2016).
Broichhagen, J. et al. Optical control of insulin secretion using an incretin switch. Angew. Chem. Int. Ed. 54, 15565–15569 (2015).
Jones, B. J. et al. Potent prearranged positive allosteric modulators of the glucagon-like peptide-1 receptor. ChemistryOpen 6, 501–505 (2017).
Frank, J. A. et al. Photoswitchable diacylglycerols enable optical control of protein kinase C. Nat. Chem. Biol. 12, 755–762 (2016).
Frank, J. A. et al. Optical control of GPR40 signalling in pancreatic β-cells. Chem. Sci. 8, 7604–7610 (2017).
Podewin, T. et al. Conditional and reversible activation of class A and B G protein-coupled receptors using tethered pharmacology. ACS Cent. Sci. 4, 166–179 (2018).
Berry, M. H. et al. Restoration of patterned vision with an engineered photoactivatable G protein-coupled receptor. Nat. Commun. 8, 1862 (2017).
Ellis-Davies, G. C. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat. Methods 4, 619–628 (2007).
Gromada, J. et al. Glucagon-like peptide 1 (7–36) amide stimulates exocytosis in human pancreatic beta-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes 47, 57–65 (1998).
Renstrom, E., Eliasson, L. & Rorsman, P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B cells. J. Physiol. 502, 105–118 (1997).
Höglinger, D., Nadler, A. & Schultz, C. Caged lipids as tools for investigating cellular signaling. Biochim. Biophys. Acta 1841, 1085–1096 (2014).
Nadler, A. et al. Exclusive photorelease of signalling lipids at the plasma membrane. Nat. Commun. 6, 10056 (2015).
Westacott, M. J., Ludin, N. W. F. & Benninger, R. K. P. Spatially organized β-cell subpopulations control electrical dynamics across islets of Langerhans. Biophys. J. 113, 1093–1108 (2017).
Tkatch, T. et al. Optogenetic control of mitochondrial metabolism and Ca2+ signaling by mitochondria-targeted opsins. Proc. Natl Acad. Sci. USA 114, E5167–E5176 (2017).
Reinbothe, T. M., Safi, F., Axelsson, A. S., Mollet, I. G. & Rosengren, A. H. Optogenetic control of insulin secretion in intact pancreatic islets with β-cell-specific expression of Channelrhodopsin-2. Islets 6, e28095 (2014). This article presents the first report showing optogenetic activation of β-cells within islets.
Briant, L. J. B. et al. δ-cells and β-cells are electrically coupled and regulate alpha-cell activity via somatostatin. J. Physiol. 596, 197–215 (2017).
Zhang, F. & Tzanakakis, E. S. Optogenetic regulation of insulin secretion in pancreatic β-cells. Sci. Rep. 7, 9357 (2017).
Grusch, M. et al. Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J. 33, 1713–1726 (2014).
Masuda, S., Nakatani, Y., Ren, S. & Tanaka, M. Blue light-mediated manipulation of transcription factor activity in vivo. ACS Chem. Biol. 8, 2649–2653 (2013).
Motta-Mena, L. B. et al. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat. Chem. Biol. 10, 196–202 (2014).
Roscioni, S. S., Migliorini, A., Gegg, M. & Lickert, H. Impact of islet architecture on beta-cell heterogeneity, plasticity and function. Nat. Rev. Endocrinol. 12, 695–709 (2016).
Ikegaya, Y. et al. Synfire chains and cortical songs: temporal modules of cortical activity. Science 304, 559–564 (2004).
Roed, S. N. et al. Functional consequences of glucagon-like peptide-1 receptor cross-talk and trafficking. J. Biol. Chem. 290, 1233–1243 (2015).
Kuna, R. S. et al. Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 305, 161–170 (2013).
Calebiro, D. et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLOS Biol. 7, e1000172 (2009). This imaging study shows that internalized GPCRs are capable of generating cAMP.
Tsvetanova, N. G. & von Zastrow, M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat. Chem. Biol. 10, 1061–1065 (2014).
Flores-Otero, J. et al. Ligand-specific endocytic dwell times control functional selectivity of the cannabinoid receptor 1. Nat. Commun. 5, 4589 (2014).
Albrecht, T., Zhao, Y., Nguyen, T. H., Campbell, R. E. & Johnson, J. D. Fluorescent biosensors illuminate calcium levels within defined beta-cell endosome subpopulations. Cell Calcium 57, 263–274 (2015).
Höglinger, D. et al. Intracellular sphingosine releases calcium from lysosomes. eLife 4, e10616 (2015).
Walter, A. M. et al. Phosphatidylinositol 4,5-bisphosphate optical uncaging potentiates exocytosis. eLife 6, e30203 (2017).
Nadler, A. et al. The fatty acid composition of diacylglycerols determines local signaling patterns. Angew. Chem. Int. Ed. 52, 6330–6334 (2013).
Stein, D. T. et al. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Invest. 100, 398–403 (1997). This paper presents the first study showing the importance of fatty acid chain length for insulin release.
Brouwers, B. et al. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab. 20, 979–990 (2014).
Zhang, Q. et al. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metab. 18, 871–882 (2013).
Cabrera, O. et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. 7, 545–554 (2008). This in vivo imaging study demonstrates the influence of neural wiring on insulin release.
Ackermann, A. M., Zhang, J., Heller, A., Briker, A. & Kaestner, K. H. High-fidelity Glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting. Mol. Metab. 6, 236–244 (2017).
Rodriguez-Diaz, R. et al. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc. Natl Acad. Sci. USA 109, 21456–21461 (2012).
Michau, A. et al. Metabolism regulates exposure of pancreatic islets to circulating molecules in vivo. Diabetes 65, 463–475 (2015).
Paschen, M. et al. Non-invasive cell type selective in vivo monitoring of insulin resistance dynamics. Sci. Rep. 6, 21448 (2016).
Ben-Othman, N. et al. Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 168, 73–85 (2017).
Chen, Y., Saulnier, J. L., Yellen, G. & Sabatini, B. L. A. PKA activity sensor for quantitative analysis of endogenous GPCR signaling via 2-photon FRET-FLIM imaging. Front. Pharmacol. 5, 56 (2014).
Acknowledgements
D.J.H. was supported by a Diabetes UK R. D. Lawrence (12/0004431) Fellowship, a Wellcome Trust Institutional Support Award and Medical Research Council (MR/N00275X/1) and Diabetes UK (17/0005681) Project Grants. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Starting Grant 715884 to D.J.H. and Advanced Grant 268795 to D.T.). C.S. is supported by TRR186 of the German Research Council (DFG). The authors apologize to the many authors whose studies could not be cited owing to space limitations.
Reviewer information
Nature Reviews Endocrinology thanks D. Eizirik and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
J.A.F., J.B., D.Y. and D.J.H. researched the data for the article, contributed to discussion of content and reviewed and edited the article before submission. C.S. and D.T. contributed to discussion of content and reviewed and edited the article before submission. D.J.H. wrote the article with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frank, J.A., Broichhagen, J., Yushchenko, D.A. et al. Optical tools for understanding the complexity of β-cell signalling and insulin release. Nat Rev Endocrinol 14, 721–737 (2018). https://doi.org/10.1038/s41574-018-0105-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-018-0105-2
This article is cited by
-
PDX1LOW MAFALOW β-cells contribute to islet function and insulin release
Nature Communications (2021)
-
Cells produced during in vitro β-cell differentiation characterized
Nature Reviews Endocrinology (2019)