Abstract
Inorganic phosphate (Pi) is essential for signal transduction and cell metabolism, and is also an essential structural component of the extracellular matrix of the skeleton. Pi is sensed in bacteria and yeast at the plasma membrane, which activates intracellular signal transduction to control the expression of Pi transporters and other genes that control intracellular Pi levels. In multicellular organisms, Pi homeostasis must be maintained in the organism and at the cellular level, requiring an endocrine and metabolic Pi-sensing mechanism, about which little is currently known. This Review will discuss the metabolic effects of Pi, which are mediated by Pi transporters, inositol pyrophosphates and SYG1–Pho81–XPR1 (SPX)-domain proteins to maintain cellular phosphate homeostasis in the musculoskeletal system. In addition, we will discuss how Pi is sensed by the human body to regulate the production of fibroblast growth factor 23 (FGF23), parathyroid hormone and calcitriol to maintain serum levels of Pi in a narrow range. New findings on the crosstalk between iron and Pi homeostasis in the regulation of FGF23 expression will also be outlined. Mutations in components of these metabolic and endocrine phosphate sensors result in genetic disorders of phosphate homeostasis, cardiomyopathy and familial basal ganglial calcifications, highlighting the importance of this newly emerging area of research.
Key points
-
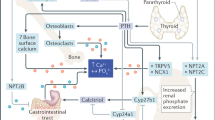
Endocrine regulation of gastrointestinal absorption, storage in the mineral deposits of the skeleton and renal excretion of inorganic phosphate (Pi) maintains the serum concentration of Pi within a narrow range.
-
Pi activates extracellular-signal-regulated kinases 1 and 2 in mammalian cells, which are required for stimulation of mitochondrial respiration and transcription of bone matrix proteins.
-
Pi stimulates the synthesis and secretion of parathyroid hormone and fibroblast growth factor 23 and blocks the synthesis of calcitriol; however, the endocrine sensor for Pi remains unknown.
-
Mutations in the endocrine regulators of Pi lead to genetic disorders characterized by abnormal bone and mineral metabolism and ectopic calcifications.
-
Identification of loss-of-function mutations in several Pi transporters highlights the importance of intracellular Pi for muscle function and vascular calcifications.
-
How intracellular Pi causes myopathy, tumour formation and changes associated with acclerated ageing is less well understood.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bevington, A., Kemp, G. J., Graham, R. & Russell, G. Phosphate-sensitive enzymes: possible molecular basis for cellular disorders of phosphate metabolism. Clin. Chem. Enzym. Comms. 4, 235–257 (1992).
Chakraborty, A., Kim, S. & Snyder, S. H. Inositol pyrophosphates as mammalian cell signals. Sci. Signal. 4, re1 (2011).
Angelova, P. R., Baev, A. Y., Berezhnov, A. V. & Abramov, A. Y. Role of inorganic polyphosphate in mammalian cells: from signal transduction and mitochondrial metabolism to cell death. Biochem. Soc. Trans. 44, 40–45 (2016).
Herman, H. & Dallemagne, M. J. The main mineral constituent of bone and teeth. Arch. Oral Biol. 5, 137–144 (1961).
Bergwitz, C. & Juppner, H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 61, 91–104 (2010).
Khoshniat, S. et al. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell. Mol. Life Sci. 68, 205–218 (2010).
Fukumoto, S. Phosphate metabolism and vitamin D. Bonekey Rep. 3, 497 (2014).
Manghat, P., Sodi, R. & Swaminathan, R. Phosphate homeostasis and disorders. Ann. Clin. Biochem. 51, 631–656 (2014).
Quarles, L. D. A systems biology preview of the relationships between mineral and metabolic complications in chronic kidney disease. Semin. Nephrol. 33, 130–142 (2013).
Brame, L. A., White, K. E. & Econs, M. J. Renal phosphate wasting disorders: clinical features and pathogenesis. Semin. Nephrol. 24, 39–47 (2004).
Prie, D., Beck, L., Silve, C. & Friedlander, G. Hypophosphatemia and calcium nephrolithiasis. Nephron Exp. Nephrol. 98, e50–54 (2004).
Lau, W. L., Pai, A., Moe, S. M. & Giachelli, C. M. Direct effects of phosphate on vascular cell function. Adv. Chron. Kidney Dis. 18, 105–112 (2011).
Scialla, J. J. & Wolf, M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat. Rev. Nephrol. 10, 268–278 (2014).
Penido, M. G. M. G. & Alon, U. S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 27, 2039–2048 (2012).
Berndt, T. & Kumar, R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiol. (Bethesda) 24, 17–25 (2009).
Sabbagh, Y. Phosphate as a sensor and signaling molecule. Clin. Nephrol. 79, 57–65 (2013).
Hsieh, Y. J. & Wanner, B. L. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 13, 198–203 (2010).
Bergwitz, C. & Juppner, H. Phosphate sensing. Adv. Chron. Kidney Dis. 18, 132–144 (2011).
Chien, M. L., Foster, J. L., Douglas, J. L. & Garcia, J. V. The amphotropic murine leukemia virus receptor gene encodes a 71-kilodalton protein that is induced by phosphate depletion. J. Virol. 71, 4564–4570 (1997).
Wang, D. et al. Alterations in the sensing and transport of phosphate and calcium by differentiating chondrocytes. J. Biol. Chem. 276, 33995–34005 (2001).
Suzuki, A., Palmer, G., Bonjour, J. P. & Caverzasio, J. Stimulation of sodium-dependent inorganic phosphate transport by activation of Gi/o-protein-coupled receptors by epinephrine in MC3T3-E1 osteoblast-like cells. Bone 28, 589–594 (2001).
Zhen, X., B., J. & Caverzasio, J. Platelet-derived growth factor stimulates sodium-dependent Pi transport in osteoblastic cells via phospholipase Cgamma and phosphatidylinositol 3’ -kinase. J. Bone Miner. Res. 12, 36–44 (1997).
Polgreen, K. E., Kemp, G. J., Leighton, B. & Radda, G. K. Modulation of Pi transport in skeletal muscle by insulin and IGF-1. Biochim. Biophys. Acta 1223, 279–284 (1994).
Suzuki, A., Palmer, G., Bonjour, J.-P. & Caverzasio, J. Stimulation of sodium-dependent phosphate transport and signaling mechanisms induced by basic fibroblast growth factor in MC3T3-E1 osteoblast-like cells. J. Bone Miner. Res. 15, 95–102 (2000).
Palmer, G., Guicheux, J. m., Bonjour, J.-P. & Caverzasio, J. Transforming growth factor-β stimulates inorganic phosphate transport and expression of the type III phosphate transporter Glvr-1 in chondrogenic ATDC5 cells*. Endocrinology 141, 2236–2243 (2000).
Lamarche, M. G., Wanner, B. L., Crepin, S. & Harel, J. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 32, 461–473 (2008).
Qi, W., Baldwin, S. A., Muench, S. P. & Baker, A. Pi sensing and signalling: from prokaryotic to eukaryotic cells. Biochem. Soc. Trans. 44, 766–773 (2016).
Brown, M. R. & Kornberg, A. The long and short of it — polyphosphate, PPK and bacterial survival. Trends Biochem. Sci. 33, 284–290 (2008).
Rao, N. N., Gomez-Garcia, M. R. & Kornberg, A. Inorganic polyphosphate: essential for growth and survival. Annu. Rev. Biochem. 78, 605–647 (2009).
Mouillon, J. M. & Persson, B. L. New aspects on phosphate sensing and signalling in Saccharomyces cerevisiae. FEMS Yeast Res. 6, 171–176 (2006).
Samyn, D. R. & Persson, B. L. Inorganic phosphate and sulfate transport in S. cerevisiae. Adv. Exp. Med. Biol. 892, 253–269 (2016).
Bergwitz, C. et al. Roles of major facilitator superfamily transporters in phosphate response in Drosophila. PLOS One 7, e31730 (2012).
Lagerstedt, J. O., Voss, J. C., Wieslander, Å. & Persson, B. L. Structural modeling of dual-affinity purified Pho84 phosphate transporter. FEBS Lett. 578, 262–268 (2004).
The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169 (2017).
Sengottaiyan, P. et al. Characterization of the biochemical and biophysical properties of the Saccharomyces cerevisiae phosphate transporter Pho89. Biochem. Biophys. Res. Commun. 436, 551–556 (2013).
Bottger, P. & Pedersen, L. Mapping of the minimal inorganic phosphate transporting unit of human PiT2 suggests a structure universal to PiT-related proteins from all kingdoms of life. BMC Biochem. 12, 21 (2011).
Werner, A. & Kinne, R. K. H. Evolution of the Na-Pi cotransport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R301–R312 (2001).
Secco, D., Wang, C., Shou, H. & Whelan, J. Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS Lett. 586, 289–295 (2012).
Saiardi, A. How inositol pyrophosphates control cellular phosphate homeostasis? Adv. Biol. Regul. 52, 351–359 (2012).
Lee, Y.-S., Huang, K., Quiocho, F. A. & O’Shea, E. K. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat. Chem. Biol. 4, 25–32 (2008).
Giovannini, D., Touhami, J., Charnet, P., Sitbon, M. & Battini, J. L. Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep. 3, 1866–1873 (2013).
Gerasimaite, R. et al. Inositol pyrophosphate specificity of the SPX-dependent polyphosphate polymerase VTC. ACS Chem. Biol. 12, 648–653 (2017).
Auesukaree, C., Tochio, H., Shirakawa, M., Kaneko, Y. & Harashima, S. Plc1p, Arg82p, and Kcs1p, enzymes involved in inositol pyrophosphate synthesis, are essential for phosphate regulation and polyphosphate accumulation in Saccharomyces cerevisiae. J. Biol. Chem. 280, 25127–25133 (2005).
Gerasimaite˙, R., Sharma, S., Desfougères, Y., Schmidt, A. & Mayer, A. Coupled synthesis and translocation restrains polyphosphate to acidocalcisome-like vacuoles and prevents its toxicity. J. Cell Sci. 127, 5093–5104 (2014).
Worley, J., Luo, X. & Capaldi, A. P. Inositol pyrophosphates regulate cell growth and the environmental stress response by activating the HDAC Rpd3L. Cell Rep. 3, 1476–1482 (2013).
Morales, R. et al. Serendipitous discovery and X-ray structure of a human phosphate binding apolipoprotein. Structure 14, 601–609 (2006).
Chavkin, N. W., Chia, J. J., Crouthamel, M. H. & Giachelli, C. M. Phosphate uptake-independent signaling functions of the type III sodium-dependent phosphate transporter, PiT-1, in vascular smooth muscle cells. Exp. Cell Res. 333, 39–48 (2015).
Beck, L. et al. Identification of a novel function of PiT1 critical for cell proliferation and independent of its phosphate transport activity. J. Biol. Chem. 284, e99959 (2009).
Wittrant, Y. et al. Inorganic phosphate regulates Glvr-1 and -2 expression: role of calcium and ERK1/2. Biochem. Biophys. Res. Commun. 381, 259–263 (2009).
Yoshiko, Y., Candeliere, G. A., Maeda, N. & Aubin, J. E. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol. Cell. Biol. 27, 4465–4474 (2007).
Papaioannou, G. et al. Raf kinases are essential for phosphate induction of ERK1/2 phosphorylation in hypertrophic chondrocytes and normal endochondral bone development. J. Biol. Chem. 292, 3164–3171 (2017).
Pesta, D. H. et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J. 30, 3378–3387 (2016).
Camalier, C. E. et al. An integrated understanding of the physiological response to elevated extracellular phosphate. J. Cell. Physiol. 228, 1536–1550 (2013).
Yamazaki, M. et al. Both FGF23 and extracellular phosphate activate Raf/MEK/ERK pathway via FGF receptors in HEK293 cells. J. Cell. Biochem. 111, 1210–1221 (2010).
Nishino, J. et al. Extracellular phosphate induces the expression of dentin matrix protein 1 through the FGF receptor in osteoblasts. J. Cell. Biochem. 118, 1151–1163 (2017).
Pauleau, A. L. et al. Unexpected role of the phosphate carrier in mitochondrial fragmentation. Cell Death And Differ. 15, 616 (2008).
Seifert, E. L., Ligeti, E., Mayr, J. A., Sondheimer, N. & Hajnoczky, G. The mitochondrial phosphate carrier: role in oxidative metabolism, calcium handling and mitochondrial disease. Biochem. Biophys. Res. Commun. 464, 369–375 (2015).
Couasnay, G. et al. Maintenance of chondrocyte survival by PIT1/SLC20A1-mediated regulation of endoplasmic reticulum homeostasis. Osteoarthr. Cartil. 24, S135 (2016).
Laver, D. R., Lenz, G. K. E. & Dulhunty, A. F. Phosphate ion channels in sarcoplasmic reticulum of rabbit skeletal muscle. J. Physiol. 535, 715–728 (2001).
Paredes, J. M. et al. Real-time phosphate sensing in living cells using fluorescence lifetime imaging microscopy (FLIM). J. Phys. Chem. B 117, 8143–8149 (2013).
Banerjee, S., Versaw, W. K. & Garcia, L. R. Imaging cellular inorganic phosphate in Caenorhabditis elegans using a genetically encoded FRET-based biosensor. PLOS One 10, e0141128 (2015).
Moore, K. L. et al. Combined NanoSIMS and synchrotron X-ray fluorescence reveal distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytol. 201, 104–115 (2014).
Braun, P. D., Schulz-Vogt, H. N., Vogts, A. & Nausch, M. Differences in the accumulation of phosphorus between vegetative cells and heterocysts in the cyanobacterium Nodularia spumigena. Sci. Rep. 8, 5651 (2018).
Kestenbaum, B. et al. Common genetic variants associate with serum phosphorus concentration. J. Am. Soc. Nephrol. 21, 1223–1232 (2010).
Koldobskiy, M. A. et al. p53-mediated apoptosis requires inositol hexakisphosphate kinase-2. Proc. Natl Acad. Sci. 107, 20947–20951 (2010).
Chakraborty, A. et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143, 897–910 (2010).
Rao, F. et al. Inositol pyrophosphates mediate the DNA-PK/ATM-p53 cell death pathway by regulating CK2 phosphorylation of Tti1/Tel2. Mol. Cell 54, 119–132 (2014).
Wild, R. et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352, 986–990 (2016).
Azevedo, C. & Saiardi, A. Eukaryotic phosphate homeostasis: the inositol pyrophosphate perspective. Trends Biochem. Sci. 42, 219–231 (2017).
Sharma, P., Patntirapong, S., Hann, S. & Hauschka, P. V. RANKL-RANK signaling regulates expression of xenotropic and polytropic virus receptor (XPR1) in osteoclasts. Biochem. Biophys. Res. Commun. 399, 129–132 (2010).
Docampo, R., de Souza, W., Miranda, K., Rohloff, P. & Moreno, S. N. J. Acidocalcisomes? conserved from bacteria to man. Nat. Rev. Micro 3, 251–261 (2005).
Pavlov, E. et al. Inorganic polyphosphate and energy metabolism in mammalian cells. J. Biol. Chem. 285, 9420–9428 (2010).
Schmelzle, T. & Hall, M. N. TOR, a central controller of cell growth. Cell 103, 253–262 (2000).
Shiba, T. et al. Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J. Biol. Chem. 278, 26788–26792 (2003).
Hernandez-Ruiz, L., Gonzalez-Garcia, I., Castro, C., Brieva, J. & Ruiz, F. Inorganic polyphosphate and specific induction of apoptosis in human plasma cells. Haematologica 91, 1180–1186 (2006).
Holmstrom, K. M. et al. Signalling properties of inorganic polyphosphate in the mammalian brain. Nat. Commun. 4, 1362 (2013).
White, K. E. et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345 (2000).
Shimada, T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. 98, 6500–6505 (2001).
Sitara, D. et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 23, 421–432 (2004).
Bingham, P. J. & Raisz, L. G. Bone growth in organ culture: effects of phosphate and other nutrients on bone and cartilage. Calcif. Tissue Res. 14, 31–48 (1974).
Kakuta, S., Golub, E. E. & Shapiro, I. M. Morphochemical analysis of phosphorus pools in calcifying cartilage. Calcif. Tissue Int. 37, 293–299 (1985).
Fujita, T. et al. Phosphate stimulates differentiation and mineralization of the chondroprogenitor clone ATDC5. Jpn J. Pharmacol. 85, 278–281 (2001).
Liu, E. S. et al. Phosphate interacts with PTHrP to regulate endochondral bone formation. Endocrinology 155, 3750–3756 (2014).
Teixeira, C. C., Mansfield, K., Hertkorn, C., Ischiropoulos, H. & Shapiro, I. M. Phosphate-induced chondrocyte apoptosis is linked to nitric oxide generation. Am. J. Physiol. Cell Physiol. 281, C833–C839 (2001).
Orfanidou, T., Malizos, K. N., Varitimidis, S. & Tsezou, A. 1,25-Dihydroxyvitamin D(3) and extracellular inorganic phosphate activate mitogen-activated protein kinase pathway through fibroblast growth factor 23 contributing to hypertrophy and mineralization in osteoarthritic chondrocytes. Exp. Biol. Med. (Maywood) 237, 241–253 (2012).
Solomon, D. H., Wilkins, R. J., Meredith, D. & Browning, J. A. Characterisation of inorganic phosphate transport in bovine articular chondrocytes. Cell. Physiol. Biochem. 20, 099–108 (2007).
Festing, M. H., Speer, M. Y., Yang, H. Y. & Giachelli, C. M. Generation of mouse conditional and null alleles of the type III sodium-dependent phosphate cotransporter PiT-1. Genesis 47, 858–863 (2009).
Beck, L. et al. The phosphate transporter PiT1 (Slc20a1) revealed as a new essential gene for mouse liver development. PLOS One 5, e9148 (2010).
Bourgine, A. et al. Mice with hypomorphic expression of the sodium-phosphate cotransporter PiT1/Slc20a1 have an unexpected normal bone mineralization. PLOS One 8, e65979 (2013).
Yadav, M. C. et al. Skeletal mineralization deficits and impaired biogenesis and function of chondrocyte-derived matrix vesicles in phospho1(−/−) and phospho1/Pi t1 double-knockout mice. J. Bone Miner. Res. 31, 1275–1286 (2016).
Suzuki, A. et al. Effects of transgenic Pit-1 overexpression on calcium phosphate and bone metabolism. J. Bone Miner. Metab. 28, 139–148 (2010).
Civitelli, R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch. Biochem. Biophys. 473, 188–192 (2008).
Staines, K. A., MacRae, V. E. & Farquharson, C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 214, 241–255 (2012).
Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 94, 25–34 (2014).
Conrads, K. A. et al. Quantitative proteomic analysis of inorganic phosphate-induced murine MC3T3-E1 osteoblast cells. Electrophoresis 25, 1342–1352 (2004).
Conrads, K. A. et al. A combined proteome and microarray investigation of inorganic phosphate-induced pre-osteoblast cells. Mol. Cell Proteom. 4, 1284–1296 (2005).
Julien, M. et al. Phosphate-dependent regulation of MGP in osteoblasts: role of ERK1/2 and Fra-1. J. Bone Miner. Res. 24, 1856–1868 (2009).
Beck, G. R. Jr., Zerler, B. & Moran, E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl Acad. Sci. USA 97, 8352–8357 (2000).
Kanatani, M., Sugimoto, T., Kano, J. & Chihara, K. IGF-I mediates the stimulatory effect of high phosphate concentration on osteoblastic cell proliferation. J. Cell. Physiol. 190, 306–312 (2002).
Hoac, B., Kiffer-Moreira, T., Millán, J. L. & McKee, M. D. Polyphosphates inhibit extracellular matrix mineralization in MC3T3-E1 osteoblast cultures. Bone 53, 478–486 (2013).
Caverzasio, J., Selz, T. & Bonjour, J. P. Characteristics of phosphate transport in osteoblastlike cells. Calcif. Tissue Int. 43, 83–87 (1988).
Lundquist, P., Murer, H. & Biber, J. Type II Na+-Pi cotransporters in osteoblast mineral formation: regulation by inorganic phosphate. Cell Physiol. Biochem. 19, 43–56 (2007).
Yates, A. J., Oreffo, R. O., Mayor, K. & Mundy, G. R. Inhibition of bone resorption by inorganic phosphate is mediated by both reduced osteoclast formation and decreased activity of mature osteoclasts. J. Bone Miner. Res. 6, 473–478 (1991).
Ha, S.-W., Park, J., Habib, M. M. & Beck, G. R. Nano-hydroxyapatite stimulation of gene expression requires Fgf receptor, phosphate transporter, and Erk1/2 signaling. ACS Appl. Mater. Interfaces 9, 39185–39196 (2017).
Akiyama, K., Kimura, T. & Shiizaki, K. Biological and clinical effects of calciprotein particles on chronic kidney disease-mineral and bone disorder. Int. J. Endocrinol. 2018, 6 (2018).
Yamada, S. et al. in American Society of Nephrology (New Orleans, LA, 2017).
Beck, S. et al. PiT2 is essential for normal endochondral and intramembranous ossification, tooth development and the maintenance of adult bone structure and strength [abstract MO0523]. J. Bone Miner. Res. 32, S339 (2017).
Larsen, F. T., Jensen, N., Autzen, J. K., Kongsfelt, I. B. & Pedersen, L. Primary brain calcification causal PiT2 transport-knockout variants can exert dominant negative effects on wild-type PiT2 transport function in mammalian cells. J. Mol. Neurosci. 61, 215–220 (2017).
Nina Bon, A. B. et al. Phosphate (Pi)-regulated heterodimerization of the high-affinity sodium-dependent Pi transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular Pi sensing independently of Pi uptake. J. Biol. Chem. 293, 2102–2114 (2017).
Segawa, H. et al. Type IIc sodium–dependent phosphate transporter regulates calcium metabolism. J. Am. Soc. Nephrol. 20, 104–113 (2009).
Tenenhouse, H. S. Regulation of phosphorus homeostasis by the type IIA Na/phosphate cotransporter. Annu. Rev. Nutr. 25, 197–214 (2005).
Shibasaki, Y. et al. Targeted deletion of the tybe IIb Na+-dependent Pi-co-transporter, NaPi-IIb, results in early embryonic lethality. Biochem. Biophys. Res. Commun. 381, 482–486 (2009).
Sabbagh, Y. et al. Intestinal Npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 20, 2348–2358 (2009).
Miyagawa, K. et al. Dysregulated gene expression in the primary osteoblasts and osteocytes isolated from hypophosphatemic Hyp mice. PLOS ONE 9, e93840 (2014).
Zhang, R. et al. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J. Bone Miner. Res. 26, 1047–1056 (2011).
Ito, N., Findlay, D. M., Anderson, P. H., Bonewald, L. F. & Atkins, G. J. Extracellular phosphate modulates the effect of 1alpha, 25-dihydroxy vitamin D3 (1,25D) on osteocyte like cells. J. Steroid Biochem. Mol. Biol. 136, 183–186 (2013).
Rhee, Y. et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49, 636–643 (2011).
Capulli, M., Paone, R. & Rucci, N. Osteoblast and osteocyte: games without frontiers. Arch. Biochem. Biophys. 561, 3–12 (2014).
Dallas, S. L., Prideaux, M. & Bonewald, L. F. The osteocyte: an endocrine cell… and more. Endocr. Rev. 34, 658–690 (2013).
Nakashima, K. & de Crombrugghe, B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 19, 458–466 (2003).
Karsenty, G. & Olson, E. N. Bone and muscle endocrine functions: unexpected paradigms of inter-organ communication. Cell 164, 1248–1256 (2016).
Teitelbaum, S. L. Bone resorption by osteoclasts. Science 289, 1504–1508 (2000).
Boyce, B. F. Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 92, 860–867 (2013).
Charles, J. F. & Aliprantis, A. O. Osteoclasts: more than ‘bone eaters’. Trends Mol. Med. 20, 449–459 (2014).
Albano, G. et al. Sodium-dependent phosphate transporters in osteoclast differentiation and function. PLOS ONE 10, e0125104 (2015).
Kanatani, M., Sugimoto, T., Kano, J., Kanzawa, M. & Chihara, K. Effect of high phosphate concentration on osteoclast differentiation as well as bone-resorbing activity. J. Cell. Physiol. 196, 180–189 (2003).
Mozar, A. et al. High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J. Cell. Physiol. 215, 47–54 (2008).
M’Baya-Moutoula, E., Louvet, L., Metzinger-Le Meuth, V., Massy, Z. A. & Metzinger, L. High inorganic phosphate concentration inhibits osteoclastogenesis by modulating miR-223. Biochim. Biophys. Acta 1852, 2202–2212 (2015).
Wang, X. et al. Dual effect of inorganic polymeric phosphate/polyphosphate on osteoblasts and osteoclasts in vitro. J. Tissue Eng. Regen Med. 7, 767–776 (2013).
Gupta, A., Guo, X. L., Alvarez, U. M. & Hruska, K. A. Regulation of sodium-dependent phosphate transport in osteoclasts. J. Clin. Invest. 100, 538–549 (1997).
Li, G., Miura, K. & Kuno, M. Extracellular phosphates enhance activities of voltage-gated proton channels and production of reactive oxygen species in murine osteoclast-like cells. Pflugers Arch. 469, 279–292 (2017).
Hayashibara, T. et al. Regulation of osteoclast differentiation and function by phosphate: potential role of osteoclasts in the skeletal abnormalities in hypophosphatemic conditions. J. Bone Miner. Res. 22, 1743–1751 (2007).
Beck, L. et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl Acad. Sci. 95, 5372–5377 (1998).
Brown, C. E., Wilkie, C. A., Meyer, M. H. & Meyer, R. A. Response of tissue phosphate content to acute dietary phosphate deprivation in the X-linked hypophosphatemic mouse. Calcif. Tissue Int. 37, 423–430 (1985).
Smith, R., Newman, R. J., Radda, G. K., Stokes, M. & Young, A. Hypophosphataemic osteomalacia and myopathy: studies with nuclear magnetic resonance spectroscopy. Clin. Sci. (Lond.) 67, 505–509 (1984).
Sinha, A., Hollingsworth, K. G., Ball, S. & Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 98, E509–E513 (2013).
Hwang, J. H. & Choi, C. S. Use of in vivo magnetic resonance spectroscopy for studying metabolic diseases. Exp. Mol. Med. 47, e139 (2015).
Itoh, H. et al. Mechanically driven ATP synthesis by F1-ATPase. Nature 427, 465 (2004).
Bose, S., French, S., Evans, F. J., Joubert, F. & Balaban, R. S. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J. Biol. Chem. 278, 39155–39165 (2003).
Phillips, D., Aponte, A. M., French, S. A., Chess, D. J. & Balaban, R. S. Succinyl-CoA synthetase is a phosphate target for the activation of mitochondrial metabolism. Biochemistry 48, 7140–7149 (2009).
Rodriguez-Zavala, J. S., Pardo, J. P. & Moreno-Sanchez, R. Modulation of 2-oxoglutarate dehydrogenase complex by inorganic phosphate, Mg(2+), and other effectors. Arch. Biochem. Biophys. 379, 78–84 (2000).
Hansford, R. G. Some properties of pyruvate and 2-oxoglutarate oxidation by blowfly flight-muscle mitochondria. Biochem. J. 127, 271–283 (1972).
Blonde, D. J., Kresack, E. J. & Kosicki, G. W. The effects of ions and freeze-thawing on supernatant and mitochondrial malate dehydrogenase. Can. J. Biochem. 45, 641–650 (1967).
Cook, W. J., Senkovich, O. & Chattopadhyay, D. An unexpected phosphate binding site in glyceraldehyde 3-phosphate dehydrogenase: crystal structures of apo, holo and ternary complex of Cryptosporidium parvum enzyme. BMC Struct. Biol. 9, 9 (2009).
Travis, S. F. et al. Alterations of red-cell glycolytic intermediates and oxygen transport as a consequence of hypophosphatemia in patients receiving intravenous hyperalimentation. N. Engl. J. Med. 285, 763–768 (1971).
Shanahan, C. M., Crouthamel, M. H., Kapustin, A. & Giachelli, C. M. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ. Res. 109, 697–711 (2011).
Rangrez, A. Y. et al. Inorganic phosphate accelerates the migration of vascular smooth muscle cells: evidence for the involvement of miR-223. PLOS One 7, e47807 (2012).
Giachelli, C. M. Vascular calcification: in vitro evidence for the role of inorganic phosphate. J. Am. Soc. Nephrol. 14, S300–304 (2003).
Shobeiri, N., Adams, M. A. & Holden, R. M. Phosphate: an old bone molecule but new cardiovascular risk factor. Br. J. Clin. Pharmacol. 77, 39–54 (2014).
Duan, P. & Bonewald, L. F. The role of the wnt/beta-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 77, 23–29 (2016).
Gaur, T. et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280, 33132–33140 (2005).
Komori, T. Signaling networks in RUNX2-dependent bone development. J. Cell. Biochem. 112, 750–755 (2011).
Cai, T. et al. WNT/beta-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp. Cell Res. 345, 206–217 (2016).
Deng, D., Diao, Z., Han, X. & Liu, W. Secreted frizzled-related protein 5 attenuates high phosphate-induced calcification in vascular smooth muscle cells by inhibiting the Wnt/ss-catenin pathway. Calcif. Tissue Int. 99, 66–75 (2016).
Allen, D. G. & Trajanovska, S. The multiple roles of phosphate in muscle fatigue. Front. Physiol. 3, 463 (2012).
Lederer, E. Regulation of serum phosphate. J. Physiol. 592, 3985–3995 (2014).
Lanske, B. & Razzaque, M. S. Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int. 86, 1072–1074 (2014).
Imel, E. A. & Econs, M. J. Fibroblast growth factor 23: roles in health and disease. J. Am. Soc. Nephrol. 16, 2565–2575 (2005).
Fukumoto, S. & Yamashita, T. Fibroblast growth factor-23 is the phosphaturic factor in tumor-induced osteomalacia and may be phosphatonin. Curr. Opin. Nephrol. Hypertens. 11, 385–389 (2002).
Liu, S., Gupta, A. & Quarles, L. D. Emerging role of fibroblast growth factor 23 in a bone-kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization. Curr. Opin. Nephrol. Hypertens. 16, 329–335 (2007).
Andrukhova, O. et al. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51, 621–628 (2012).
Olauson, H. et al. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLOS Genet. 9, e1003975 (2013).
Ichikawa, S. et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 117, 2684–2691 (2007).
Tsujikawa, H., Kurotaki, Y., Fujimori, T., Fukuda, K. & Nabeshima, Y.-I. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 17, 2393–2403 (2003).
Ferrari, S. L., Bonjour, J. P. & Rizzoli, R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J. Clin. Endocrinol. Metab. 90, 1519–1524 (2005).
Yamazaki, Y. et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J. Bone Miner. Res. 23, 1509–1518 (2008).
Perwad, F. & Portale, A. A. Vitamin D metabolism in the kidney: regulation by phosphorus and fibroblast growth factor 23. Mol. Cell. Endocrinol. 347, 17–24 (2011).
Kägi, L. et al. Regulation of vitamin D metabolizing enzymes in murine renal and extrarenal tissues by dietary phosphate, FGF23, and 1,25(OH)2D3. PLOS One 13, e0195427 (2018).
Feng, J. Q., Ye, L. & Schiavi, S. Do osteocytes contribute to phosphate homeostasis? Curr. Opin. Nephrol. Hypertens. 18, 285–291 (2009).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45 (1997).
Lindberg, K. et al. The kidney is the principal organ mediating Klotho effects. J. Am. Soc. Nephrol. 25, 2169–2175 (2014).
Ide, N. et al. In vivo evidence for a limited role of proximal tubular Klotho in renal phosphate handling. Kidney Int. 90, 348–362 (2016).
Martin, A. et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 25, 2551–2562 (2011).
Farrow, E. G. et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc. Natl Acad. Sci. 108, E1146–E1155 (2011).
Tagliabracci, V. S. et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl Acad. Sci. 111, 5520–5525 (2014).
Rankin, E. B. et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149, 63–74 (2012).
Clinkenbeard, E. L. et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica 102, e427–e430 (2017).
Coe, L. M. et al. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J. Biol. Chem. 289, 9795–9810 (2014).
Kyono, A., Avishai, N., Ouyang, Z., Landreth, G. E. & Murakami, S. FGF and ERK signaling coordinately regulate mineralization-related genes and play essential roles in osteocyte differentiation. J. Bone Miner. Metab. 30, 19–30 (2012).
Woo, S. M., Rosser, J., Dusevich, V., Kalajzic, I. & Bonewald, L. F. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J. Bone Miner. Res. 26, 2634–2646 (2011).
Lee, J. W., Yamaguchi, A. & Iimura, T. Functional heterogeneity of osteocytes in FGF23 production: the possible involvement of DMP1 as a direct negative regulator. Bonekey Rep. 3, 543 (2014).
Hori, M., Kinoshita, Y., Taguchi, M. & Fukumoto, S. Phosphate enhances Fgf23 expression through reactive oxygen species in UMR-106 cells. J. Bone Miner. Metab. 34, 132–139 (2016).
Ludmilla, B. et al. Advanced glycation end products stimulate gene expression of fibroblast growth factor 23. Mol. Nutr. Food Res. 61, 1601019 (2017).
Nielsen, P. K., Feldt-Rasmussen, U. & Olgaard, K. A direct effect in vitro of phosphate on PTH release from bovine parathyroid tissue slices but not from dispersed parathyroid cells. Nephrol. Dialysis Transplant. 11, 1762–1768 (1996).
Silver, J. & Naveh-Many, T. Phosphate and the parathyroid. Kidney Int. 75, 898–905 (2009).
Maeda, A. et al. Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc. Natl Acad. Sci. 110, 5864–5869 (2013).
Kulkarni, N. H. et al. Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell. Biochem. 95, 1178–1190 (2005).
Meir, T. et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 86, 1106–1115 (2014).
Nilsson, I. L. et al. FGF23, metabolic risk factors, and blood pressure in patients with primary hyperparathyroidism undergoing parathyroid adenomectomy. Surgery 159, 211–217 (2016).
Witteveen, J. E., van Lierop, A. H., Papapoulos, S. E. & Hamdy, N. A. Increased circulating levels of FGF23: an adaptive response in primary hyperparathyroidism? Eur. J. Endocrinol. 166, 55–60 (2012).
Horwitz, M. J. et al. Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J. Bone Miner. Res. 20, 1792–1803 (2005).
Wysolmerski, J. J. Parathyroid hormone-related protein: an update. J. Clin. Endocrinol. Metab. 97, 2947–2956 (2012).
Zhang, C. et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2, e1600241 (2016).
Clinkenbeard, E. et al. Erythropoietin and FGF23 cross-talk during iron-deficiency anemia [abstract 1117]. J. Bone Miner. Res. 30, S39 (2015).
David, V., Francis, C. & Babitt, J. L. Ironing out the cross talk between FGF23 and inflammation. Am. J. Physiol. Renal Physiol. 312, F1–F8 (2017).
Yamamoto, S., Okada, Y., Mori, H., Fukumoto, S. & Tanaka, Y. Fibroblast growth factor 23-related osteomalacia caused by the prolonged administration of saccharated ferric oxide. Intern. Med. 51, 2375–2378 (2012).
Schouten, B. J., Hunt, P. J., Livesey, J. H., Frampton, C. M. & Soule, S. G. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J. Clin. Endocrinol. Metab. 94, 2332–2337 (2009).
Hryszko, T., Rydzewska-Rosolowska, A., Brzosko, S., Koc-Zorawska, E. & Mysliwiec, M. Low molecular weight iron dextran increases fibroblast growth factor-23 concentration, together with parathyroid hormone decrease in hemodialyzed patients. Ther. Apheresis Dialysis 16, 146–151 (2012).
Lewerin, C. et al. Low serum iron is associated with high serum intact FGF23 in elderly men: the Swedish MrOS study. Bone 98, 1–8 (2017).
Melda, O. et al. A novel distal enhancer mediates inflammation-, PTH-, and early onset murine kidney disease-induced expression of the mouse Fgf23 gene. JBMR Plus 2, 31–46 (2018).
Bora, S. A., Kennett, M. J., Smith, P. B., Patterson, A. D. & Cantorna, M. T. The gut microbiota regulates endocrine vitamin D metabolism through fibroblast growth factor 23. Front. Immunol. 9, 408 (2018).
Bär, L. et al. Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc. Natl Acad. Sci. 115, 5804–5809 (2018).
Zhang, B. et al. Up-regulation of FGF23 release by aldosterone. Biochem. Biophys. Res. Commun. 470, 384–390 (2016).
Pathare, G., Anderegg, M., Albano, G., Lang, F. & Fuster, D. G. Elevated FGF23 levels in mice lacking the thiazide-sensitive NaCl cotransporter (NCC). Sci. Rep. 8, 3590 (2018).
Bikle, D. D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 21, 319–329 (2014).
Norman, A. W. The history of the discovery of vitamin D and its daughter steroid hormone. Ann. Nutr. Metab. 61, 199–206 (2012).
Petkovich, M. & Jones, G. CYP24A1 and kidney disease. Curr. Opin. Nephrol. Hypertens. 20, 337–344 (2011).
Jones, G., Prosser, D. E. & Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 523, 9–18 (2012).
Carpenter, T. O. & Shiratori, T. Renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity and mitochondrial phosphate transport in Hyp mice. Am. J. Physiol. 259, E814–821 (1990).
Kaufmann, M., Lee, S. M., Pike, J. W. & Jones, G. A. High-calcium and phosphate rescue diet and VDR-expressing transgenes normalize serum vitamin D metabolite profiles and renal Cyp27b1 and Cyp24a1 expression in VDR null mice. Endocrinology 156, 4388–4397 (2015).
Masuda, S. et al. Altered pharmacokinetics of 1alpha, 25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology 146, 825–834 (2005).
Vanhooke, J. L. et al. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc. Natl Acad. Sci. USA 103, 75–80 (2006).
Gattineni, J. & Friedman, P. A. Regulation of hormone-sensitive renal phosphate transport. Vitam. Horm. 98, 249–306 (2015).
Steingrimsdottir, L., Gunnarsson, O., Indridason, O. S., Franzson, L. & Sigurdsson, G. Relationship between serum parathyroid hormone levels, vitamin d sufficiency, and calcium intake. JAMA 294, 2336–2341 (2005).
Condamine, L., Menaa, C., Vrtovsnik, F., Friedlander, G. & Garabédian, M. Local action of phosphate depletion and insulin-like growth factor 1 on in vitro production of 1,25-dihydroxyvitamin D by cultured mammalian kidney cells. J. Clin. Invest. 94, 1673–1679 (1994).
Zhang, M. Y. H. et al. Dietary phosphorus transcriptionally regulates 25-Hydroxyvitamin D-1α-hydroxylase gene expression in the proximal renal tubule. Endocrinology 143, 587–595 (2002).
Tenenhouse, H. S., Martel, J., Gauthier, C., Zhang, M. Y. H. & Portale, A. A. Renal expression of the sodium/phosphate cotransporter gene, Npt2, is not required for regulation of renal 1α-hydroxylase by phosphate. Endocrinology 142, 1124–1129 (2001).
Schlingmann, K. P. et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 365, 410–421 (2011).
Kaneko, I. et al. Hypophosphatemia in vitamin D receptor null mice: effect of rescue diet on the developmental changes in renal Na+ -dependent phosphate cotransporters. Pflugers Arch. 461, 77–90 (2011).
Christakos, S., Ajibade, D. V., Dhawan, P., Fechner, A. J. & Mady, L. J. Vitamin D: metabolism. Rheum. Dis. Clin. North Am. 38, 1–11, vii (2012).
Lee, G. J. & Marks, J. Intestinal phosphate transport: a therapeutic target in chronic kidney disease and beyond? Pediatr. Nephrol. 30, 363–371 (2015).
Sabbagh, Y., Giral, H., Caldas, Y., Levi, M. & Schiavi, S. C. Intestinal phosphate transport. Adv. Chron. Kidney Dis. 18, 85–90 (2011).
Knöpfel, T. et al. The intestinal phosphate transporter NaPi-IIb (Slc34a2) is required to protect bone during dietary phosphate restriction. Sci. Rep. 7, 11018 (2017).
Berndt, T. et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc. Natl Acad. Sci. USA 104, 11085–11090 (2007).
Scanni, R., vonRotz, M., Jehle, S., Hulter, H. N. & Krapf, R. The human response to acute enteral and parenteral phosphate loads. J. Am. Soc. Nephrol. 25, 2730–2739 (2014).
Razzaque, M. S. Phosphate toxicity: new insights into an old problem. Clin. Sci. 120, 91–97 (2011).
Drezner, M. K. PHEX gene and hypophosphatemia. Kidney Int. 57, 9–18 (2000).
Hautmann, A. H., Hautmann, M. G., Kolbl, O., Herr, W. & Fleck, M. Tumor-Induced osteomalacia: an up-to-date review. Curr. Rheumatol Rep. 17, 512 (2015).
Lee, J. C. et al. Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J. Pathol. 235, 539–545 (2015).
Lee, J. C. et al. Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod. Pathol. 29, 1335–1346 (2016).
Wohrle, S. et al. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J. Bone Miner. Res. 28, 899–911 (2013).
Xiao, Z. et al. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLOS One 9, e104154 (2014).
Miller, C. B. et al. Response of tumor-induced osteomalacia (TIO) to the FGFR inhibitor BGJ398. J. Clin. Oncol. 34, e22500–e22500 (2016).
Akl, M. R. et al. Molecular and clinical significance of fibroblast growth factor 2 (FGF2 /bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 7, 44735–44762 (2016).
De Beur, S. J. et al. Effects of burosumab (KRN23), a human monoclonal antibody to FGF23, in patients with tumor-induced osteomalacia (TIO) or epidermal nevus syndrome (ENS) [abstract SU0325]. J. Bone Miner. Res. 32, S280 (2017).
Francis, F. et al. A gene (PEX) with homologies to endopeptidases is mutated in patients with X–linked hypophosphatemic rickets. Nat. Genet. 11, 130 (1995).
Bai, X.-Y., Miao, D., Goltzman, D. & Karaplis, A. C. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J. Biol. Chem. 278, 9843–9849 (2003).
Lorenz-Depiereux, B., Schnabel, D., Tiosano, D., Häusler, G. & Strom, T. M. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am. J. Hum. Genet. 86, 267–272 (2010).
Simpson, M. A. et al. Mutations in FAM20C also identified in non-lethal osteosclerotic bone dysplasia. Clin. Genet. 75, 271–276 (2009).
Rafaelsen, S. H. et al. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23–related hypophosphatemia, dental anomalies, and ectopic calcification. J. Bone Miner. Res. 28, 1378–1385 (2013).
Nitschke, Y. & Rutsch, F. Inherited arterial calcification syndromes: etiologies and treatment concepts. Curr. Osteoporosis Rep. 15, 255–270 (2017).
Wang, X. et al. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLOS Genet. 8, e1002708 (2012).
Topaz, O. et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 36, 579 (2004).
Anand, G. & Schmid, C. Severe hypophosphataemia after intravenous iron administration. BMJ Case Rep. 2017, bcr2016219160 (2017).
Wolf, M., Koch, T. A. & Bregman, D. B. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 28, 1793–1803 (2013).
Wolf, M. & White, K. E. Coupling FGF23 production and cleavage: iron deficiency, rickets and kidney disease. Curr. Opin. Nephrol. Hypertension 23, 411–419 (2014).
Dumitrescu, C. E. & Collins, M. T. McCune-Albright syndrome. Orphanet J. Rare Dis. 3, 12 (2008).
Zhu, Y. et al. Ablation of the stimulatory G protein alpha-subunit in renal proximal tubules leads to parathyroid hormone-resistance with increased renal Cyp24a1 mRNA abundance and reduced serum 1,25-Dihydroxyvitamin D. Endocrinology 157, 497–507 (2016).
He, Q. et al. The extra-large G protein alpha-subunit (XLαs) mediates FGF23 production by maintaining FGFR1 expression and MAPK signaling in bone [abstract 1138]. J. Bone Miner. Res. 32, S47 (2017).
Carpenter, T. O. The expanding family of hypophosphatemic syndromes. J. Bone Miner. Metab. 30, 1–9 (2012).
Sharma, A. Physiology of the Developing Kidney: Disorders and Therapy of Calcium and Phosphorous Homeostasis 291–339 (Springer Berlin Heidelberg, 2016).
White, K. E., Hum, J. M. & Econs, M. J. Hypophosphatemic rickets: revealing novel control points for phosphate homeostasis. Curr. Osteoporos. Rep. 12, 252–262 (2014).
Carpenter, T. O. et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J. Clin. Invest. 124, 1587–1597 (2014).
Johnson, K. et al. Therapeutic Effects of FGF23 c-tail Fc in a murine pre-clinical model of X-linked hypophosphatemia via the selective modulation of phosphate reabsorption. J. Bone Miner. Res. 32, 2062–2073 (2017).
Bhattacharyya, N., Chong, W. H., Gafni, R. I. & Collins, M. T. Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol. Metab. 23, 610–618 (2012).
Bergwitz, C. et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 78, 179–192 (2006).
Lorenz-Depiereux, B. et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am. J. Hum. Genet. 78, 193–201 (2006).
Caballero, D. et al. Intraperitoneal pyrophosphate treatment reduces renal calcifications in Npt2a null mice. PLOS One 12, e0180098 (2017).
Li, Y. et al. Response of Npt2a knockout mice to dietary calcium and phosphorus. PLOS One 12, e0176232 (2017).
Caballero, D., Li, Y., Ponsetto, J., Zhu, C. & Bergwitz, C. Impaired urinary osteopontin excretion in Npt2a −/− mice. Am. J. Physiol. Renal Physiol. 312, F77–F83 (2017).
Bhoj, E. J. et al. Pathologic variants of the mitochondrial phosphate carrier SLC25A3: two new patients and expansion of the cardiomyopathy/skeletal myopathy phenotype with and without lactic acidosis. JIMD Rep. 19, 59–66 (2015).
Mayr, J. A. et al. Deficiency of the mitochondrial phosphate carrier presenting as myopathy and cardiomyopathy in a family with three affected children. Neuromuscul. Disord. 21, 803–808 (2011).
Adachi, J. D. et al. Management of corticosteroid-induced osteoporosis. Semin. Arthritis Rheum. 29, 228–251 (2000).
Maldonado, E. N. & Lemasters, J. J. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 19 Pt A, 78–84 (2014).
Kwong, J. Q. et al. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 21, 1209–1217 (2014).
Lemos, R. R. et al. Update and mutational analysis of SLC20A2: a major cause of primary familial brain calcification. Hum. Mutat. 36, 489–495 (2015).
Legati, A. et al. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 47, 579–581 (2015).
Nan, H. et al. Novel SLC20A2 mutation in primary familial brain calcification with disturbance of sustained phonation and orofacial apraxia. J. Neurol. Sci. 390, 1–3 (2018).
Anheim, M. et al. XPR1 mutations are a rare cause of primary familial brain calcification. J. Neurol. 263, 1559–1564 (2016).
Keller, A. et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 45, 1077–1082 (2013).
Yao, X.-P. et al. Analysis of gene expression and functional characterization of XPR1: a pathogenic gene for primary familial brain calcification. Cell Tissue Res. 370, 267–273 (2017).
Jensen, N. et al. Mice knocked out for the primary brain calcification associated gene Slc20a2 show unimpaired pre-natal survival but retarded growth and nodules in the brain that grow and calcify over time. Am. J. Pathol. https://doi.org/10.1016/j.ajpath.2018.04.010 (2018).
Wallingford, M. C. et al. SLC20A2 deficiency in mice leads to elevated phosphate levels in cerbrospinal fluid and glymphatic pathway-associated arteriolar calcification, and recapitulates human idiopathic basal ganglia calcification. Brain Pathol. 27, 64–76 (2017).
Jensen, N., Autzen, J. K. & Pedersen, L. Slc20a2 is critical for maintaining a physiologic inorganic phosphate level in cerebrospinal fluid. Neurogenetics 17, 125–130 (2016).
Bergen, A. A. B. et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat. Genet. 25, 228 (2000).
Klement, J. F. et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol. Cell. Biol. 25, 8299–8310 (2005).
Li, X., Yang, H. Y. & Giachelli, C. M. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ. Res. 98, 905–912 (2006).
Bastepe, M. & Juppner, H. Inherited hypophosphatemic disorders in children and the evolving mechanisms of phosphate regulation. Rev. Endocr. Metab. Disord. 9, 171–180 (2008).
Fukumoto, S. FGF23-FGF receptor/Klotho pathway as a new drug target for disorders of bone and mineral metabolism. Calcif. Tissue Int. 98, 334–340 (2016).
Moniot, S., Elias, M., Kim, D., Scott, K. & Chabriere, E. Crystallization, diffraction data collection and preliminary crystallographic analysis of DING protein from Pseudomonas fluorescens. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 63, 590–592 (2007).
Choi, P. H., Sureka, K., Woodward, J. J. & Tong, L. Molecular basis for the recognition of cyclic-di-AMP by PstA, a P(II)-like signal transduction protein. Microbiologyopen 4, 361–374 (2015).
Hudek, L., Premachandra, D., Webster, W. A. J. & Bräu, L. Role of phosphate transport system component PstB1 in phosphate internalization by Nostoc punctiforme. Appl. Environ. Microbiol. 82, 6344–6356 (2016).
Murer, H., Forster, I. & Biber, J. The sodium phosphate cotransporter family SLC34. Pflügers Arch. 447, 763–767 (2004).
Patrice, H. et al. Redundancy in the function of mitochondrial phosphate transport in Saccharomyces cerevisiae and Arabidopsis thaliana. Mol. Microbiol. 51, 307–317 (2004).
Secco, D. et al. The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol. 193, 842–851 (2012).
Yuan, Q. et al. PTH ablation ameliorates the anomalies of Fgf23-deficient mice by suppressing the elevated vitamin D and calcium levels. Endocrinology 152, 4053–4061 (2011).
Kido, S., Kaneko, I., Tatsumi, S., Segawa, H. & Miyamoto, K. Vitamin D and type II sodium-dependent phosphate cotransporters. Contrib. Nephrol. 180, 86–97 (2013).
Kamenický, P., Mazziotti, G., Lombès, M., Giustina, A. & Chanson, P. Growth hormone, insulin-like growth factor-1, and the kidney: pathophysiological and clinical implications. Endocr. Rev. 35, 234–281 (2014).
Acknowledgements
C.B. is supported by the Yale O’Brien Center (P30DK079310), and S.C. is supported by a postdoctoral fellowship from the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (T32DK007058-42).
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, provided substantial contribution to the discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Phosphaturic hormone
-
Hormone causing excretion of phosphate in the urine.
- Hypomorphic alleles
-
Genes that have a mutation that causes a partial loss of gene function.
- Haploinsufficiency
-
Refers to complete loss of function of one copy of a gene when the remaining functional copy of the gene is not adequate to produce the needed gene product to preserve normal function.
- 5/6 nephrectomy
-
Model of progressive renal failure with reduced nephron number achieved by either infarction or surgical excision of both poles and removal of the contralateral kidney.
- Pulmonary alveolar microlithiasis
-
A rare autosomal recessive disease of widespread intra-alveolar accumulation of minute calcium phosphate calculi called microliths caused by homozygous loss-of-function mutations in SLC34A2 (which encodes NPT2b).
- Tumoural calcinosis
-
Group of rare autosomal recessive metabolic disorders characterized by the development of severe ectopic calcifications in soft tissues due to homozygous loss-of-function mutations in the GALNT3, FGF23 or KL genes.
- Osteomalacia
-
Osteomalacia is a rare disorder of bone metabolism leading to reduced bone matrix mineralization.
- Phosphatonins
-
Phosphatonins is the collective term used for major regulators of Pi homeostasis, which generally function as phosphaturic hormones and lower blood levels of Pi.
Rights and permissions
About this article
Cite this article
Chande, S., Bergwitz, C. Role of phosphate sensing in bone and mineral metabolism. Nat Rev Endocrinol 14, 637–655 (2018). https://doi.org/10.1038/s41574-018-0076-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-018-0076-3
This article is cited by
-
Emerging concepts on the FGF23 regulation and activity
Molecular and Cellular Biochemistry (2024)
-
Disease Manifestations and Complications in Dutch X-Linked Hypophosphatemia Patients
Calcified Tissue International (2024)
-
Adjusting phosphate feeding regimen according to daily rhythm increases eggshell quality via enhancing medullary bone remodeling in laying hens
Journal of Animal Science and Biotechnology (2023)
-
Sexual dimorphisms in serum calcium and phosphate concentrations in the Rotterdam Study
Scientific Reports (2023)
-
Spatial distribution of elements during osteoarthritis disease progression using synchrotron X-ray fluorescence microscopy
Scientific Reports (2023)