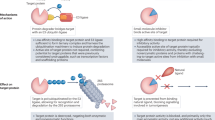
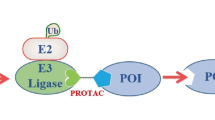
Abstract
Over the past 3 years, the first bivalent protein degraders intentionally designed for targeted protein degradation (TPD) have advanced to clinical trials, with an initial focus on established targets. Most of these clinical candidates are designed for oral administration, and many discovery efforts appear to be similarly focused. As we look towards the future, we propose that an oral-centric discovery paradigm will overly constrain the chemical designs that are considered and limit the potential to drug novel targets. In this Perspective, we summarize the current state of the bivalent degrader modality and propose three categories of degrader designs, based on their likely route of administration and requirement for drug delivery technologies. We then describe a vision for how parenteral drug delivery, implemented early in research and supported by pharmacokinetic–pharmacodynamic modelling, can enable exploration of a broader drug design space, expand the scope of accessible targets and deliver on the promise of protein degraders as a therapeutic modality.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hopkins, A. L. & Groom, C. R. The druggable genome. Nat. Rev. Drug Discov. 1, 727–730 (2002).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Churcher, I. Protac-induced protein degradation in drug discovery: breaking the rules or just making new ones? J. Med. Chem. 61, 444–452 (2018).
Maple, H. J., Clayden, N., Baron, A., Stacey, C. & Felix, R. Developing degraders: principles and perspectives on design and chemical space. MedChemComm 10, 1755–1764 (2019).
Pettersson, M. & Crews, C. M. Proteolysis targeting chimeras (PROTACs) — past, present and future. Drug Discov. Today Technol. 31, 15–27 (2019).
Schapira, M., Calabrese, M. F., Bullock, A. N. & Crews, C. M. Targeted protein degradation: expanding the toolbox. Nat. Rev. Drug Discov. 18, 949–963 (2019).
Rambacher, K. M., Calabrese, M. F. & Yamaguchi, M. Perspectives on the development of first-in-class protein degraders. Future Med. Chem. 13, 1203–1226 (2021).
Alabi, S. B. & Crews, C. M. Major advances in targeted protein degradation: PROTACs, LYTACs, and MADTACs. J. Biol. Chem. 296, 100647 (2021).
Békés, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 21, 181–200 (2022).
Sakamoto, K. M. et al. Protacs: chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc. Natl Acad. Sci. USA 98, 8554–8559 (2001).
Okuhira, K. et al. Specific degradation of CRABP-II via cIAP1-mediated ubiquitylation induced by hybrid molecules that crosslink cIAP1 and the target protein. FEBS Lett. 585, 1147–1152 (2011).
Banik, S. M. et al. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 584, 291–297 (2020).
Ahn, G. et al. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat. Chem. Biol. 17, 937–946 (2021).
Lin, J. et al. Emerging protein degradation strategies: expanding the scope to extracellular and membrane proteins. Theranostics 11, 8337–8349 (2021).
Samarasinghe, K. T. G. et al. Targeted degradation of transcription factors by TRAFTACs: TRAnscription Factor TArgeting Chimeras. Cell Chem. Biol. 28, 648–661 (2021).
Bondeson, D. P. et al. Lessons in PROTAC design from selective degradation with a promiscuous warhead. Cell Chem. Biol. 25, 78–87.e5 (2018).
Gadd, M. S. et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat. Chem. Biol. 13, 514–521 (2017).
Cantrill, C. et al. Fundamental aspects of DMPK optimization of targeted protein degraders. Drug. Discov. Today 25, 969–982 (2020).
Liu, X. et al. Assays and technologies for developing proteolysis targeting chimera degraders. Future Med. Chem. 12, 1155–1179 (2020).
Pike, A., Williamson, B., Harlfinger, S., Martin, S. & McGinnity, D. F. Optimising proteolysis-targeting chimeras (PROTACs) for oral drug delivery: a drug metabolism and pharmacokinetics perspective. Drug Discov. Today 25, 1793–1800 (2020).
Rodriguez-Rivera, F. P. & Levi, S. M. Unifying catalysis framework to dissect proteasomal degradation paradigms. ACS Cent. Sci. 7, 1117–1125 (2021).
Farnaby, W. et al. BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nat. Chem. Biol. 15, 672–680 (2019).
Iconomou, M. & Saunders, D. N. Systematic approaches to identify E3 ligase substrates. Biochemical J. 473, 4083–4101 (2016).
Khan, S. et al. A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 25, 1938–1947 (2019).
Troup, R. I., Fallan, C. & Baud, M. G. J. Current strategies for the design of PROTAC linkers: a critical review. Explor. Target. Antitumor Ther. 1, 273–312 (2020).
Morgan, P. et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov. Today 17, 419–424 (2012).
Bartlett, D. W. & Gilbert, A. M. A kinetic proofreading model for bispecific protein degraders. J. Pharmacokinet. Pharmacodyn. 48, 149–163 (2021).
Bartlett, D. W. & Gilbert, A. M. Translational PK–PD for targeted protein degradation. Chem. Soc. Rev. 51, 3477–3486 (2022).
Nowak, R. P. & Jones, L. H. Target validation using PROTACs: applying the four pillars framework. SLAS Discov. 26, 474–483 (2021).
Edmondson, S. D., Yang, B. & Fallan, C. Proteolysis targeting chimeras (PROTACs) in ‘beyond rule-of-five’ chemical space: recent progress and future challenges. Bioorg. Med. Chem. Lett. 29, 1555–1564 (2019).
Shultz, M. D. Two decades under the influence of the rule of five and the changing properties of approved oral drugs. J. Med. Chem. 62, 1701–1714 (2019).
Ermondi, G., Vallaro, M. & Caron, G. Degraders early developability assessment: face-to-face with molecular properties. Drug Discov. Today 25, 1585–1591 (2020).
Ermondi, G., Vallaro, M., Goetz, G., Shalaeva, M. & Caron, G. Updating the portfolio of physicochemical descriptors related to permeability in the beyond the Rule of 5 chemical space. Eur. J. Pharm. Sci. 146, 105274 (2020).
Rossi Sebastiano, M. et al. Impact of dynamically exposed polarity on permeability and solubility of chameleonic drugs beyond the Rule of 5. J. Med. Chem. 61, 4189–4202 (2018).
Klein, V. G. et al. Understanding and improving the membrane permeability of VH032-based PROTACs. ACS Med. Chem. Lett. 11, 1732–1738 (2020).
Atilaw, Y. et al. Solution conformations shed light on PROTAC cell permeability. ACS Med. Chem. Lett. 12, 107–114 (2021).
Kofink, C. et al. A selective and orally bioavailable VHL-recruiting PROTAC achieves SMARCA2 degradation in vivo. Nat. Commun. 13, 5969 (2022).
Petrylak, D. P. et al. First-in-human phase I study of ARV-110, an androgen receptor (AR) PROTAC degrader in patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC) following enzalutamide (ENZ) and/or abiraterone (ABI). J. Clin. Oncol. https://ascopubs.org/doi/10.1200/JCO.2020.38.15_suppl.3500 (2020).
Jin, J. et al. The peptide PROTAC modality: a novel strategy for targeted protein ubiquitination. Theranostics 10, 10141–10153 (2020).
Jiang, Y. et al. Development of stabilized peptide-based PROTACs against estrogen receptor α. ACS Chem. Biol. 13, 628–635 (2018).
Lu, M. et al. Discovery of a Keap1-dependent peptide PROTAC to knockdown Tau by ubiquitination–proteasome degradation pathway. Eur. J. Med. Chem. 146, 251–259 (2018).
Qu, J. et al. Specific knockdown of α-synuclein by peptide-directed proteasome degradation rescued its associated neurotoxicity. Cell Chem. Biol. 27, 751–762 (2020).
Testa, A., Hughes, S. J., Lucas, X., Wright, J. E. & Ciulli, A. Structure-based design of a macrocyclic PROTAC. Angew. Chem. Int. Ed. 59, 1727–1734 (2020).
Fulcher, L. J. et al. An affinity-directed protein missile system for targeted proteolysis. Open Biol. 6, 160255 (2016).
Röth, S. et al. Targeting endogenous K-RAS for degradation through the affinity-directed protein missile system. Cell Chem. Biol. 27, 1151–1163 (2020).
Simpson, L. M. et al. Inducible degradation of target proteins through a tractable affinity-directed protein missile system. Cell Chem. Biol. 27, 1164–1180 (2020).
Caussinus, E., Kanca, O. & Affolter, M. Fluorescent fusion protein knockout mediated by anti-GFP nanobody. Nat. Struct. Mol. Biol. 19, 117–121 (2012).
Portnoff, A. D., Stephens, E. A., Varner, J. D. & DeLisa, M. P. Ubiquibodies, synthetic E3 ubiquitin ligases endowed with unnatural substrate specificity for targeted protein silencing. J. Biol. Chem. 289, 7844–7855 (2014).
Lim, S. et al. bioPROTACs as versatile modulators of intracellular therapeutic targets including proliferating cell nuclear antigen (PCNA). Proc. Natl Acad. Sci. USA 117, 5791–5800 (2020).
Shao, J. et al. Destruction of DNA-binding proteins by programmable oligonucleotide PROTAC (O’PROTAC): effective targeting of LEF1 and ERG. Adv. Sci. 8, 2102555 (2021).
Liu, J. et al. TF-PROTACs enable targeted degradation of transcription factors. J. Am. Chem. Soc. 143, 8902–8910 (2021).
Van den Mooter, G. The use of amorphous solid dispersions: a formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 9, e79–e85 (2012).
Patel, V., Lalani, R., Bardoliwala, D., Ghosh, S. & Misra, A. Lipid-based oral formulation strategies for lipophilic drugs. AAPS PharmSciTech 19, 3609–3630 (2018).
Tran, P. et al. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics 11, 132 (2019).
Schittny, A., Huwyler, J. & Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: a review. Drug Deliv. 27, 110–127 (2020).
Phan, T. N. Q., Le-Vinh, B., Efiana, N. A. & Bernkop-Schnürch, A. Oral self-emulsifying delivery systems for systemic administration of therapeutic proteins: science fiction? J. Drug Target. 27, 1017–1024 (2019).
McCartney, F. et al. Labrasol® is an efficacious intestinal permeation enhancer across rat intestine: ex vivo and in vivo rat studies. J. Control. Rel. 310, 115–126 (2019).
Brayden, D. J., Hill, T. A., Fairlie, D. P., Maher, S. & Mrsny, R. J. Systemic delivery of peptides by the oral route: formulation and medicinal chemistry approaches. Adv. Drug Deliv. Rev. 157, 2–36 (2020).
Overgaard, R. V., Navarria, A., Ingwersen, S. H., Bækdal, T. A. & Kildemoes, R. J. Clinical pharmacokinetics of oral semaglutide: analyses of data from clinical pharmacology trials. Clin. Pharmacokinet. 60, 1335–1348 (2021).
Brayden, D. J. & Maher, S. Transient permeation enhancer® (TPE®) technology for oral delivery of octreotide: a technological evaluation. Expert. Opin. Drug Deliv. 18, 1501–1512 (2021).
Bækdal, T. A. et al. Effect of various dosing conditions on the pharmacokinetics of oral semaglutide, a human glucagon-like peptide-1 analogue in a tablet formulation. Diabetes Ther. 12, 1915–1927 (2021).
Lau, J. et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 58, 7370–7380 (2015).
Bucheit, J. D. et al. Oral semaglutide: a review of the first oral glucagon-like peptide 1 receptor agonist. Diabetes Technol. Ther. 22, 10–18 (2020).
Deng, F. & Bae, Y. H. Bile acid transporter-mediated oral drug delivery. J. Control. Rel. 327, 100–116 (2020).
Maher, S., Ryan, K. B., Ahmad, T., O’Driscoll, C. M. & Brayden, D. J. in Nanostructured Biomaterials for Overcoming Biological Barriers (eds Alonso, M. J. & Csaba, N. S.) 39–62 (Royal Society of Chemistry, 2012).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019).
Dhalla, A. K. et al. A robotic pill for oral delivery of biotherapeutics: safety, tolerability, and performance in healthy subjects. Drug Deliv. Transl. Res. 12, 294–305 (2022).
Los, M. et al. Expression pattern of the von Hippel–Lindau protein in human tissues. Lab. Invest. 75, 231–238 (1996).
Luo, X. et al. Profiling of diverse tumor types establishes the broad utility of VHL-based ProTaCs and triages candidate ubiquitin ligases. iScience 25, 103985 (2022).
Yamanaka, S. et al. Thalidomide and its metabolite 5-hydroxythalidomide induce teratogenicity via the cereblon neosubstrate PLZF. EMBO J. 40, e105375 (2021).
Asatsuma-Okumura, T. et al. p63 is a cereblon substrate involved in thalidomide teratogenicity. Nat. Chem. Biol. 15, 1077–1084 (2019).
Matyskiela, M. E. et al. SALL4 mediates teratogenicity as a thalidomide-dependent cereblon substrate. Nat. Chem. Biol. 14, 981–987 (2018).
Sievers, Q. L. et al. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science 362, eaat0572 (2018).
Liu, X. et al. Discovery of XL01126: a potent, fast, cooperative, selective, orally bioavailable, and blood–brain barrier penetrant PROTAC degrader of leucine-rich repeat kinase 2. J. Am. Chem. Soc. 144, 16930–16952 (2022).
Posternak, G. et al. Functional characterization of a PROTAC directed against BRAF mutant V600E. Nat. Chem. Biol. 16, 1170–1178 (2020).
Alabi, S. et al. Mutant-selective degradation by BRAF-targeting PROTACs. Nat. Commun. 12, 920 (2021).
Kramer, L. T. & Zhang, X. Expanding the landscape of E3 ligases for targeted protein degradation. Curr. Res. Chem. Biol. 2, 100020 (2022).
Leeson, P. D. et al. Target-based evaluation of “drug-like” properties and ligand efficiencies. J. Med. Chem. 64, 7210–7230 (2021).
Imaide, S. et al. Trivalent PROTACs enhance protein degradation via combined avidity and cooperativity. Nat. Chem. Biol. 17, 1157–1167 (2021).
Riching, K. M., Caine, E. A., Urh, M. & Daniels, D. L. The importance of cellular degradation kinetics for understanding mechanisms in targeted protein degradation. Chem. Soc. Rev. 51, 6210–6221 (2022).
Riching, K. M. et al. CDK family PROTAC profiling reveals distinct kinetic responses and cell cycle–dependent degradation of CDK2. SLAS Discov. 26, 560–569 (2021).
Kaminskas, L. M., Boyd, B. J. & Porter, C. J. Dendrimer pharmacokinetics: the effect of size, structure and surface characteristics on ADME properties. Nanomedicine 6, 1063–1084 (2011).
Allen, T. M. & Cullis, P. R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 65, 36–48 (2013).
Ashton, S. et al. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci. Transl. Med. 8, 325ra317 (2016).
Patterson, C. M. et al. Design and optimisation of dendrimer-conjugated Bcl-2/xL inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun. Biol. 4, 112 (2021).
Gabizon, A., Shmeeda, H. & Barenholz, Y. Pharmacokinetics of pegylated liposomal doxorubicin. Clin. Pharmacokinet. 42, 419–436 (2003).
Jiang, W., Lionberger, R. & Yu, L. X. In vitro and in vivo characterizations of PEGylated liposomal doxorubicin. Bioanalysis 3, 333–344 (2011).
Lamb, Y. N. & Scott, L. J. Liposomal irinotecan: a review in metastatic pancreatic adenocarcinoma. Drugs 77, 785–792 (2017).
Blair, H. A. Daunorubicin/cytarabine liposome: a review in acute myeloid leukaemia. Drugs 78, 1903–1910 (2018).
Sun, D., Zhou, S. & Gao, W. What went wrong with anticancer nanomedicine design and how to make it right. ACS Nano 14, 12281–12290 (2020).
Crommelin, D. J. A., van Hoogevest, P. & Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Rel. 318, 256–263 (2020).
Saraswat, A. et al. Nanoformulation of proteolysis targeting chimera targeting ‘undruggable’ c-Myc for the treatment of pancreatic cancer. Nanomedicine 15, 1761–1777 (2020).
Donahue, N. D., Acar, H. & Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 143, 68–96 (2019).
Dragovich, P. S. Degrader–antibody conjugates. Chem. Soc. Rev. 51, 3886–3897 (2022).
Chari, R. V. J., Miller, M. L. & Widdison, W. C. Antibody–drug conjugates: an emerging concept in cancer therapy. Angew. Chem. Int. Ed. 53, 3796–3827 (2014).
Beck, A., Goetsch, L., Dumontet, C. & Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 (2017).
Sau, S., Alsaab, H. O., Kashaw, S. K., Tatiparti, K. & Iyer, A. K. Advances in antibody–drug conjugates: a new era of targeted cancer therapy. Drug Discov. Today 22, 1547–1556 (2017).
Dragovich, P. S. et al. Antibody-mediated delivery of chimeric protein degraders which target estrogen receptor α (ERα). Bioorg. Med. Chem. Lett. 30, 126907 (2020).
Pillow, T. H. et al. Antibody conjugation of a chimeric BET degrader enables in vivo activity. ChemMedChem 15, 17–25 (2020).
Dragovich, P. S. et al. Antibody-mediated delivery of chimeric BRD4 degraders. Part 1: exploration of antibody linker, payload loading, and payload molecular properties. J. Med. Chem. 64, 2534–2575 (2021).
Dragovich, P. S. et al. Antibody-mediated delivery of chimeric BRD4 degraders. Part 2: improvement of in vitro antiproliferation activity and in vivo antitumor efficacy. J. Med. Chem. 64, 2576–2607 (2021).
Maneiro, M. A. et al. Antibody–PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem. Biol. 15, 1306–1312 (2020).
Cotton, A. D., Nguyen, D. P., Gramespacher, J. A., Seiple, I. B. & Wells, J. A. Development of antibody-based PROTACs for the degradation of the cell-surface immune checkpoint protein PD-L1. J. Am. Chem. Soc. 143, 593–598 (2021).
Hrkach, J. et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 4, 128ra139 (2012).
Johnston, M. C. & Scott, C. J. Antibody conjugated nanoparticles as a novel form of antibody drug conjugate chemotherapy. Drug Discov. Today Technol. 30, 63–69 (2018).
Di, J., Xie, F. & Xu, Y. When liposomes met antibodies: drug delivery and beyond. Adv. Drug Deliv. Rev. 154-155, 151–162 (2020).
Cimas, F. J. et al. Controlled delivery of BET-PROTACs: in vitro evaluation of MZ1-loaded polymeric antibody conjugated nanoparticles in breast cancer. Pharmaceutics 12, 986 (2020).
Usach, I., Martinez, R., Festini, T. & Peris, J.-E. Subcutaneous injection of drugs: literature review of factors influencing pain sensation at the injection site. Adv. Ther. 36, 2986–2996 (2019).
Badkar, A. V., Gandhi, R. B., Davis, S. P. & LaBarre, M. J. Subcutaneous delivery of high-dose/volume biologics: current status and prospect for future advancements. Drug Des. Devel. Ther. 15, 159–170 (2021).
Wright, J. C. & Burgess, D. J. (eds) Long Acting Injections and Implants (Springer, 2012).
Hillery, A. & Park, K. (eds) Drug Delivery: Fundamentals and Applications (CRC, 2016).
O’Brien, M. N., Jiang, W., Wang, Y. & Loffredo, D. M. Challenges and opportunities in the development of complex generic long-acting injectable drug products. J. Control. Rel. 336, 144–158 (2021).
Shah, J. C. & Hong, J. Model for long acting injectables (depot formulation) based on pharmacokinetics and physical chemical properties. AAPS J. 24, 44 (2022).
DeYoung, M. B., MacConnell, L., Sarin, V., Trautmann, M. & Herbert, P. Encapsulation of exenatide in poly-(d,l-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes Technol. Ther. 13, 1145–1154 (2011).
Park, K. et al. Injectable, long-acting PLGA formulations: analyzing PLGA and understanding microparticle formation. J. Control. Rel. 304, 125–134 (2019).
Robertson, J. F. R. & Harrison, M. Fulvestrant: pharmacokinetics and pharmacology. Br. J. Cancer 90, S7–S10 (2004).
Miah, A. H. et al. Optimization of a series of RIPK2 PROTACs. J. Med. Chem. 64, 12978–13003 (2021).
Vinogradov, A. A., Yin, Y. & Suga, H. Macrocyclic peptides as drug candidates: recent progress and remaining challenges. J. Am. Chem. Soc. 141, 4167–4181 (2019).
Guo, Y. et al. An integrated strategy for assessing the metabolic stability and biotransformation of macrocyclic peptides in drug discovery toward oral delivery. Anal. Chem. 94, 2032–2041 (2022).
Qian, Z., Dougherty, P. G. & Pei, D. Targeting intracellular protein–protein interactions with cell-permeable cyclic peptides. Curr. Opin. Chem. Biol. 38, 80–86 (2017).
Milletti, F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov. Today 17, 850–860 (2012).
Xie, J. et al. Cell-penetrating peptides in diagnosis and treatment of human diseases: from preclinical research to clinical application. Front. Pharmacol. 11, 00697 (2020).
Kulkarni, J. A. et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643 (2021).
Cullis, P. R. & Hope, M. J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475 (2017).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Francia, V., Schiffelers, R. M., Cullis, P. R. & Witzigmann, D. The biomolecular corona of lipid nanoparticles for gene therapy. Bioconjug. Chem. 31, 2046–2059 (2020).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021).
Luo, W.-C. et al. Impact of formulation on the quality and stability of freeze-dried nanoparticles. Eur. J. Pharm. Biopharm. 169, 256–267 (2021).
Dugal-Tessier, J., Thirumalairajan, S. & Jain, N. Antibody–oligonucleotide conjugates: a twist to antibody–drug conjugates. J. Clin. Med. 10, 838 (2021).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997).
Tuntland, T. et al. Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at novartis institute of biomedical research. Front. Pharmacol. 5, 174–174 (2014).
Benet, L. Z. & Zia-Amirhosseini, P. Basic principles of pharmacokinetics. Toxicol. Pathol. 23, 115–123 (1995).
Kondic, A. et al. Navigating between right, wrong, and relevant: the use of mathematical modeling in preclinical decision making. Front. Pharmacol. 13, 860881 (2022).
Derendorf, H. & Meibohm, B. Modeling of pharmacokinetic/pharmacodynamic (PK/PD) relationships: concepts and perspectives. Pharm. Res. 16, 176–185 (1999).
Azer, K. et al. History and future perspectives on the discipline of quantitative systems pharmacology modeling and its applications. Front. Physiol. 12, 637999 (2021).
Acknowledgements
The authors acknowledge their many colleagues who helped shape their thoughts on drug design and drug delivery strategies for protein degraders, including R. Green, R. Jaini, M. Landis, D. Loffredo and M. Ticehurst. In addition, the authors thank M. Landis, P. Dragovich, K. Nagapudi, J. Montgomery and M. Calabrese for their help with reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
M.N.O.L., S.L., M.F.B. and D.W.B. all wrote and edited the perspective. D.W.B. performed PK–PD simulations.
Corresponding author
Ethics declarations
Competing interests
M.N.O.L. is an employee of Genentech, Inc. S.L. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. M.F.B. and D.W.B. are employees of Pfizer, Inc.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Alessio Ciulli, John Harling and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Clearance
-
(CL). The rate at which a drug is eliminated from the body (for example, metabolized, excreted). It represents the volume of plasma (or blood) that can be cleared of drug per unit of time and has units of volume per time.
- DC50
-
The concentration of degrader that results in half of its maximum achievable protein of interest degradation (Dmax/2).
- Dmax
-
The maximum amount of degradation that can be achieved for a protein of interest (POI) with a given degrader, expressed as a percentage relative to the steady-state unperturbed concentration of the POI.
- Drug design space
-
The range of possible chemical structures that are considered by medicinal chemists during drug discovery, as constrained by physicochemical properties and synthetic capabilities.
- Excipients
-
The components within a drug product formulation other than the active pharmaceutical ingredient.
- Half-life
-
(t1/2). The time it takes for a given property to be reduced by half. For example, the drug t1/2 or protein of interest (POI) t1/2 represents the time for the drug concentration or the POI concentration, respectively, to reach half of the original value.
- Lipophilicity
-
The affinity of a compound for a hydrophobic, or lipid-like, environment. Traditionally, lipophilicity is measured by an octanol/water partition coefficient, or logP. In this experiment, a compound is added to an immiscible mixture of a hydrophobic solvent (for example, octanol) and a hydrophilic solvent (for example, water), and the relative amount that is solubilized in each phase is quantified. Additional tools now exist to evaluate lipophilicity and the results are dependent on the method used.
- Parenteral
-
Any route of administration outside the enteral or gastrointestinal tract (that is, not oral, gastric, duodenal or rectal administration). Typically used in reference to injectable routes of administration such as intravenous, intramuscular and subcutaneous.
- Peak to trough
-
The maximum fold change in drug concentration observed during the dosing interval. It is defined as the ratio of the maximum (peak) concentration to the minimum (trough) concentration.
- Protein of interest
-
(POI). The endogenous protein intended for targeted protein degradation.
- Target binding moiety
-
(TBM). The portion of a bivalent protein degrader that binds to the protein of interest.
- Ternary complex
-
The three-component complex required to drive protein degradation, comprising a bivalent protein degrader bound to a protein of interest at one terminus and an E3 ubiquitin ligase at the other end.
- Therapeutic index
-
The ratio of the drug dose that results in toxicity to the drug dose that is required for efficacy.
- Volume of distribution
-
(Vd). The apparent volume into which an administered drug must distribute to achieve a given concentration in plasma (or blood).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Brien Laramy, M.N., Luthra, S., Brown, M.F. et al. Delivering on the promise of protein degraders. Nat Rev Drug Discov 22, 410–427 (2023). https://doi.org/10.1038/s41573-023-00652-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-023-00652-2