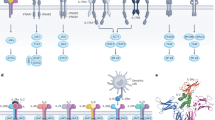
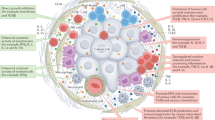
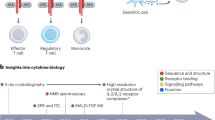
Abstract
Cytokines are secreted signalling proteins that play essential roles in the initiation, maintenance and resolution of immune responses. Although the unique ability of cytokines to control immune function has garnered clinical interest in the context of cancer, autoimmunity and infectious disease, the use of cytokine-based therapeutics has been limited. This is due, in part, to the ability of cytokines to act on many cell types and impact diverse biological functions, resulting in dose-limiting toxicity or lack of efficacy. Recent studies combining structural biology, protein engineering and receptor pharmacology have unlocked new insights into the mechanisms of cytokine receptor activation, demonstrating that many aspects of cytokine function are highly tunable. Here, we discuss the pharmacological principles underlying these efforts to overcome cytokine pleiotropy and enhance the therapeutic potential of this important class of signalling molecules.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Akdis, M. et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 127, 701–721.e1–e70 (2011).
Rider, P., Carmi, Y. & Cohen, I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int. J. Cell Biol. 2016, 9259646 (2016).
Donnelly, R. P., Young, H. A. & Rosenberg, A. S. An overview of cytokines and cytokine antagonists as therapeutic agents. Ann. N. Y. Acad. Sci. 1182, 1–13 (2009).
Pires, I. S., Hammond, P. T. & Irvine, D. J. Engineering strategies for immunomodulatory cytokine therapies — challenges and clinical progress. Adv. Ther. https://doi.org/10.1002/adtp.202100035 (2021).
Holder, P. G. et al. Engineering interferons and interleukins for cancer immunotherapy. Adv. Drug Deliv. Rev. 182, 114112 (2022).
Cunningham, B. C. et al. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science 254, 821–825 (1991).
Watowich, S. S., Hilton, D. J. & Lodish, H. F. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol. Cell Biol. 14, 3535–3549 (1994).
Kossiakoff, A. A. & De Vos, A. M. Structural basis for cytokine hormone-receptor recognition and receptor activation. Adv. Protein Chem. 52, 67–108 (1998).
Brooks, A. J. et al. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science 344, 1249783 (2014).
Wilmes, S. et al. Mechanism of homodimeric cytokine receptor activation and dysregulation by oncogenic mutations. Science 367, 643–652 (2020).
Glassman, C. R. et al. Structure of a Janus kinase cytokine receptor complex reveals the basis for dimeric activation. Science 376, 163–169 (2022).
Ihle, J. N., Witthuhn, B. A., Quelle, F. W., Yamamoto, K. & Silvennoinen, O. Signaling through the hematopoietic cytokine receptors. Annu. Rev. Immunol. 13, 369–398 (1995).
O’Shea, J. J. & Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36, 542–550 (2012).
Levy, D. E. & Darnell, J. E. Jr. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3, 651–662 (2002).
Morris, R., Kershaw, N. J. & Babon, J. J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 27, 1984–2009 (2018).
Wells, J. A. & de Vos, A. M. Hematopoietic receptor complexes. Annu. Rev. Biochem. 65, 609–634 (1996).
Wang, X., Lupardus, P., Laporte, S. L. & Garcia, K. C. Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 27, 29–60 (2009).
Rickert, M., Wang, X., Boulanger, M. J., Goriatcheva, N. & Garcia, K. C. The structure of interleukin-2 complexed with its α receptor. Science 308, 1477–1480 (2005).
Boulanger, M. J. & Garcia, K. C. Shared cytokine signaling receptors: structural insights from the gp130 system. Adv. Protein Chem. 68, 107–146 (2004).
van Boxel-Dezaire, A. H., Rani, M. R. & Stark, G. R. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25, 361–372 (2006).
Donnelly, R. P. & Kotenko, S. V. Interferon-λ: a new addition to an old family. J. Interferon Cytokine Res. 30, 555–564 (2010).
Malek, T. R. The biology of interleukin-2. Annu. Rev. Immunol. 26, 453–479 (2008).
Lai, Y. & Dong, C. Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases. Int. Immunol. 28, 181–188 (2016).
Metcalf, D. Hematopoietic cytokines. Blood 111, 485–491 (2008).
Borden, E. C. et al. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 6, 975–990 (2007).
Gillis, S. & Smith, K. A. Long term culture of tumour-specific cytotoxic T cells. Nature 268, 154–156 (1977).
Morgan, D. A., Ruscetti, F. W. & Gallo, R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 193, 1007–1008 (1976).
Rosenberg, S. A., Yang, J. C., White, D. E. & Steinberg, S. M. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann. Surg. 228, 307–319 (1998).
McDermott, D. F. & Atkins, M. B. Application of IL-2 and other cytokines in renal cancer. Expert Opin. Biol. Ther. 4, 455–468 (2004).
Legha, S. S., Gianan, M. A., Plager, C., Eton, O. E. & Papadopoulous, N. E. Evaluation of interleukin-2 administered by continuous infusion in patients with metastatic melanoma. Cancer 77, 89–96 (1996).
Waldhauer, I. et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. MAbs 13, 1913791 (2021).
Ptacin, J. L. et al. An engineered IL-2 reprogrammed for anti-tumor therapy using a semi-synthetic organism. Nat. Commun. 12, 4785 (2021).
Arellano, M. & Lonial, S. Clinical uses of GM-CSF, a critical appraisal and update. Biologics 2, 13–27 (2008).
Ng, T., Marx, G., Littlewood, T. & Macdougall, I. Recombinant erythropoietin in clinical practice. Postgrad. Med. J. 79, 367–376 (2003).
Tahtinen, S. et al. Favorable alteration of tumor microenvironment by immunomodulatory cytokines for efficient T-cell therapy in solid tumors. PLoS ONE 10, e0131242 (2015).
Liu, B. L. et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 10, 292–303 (2003).
Andtbacka, R. H. I. et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte–macrophage colony-stimulating factor in unresectable stage III–IV melanoma. J. Immunother. Cancer 7, 145 (2019).
Pegram, H. J. et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119, 4133–4141 (2012).
Zitvogel, L. et al. Cancer immunotherapy of established tumors with IL-12. Effective delivery by genetically engineered fibroblasts. J. Immunol. 155, 1393–1403 (1995).
Hsu, E. J. et al. A cytokine receptor-masked IL2 prodrug selectively activates tumor-infiltrating lymphocytes for potent antitumor therapy. Nat. Commun. 12, 2768 (2021).
Momin, N. et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaw2614 (2019).
Pasche, N., Wulhfard, S., Pretto, F., Carugati, E. & Neri, D. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin. Cancer Res. 18, 4092–4103 (2012).
Mansurov, A. et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat. Biomed. Eng. 4, 531–543 (2020).
Neri, D. & Sondel, P. M. Immunocytokines for cancer treatment: past, present and future. Curr. Opin. Immunol. 40, 96–102 (2016).
Mock, J. et al. An engineered 4-1BBL fusion protein with “activity on demand”. Proc. Natl Acad. Sci. USA 117, 31780–31788 (2020).
Pogue, S. L. et al. Targeting attenuated interferon-α to myeloma cells with a CD38 antibody induces potent tumor regression with reduced off-target activity. PLoS ONE 11, e0162472 (2016).
Xu, Y. et al. An engineered IL15 cytokine mutein fused to an anti-PD1 improves intratumoral T-cell function and antitumor immunity. Cancer Immunol. Res. 9, 1141–1157 (2021).
O’Shea, J. J. & Murray, P. J. Cytokine signaling modules in inflammatory responses. Immunity 28, 477–487 (2008).
Regis, G., Pensa, S., Boselli, D., Novelli, F. & Poli, V. Ups and downs: the STAT1:STAT3 seesaw of interferon and gp130 receptor signalling. Semin. Cell Dev. Biol. 19, 351–359 (2008).
Rosenbaum, D. M., Rasmussen, S. G. & Kobilka, B. K. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009).
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schioth, H. B. & Gloriam, D. E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 (2017).
Weis, W. I. & Kobilka, B. K. The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem. 87, 897–919 (2018).
Wingler, L. M. & Lefkowitz, R. J. Conformational basis of G protein-coupled receptor signaling versatility. Trends Cell Biol. 30, 736–747 (2020).
Wootten, D., Christopoulos, A., Marti-Solano, M., Babu, M. M. & Sexton, P. M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 19, 638–653 (2018).
Wacker, D., Stevens, R. C. & Roth, B. L. How ligands illuminate GPCR molecular pharmacology. Cell 170, 414–427 (2017).
Tan, H. S. & Habib, A. S. Oliceridine: a novel drug for the management of moderate to severe acute pain — a review of current evidence. J. Pain. Res. 14, 969–979 (2021).
Gonzalez-Navajas, J. M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Jaitin, D. A. et al. Inquiring into the differential action of interferons (IFNs): an IFN-α2 mutant with enhanced affinity to IFNAR1 is functionally similar to IFN-β. Mol. Cell Biol. 26, 1888–1897 (2006).
Wilmes, S. et al. Receptor dimerization dynamics as a regulatory valve for plasticity of type I interferon signaling. J. Cell Biol. 209, 579–593 (2015).
Thomas, C. et al. Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell 146, 621–632 (2011).
Jaks, E., Gavutis, M., Uze, G., Martal, J. & Piehler, J. Differential receptor subunit affinities of type I interferons govern differential signal activation. J. Mol. Biol. 366, 525–539 (2007). This work demonstrates that differences in IFNAR1/2 affinity and complex stability enable distinct functions of type 1 interferons.
Khabar, K. S. et al. Expressed gene clusters associated with cellular sensitivity and resistance towards anti-viral and anti-proliferative actions of interferon. J. Mol. Biol. 342, 833–846 (2004).
Zurawski, S. M., Vega, F. Jr, Huyghe, B. & Zurawski, G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 12, 2663–2670 (1993).
Ozaki, K. & Leonard, W. J. Cytokine and cytokine receptor pleiotropy and redundancy. J. Biol. Chem. 277, 29355–29358 (2002).
Spangler, J. B., Moraga, I., Mendoza, J. L. & Garcia, K. C. Insights into cytokine-receptor interactions from cytokine engineering. Annu. Rev. Immunol. 33, 139–167 (2015).
Kalie, E., Jaitin, D. A., Abramovich, R. & Schreiber, G. An interferon α2 mutant optimized by phage display for IFNAR1 binding confers specifically enhanced antitumor activities. J. Biol. Chem. 282, 11602–11611 (2007).
Mendoza, J. L. et al. The IFN-λ–IFN-λ–R1-IL-10Rβ complex reveals structural features underlying type III IFN functional plasticity. Immunity 46, 379–392 (2017).
Levin, A. M. et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 484, 529–533 (2012). This work presents the first use of yeast display to engineer a super agonist variant of IL-2 (super-2).
Rao, B. M., Girvin, A. T., Ciardelli, T., Lauffenburger, D. A. & Wittrup, K. D. Interleukin-2 mutants with enhanced α-receptor subunit binding affinity. Protein Eng. 16, 1081–1087 (2003).
Junttila, I. S. et al. Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines. Nat. Chem. Biol. 8, 990–998 (2012).
Martinez-Fabregas, J. et al. Kinetics of cytokine receptor trafficking determine signaling and functional selectivity. eLife https://doi.org/10.7554/eLife.49314 (2019).
Saxton, R. A. et al. Structure-based decoupling of the pro- and anti-inflammatory functions of interleukin-10. Science https://doi.org/10.1126/science.abc8433 (2021). This work presents insights from the cryo-electron microscopy structure of the IL-10R complex that enable the design of myeloid selective anti-inflammatory IL-10 variants with diminished immunostimulatory effects.
Gorby, C. et al. Engineered IL-10 variants elicit potent immunomodulatory effects at low ligand doses. Sci. Signal. https://doi.org/10.1126/scisignal.abc0653 (2020).
Moraga, I. et al. Instructive roles for cytokine-receptor binding parameters in determining signaling and functional potency. Sci. Signal. 8, ra114 (2015).
Saxton, R. A. et al. The tissue protective functions of interleukin-22 can be decoupled from pro-inflammatory actions through structure-based design. Immunity 54, 660–672 e669 (2021). This work presents the rational design of STAT3-biased IL-22 variants that drive tissue protection without inflammation.
Piehler, J., Thomas, C., Garcia, K. C. & Schreiber, G. Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunol. Rev. 250, 317–334 (2012).
Waldmann, T. A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 (2006).
McKay, D. B. Response. Science 257, 412–413 (1992).
Ren, J. et al. Interleukin-2 superkines by computational design. Proc. Natl Acad. Sci. USA 119, e2117401119 (2022).
Silva, D. A. et al. De novo design of potent and selective mimics of IL-2 and IL-15. Nature 565, 186–191 (2019). This work is the first example of a de novo designed cytokine therapeutic (neo-2/15), now in phase I clinical trials (NL-201, developed by Neoleukin).
Hecht, J. R. et al. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J. Clin. Oncol. 39, 1108–1118 (2021).
Ivashkiv, L. B. & Donlin, L. T. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 (2014).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Mitra, S. et al. Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity 42, 826–838 (2015). This work designs high-affinity partial agonist variants of IL-2 with increased affinity for IL-2Rβ and reduced affinity for γc.
Mo, F. et al. An engineered IL-2 partial agonist promotes CD8+ T cell stemness. Nature 597, 544–548 (2021). This work identifies a partial agonist variant of IL-2 that selectively expands stem-like CD8+ T cells ex vivo.
Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Mendoza, J. L. et al. Structure of the IFNγ receptor complex guides design of biased agonists. Nature 567, 56–60 (2019).
Sadlack, B. et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75, 253–261 (1993).
Sadlack, B. et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 25, 3053–3059 (1995).
Suzuki, H. et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268, 1472–1476 (1995).
Willerford, D. M. et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3, 521–530 (1995).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Boyman, O., Kovar, M., Rubinstein, M. P., Surh, C. D. & Sprent, J. Selective stimulation of T cell subsets with antibody–cytokine immune complexes. Science 311, 1924–1927 (2006).
Spangler, J. B. et al. Antibodies to interleukin-2 elicit selective T cell subset potentiation through distinct conformational mechanisms. Immunity 42, 815–825 (2015).
Peterson, L. B. et al. A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease. J. Autoimmun. 95, 1–14 (2018).
Khoryati, L. et al. An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aba5264 (2020).
Glassman, C. R. et al. Calibration of cell-intrinsic interleukin-2 response thresholds guides design of a regulatory T cell biased agonist. eLife https://doi.org/10.7554/eLife.65777 (2021). This work identifies partial agonist IL-2 variants that selectively expand Treg cells.
Arenas-Ramirez, N. et al. Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2. Sci. Transl. Med. 8, 367ra166 (2016).
Sahin, D. et al. An IL-2-grafted antibody immunotherapy with potent efficacy against metastatic cancer. Nat. Commun. 11, 6440 (2020).
Spangler, J. B. et al. Engineering a single-agent cytokine/antibody fusion that selectively expands regulatory T cells for autoimmune disease therapy. J. Immunol. 201, 2094–2106 (2018).
Junttila, I. S. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 9, 888 (2018).
Junttila, I. S. et al. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Rα, IL-13α1, and γc regulates relative cytokine sensitivity. J. Exp. Med. 205, 2595–2608 (2008).
Woytschak, J. et al. Type 2 interleukin-4 receptor signaling in neutrophils antagonizes their expansion and migration during infection and inflammation. Immunity 45, 172–184 (2016).
Impellizzieri, D. et al. IL-4 receptor engagement in human neutrophils impairs their migration and extracellular trap formation. J. Allergy Clin. Immunol. 144, 267–279.e4 (2019).
Fiorentino, D. F., Zlotnik, A., Mosmann, T. R., Howard, M. & O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147, 3815–3822 (1991).
Fujii, S., Shimizu, K., Shimizu, T. & Lotze, M. T. Interleukin-10 promotes the maintenance of antitumor CD8+ T-cell effector function in situ. Blood 98, 2143–2151 (2001).
Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 (2003).
Brunda, M. J. et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J. Exp. Med. 178, 1223–1230 (1993).
Carson, W. E. et al. Coadministration of interleukin-18 and interleukin-12 induces a fatal inflammatory response in mice: critical role of natural killer cell interferon-γ production and STAT-mediated signal transduction. Blood 96, 1465–1473 (2000).
Glassman, C. R. et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell 184, 983–999 e924 (2021). This work presents the dtructure-based design of T cell selective IL-12 variants.
Kim, A. R. et al. Functional selectivity in cytokine signaling revealed through a pathogenic EPO mutation. Cell 168, 1053–1064 e1015 (2017). This work discusses the discovery of a natural cytokine variant that elicits biased receptor signalling.
Dudakov, J. A., Hanash, A. M. & van den Brink, M. R. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785 (2015).
Sabat, R., Ouyang, W. & Wolk, K. Therapeutic opportunities of the IL-22–IL-22R1 system. Nat. Rev. Drug Discov. 13, 21–38 (2014).
Ouyang, W. & O’Garra, A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity 50, 871–891 (2019).
Mantovani, A., Locati, M., Vecchi, A., Sozzani, S. & Allavena, P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 22, 328–336 (2001).
Dinarello, C. A., Novick, D., Kim, S. & Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 4, 289 (2013).
Srivastava, S., Salim, N. & Robertson, M. J. Interleukin-18: biology and role in the immunotherapy of cancer. Curr. Med. Chem. 17, 3353–3357 (2010).
Zhou, T. et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature 583, 609–614 (2020). This work presents the directed evolution of an IL-18 variant that is no longer inhibited by the soluble decoy receptor IL-18BP, displaying superior antitumour effects in mouse models.
Harris, K. E. et al. A bispecific antibody agonist of the IL-2 heterodimeric receptor preferentially promotes in vivo expansion of CD8 and NK cells. Sci. Rep. 11, 10592 (2021).
Yea, K. et al. Agonist antibody that induces human malignant cells to kill one another. Proc. Natl Acad. Sci. USA 112, E6158–E6165 (2015).
Nakano, K. et al. Effective screening method of agonistic diabodies based on autocrine growth. J. Immunol. Methods 347, 31–35 (2009).
Moraga, I. et al. Tuning cytokine receptor signaling by re-orienting dimer geometry with surrogate ligands. Cell 160, 1196–1208 (2015). This work demonstrates that altering the receptor geometry and orientation can influence downstream signalling.
Yen, M. et al. Facile discovery of surrogate cytokine agonists. Cell 185, 1414–1430.e19 (2022). This work presents a platform for the identification of nanobody-based surrogate cytokine receptor agonists.
Pluckthun, A. Designed ankyrin repeat proteins (DARPins): binding proteins for research, diagnostics, and therapy. Annu. Rev. Pharmacol. Toxicol. 55, 489–511 (2015).
Mohan, K. et al. Topological control of cytokine receptor signaling induces differential effects in hematopoiesis. Science https://doi.org/10.1126/science.aav7532 (2019). This work uses synthetic DARPins to systematically control cytokine receptor geometry, yielding partial and biased agonism.
Moraga, I. et al. Synthekines are surrogate cytokine and growth factor agonists that compel signaling through non-natural receptor dimers. eLife https://doi.org/10.7554/eLife.22882 (2017). This work demonstrates that induced dimerization of non-natural cytokine receptor pairs can drive JAK/STAT signalling.
Engelowski, E. et al. Synthetic cytokine receptors transmit biological signals using artificial ligands. Nat. Commun. 9, 2034 (2018).
Kallen, K. J. et al. Receptor recognition sites of cytokines are organized as exchangeable modules. Transfer of the leukemia inhibitory factor receptor-binding site from ciliary neurotrophic factor to interleukin-6. J. Biol. Chem. 274, 11859–11867 (1999).
Findeisen, M. et al. Treatment of type 2 diabetes with the designer cytokine IC7Fc. Nature 574, 63–68 (2019).
Yang, J. C. et al. The use of polyethylene glycol-modified interleukin-2 (PEG-IL-2) in the treatment of patients with metastatic renal cell carcinoma and melanoma. A phase I study and a randomized prospective study comparing IL-2 alone versus IL-2 combined with PEG-IL-2. Cancer 76, 687–694 (1995).
Roybal, K. T. et al. Engineering T cells with customized therapeutic response programs using synthetic Notch receptors. Cell 167, 419–432.e16 (2016).
Zhang, B. et al. Site-specific PEGylation of interleukin-2 enhances immunosuppression via the sustained activation of regulatory T cells. Nat. Biomed. Eng. 5, 1288–1305 (2021).
Casadevall, N. et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 346, 469–475 (2002).
Ettinger, M. P. et al. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: a randomized, dose-ranging study. JAMA 289, 1826–1832 (2003).
Hermeling, S., Crommelin, D. J., Schellekens, H. & Jiskoot, W. Structure–immunogenicity relationships of therapeutic proteins. Pharm. Res. 21, 897–903 (2004).
Andreatta, M. & Nielsen, M. Gapped sequence alignment using artificial neural networks: application to the MHC class I system. Bioinformatics 32, 511–517 (2016).
Liu, D. V., Maier, L. M., Hafler, D. A. & Wittrup, K. D. Engineered interleukin-2 antagonists for the inhibition of regulatory T cells. J. Immunother. 32, 887–894 (2009).
Kuziel, W. A., Ju, G., Grdina, T. A. & Greene, W. C. Unexpected effects of the IL-2 receptor α subunit on high affinity IL-2 receptor assembly and function detected with a mutant IL-2 analog. J. Immunol. 150, 3357–3365 (1993).
Rath, T. et al. Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics. Crit. Rev. Biotechnol. 35, 235–254 (2015).
Acknowledgements
K.C.G. is an investigator of the Howard Hughes Medical Institute (HHMI). This work was supported by National Institutes of Health (NIH) grant R01-AI51321 (K.C.G.) as well as funding from the Helen Hay Whitney Foundation (R.A.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
K.C.G. is the founder of Synthekine Therapeutics. R.A.S., C.R.G. and K.C.G. are inventors on patents relating to molecules discussed in this Review that are being commercialized.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Receptor tyrosine kinases
-
(RTKs). Cell surface receptors with intrinsic tyrosine kinase activity.
- Janus kinases
-
(JAKs). A family of cytosolic tyrosine kinases that associate with cytokine receptor intracellular domains.
- EC50
-
The ligand concentration that induces half-maximal response.
- E max
-
The maximal response elicited by saturating ligand concentrations.
- Regulatory T cells
-
(Treg cells). A subpopulation of immunosuppressive T cells important for maintaining self-tolerance and homeostasis.
- Cellular pleiotropy
-
Pleiotropy arising from the ability of one molecule to stimulate multiple distinct cell types or tissues.
- Functional pleiotropy
-
Pleiotropy arising from the ability of one molecule to activate multiple signalling pathways and, thereby, induce multiple functional responses on a target cell type.
- G proteins
-
Guanine nucleotide binding proteins that mediate signal transduction in a GTP-dependent manner.
- Diamond–Blackfan anaemia
-
A genetic disorder resulting in reduced production of red blood cells.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saxton, R.A., Glassman, C.R. & Garcia, K.C. Emerging principles of cytokine pharmacology and therapeutics. Nat Rev Drug Discov 22, 21–37 (2023). https://doi.org/10.1038/s41573-022-00557-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-022-00557-6
This article is cited by
-
Liposomes, transfersomes and niosomes: production methods and their applications in the vaccinal field
Journal of Translational Medicine (2024)
-
Dictionary of immune responses to cytokines at single-cell resolution
Nature (2024)
-
Molecular Engineering of Interleukin-2 for Enhanced Therapeutic Activity in Autoimmune Diseases
BioDrugs (2024)
-
CEP55: an immune-related predictive and prognostic molecular biomarker for multiple cancers
BMC Pulmonary Medicine (2023)
-
Exploring hub pyroptosis-related genes, molecular subtypes, and potential drugs in ankylosing spondylitis by comprehensive bioinformatics analysis and molecular docking
BMC Musculoskeletal Disorders (2023)