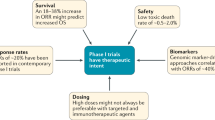
Abstract
Recent progress in understanding the molecular basis of cellular processes, identification of promising therapeutic targets and evolution of the regulatory landscape makes this an exciting and unprecedented time to be in the field of oncology drug development. However, high costs, long development timelines and steep rates of attrition continue to afflict the drug development process. Lack of predictive preclinical models is considered one of the key reasons for the high rate of attrition in oncology. Generating meaningful and predictive results preclinically requires a firm grasp of the relevant biological questions and alignment of the model systems that mirror the patient context. In doing so, the ability to conduct both forward translation, the process of implementing basic research discoveries into practice, as well as reverse translation, the process of elucidating the mechanistic basis of clinical observations, greatly enhances our ability to develop effective anticancer treatments. In this Review, we outline issues in preclinical-to-clinical translatability of molecularly targeted cancer therapies, present concepts and examples of successful reverse translation, and highlight the need to better align tumour biology in patients with preclinical model systems including tracking of strengths and weaknesses of preclinical models throughout programme development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bedair, A. & Mansour, F. R. Insights into the FDA 2018 new drug approvals. Curr. Drug Discov. Technol. 18, 293–306 (2019).
New Drug Therapy Approvals 2019 (FDA, 2019); https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2019.
Scannell, J. W., Blanckley, A., Boldon, H. & Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 11, 191–200 (2012).
Kunnumakkara, A. B. et al. Cancer drug development: the missing links. Exp. Biol. Med. 244, 663–689 (2019).
Wong, C. H., Siah, K. W. & Lo, A. W. Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286 (2019).
Jardim, D. L., Groves, E. S., Breitfeld, P. P. & Kurzrock, R. Factors associated with failure of oncology drugs in late-stage clinical development: a systematic review. Cancer Treat. Rev. 52, 12–21 (2017).
Lightfoot, J. T., Bamman, M. M. & Booth, F. W. Translation goes both ways: the power of reverse translation from human trials into animal models. Transl. J. Am. Coll. Sports Med. 2, 29–31 (2017).
Kurzrock, R., Kantarjian, H. M., Kesselheim, A. S. & Sigal, E. V. New drug approvals in oncology. Nat. Rev. Clin. Oncol. 17, 140–146 (2020).
Herbst, R. S. et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial — INTACT 2. J. Clin. Oncol. 22, 785–794 (2004).
Giaccone, G. et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial — INTACT 1. J. Clin. Oncol. 22, 777–784 (2004).
Pao, W. et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA 101, 13306–13311 (2004).
Politi, K. et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 20, 1496–1510 (2006).
Greulich, C. et al. Oncogenic transformation by inhibitor-sensitive and-resistant EGFR mutants. PLoS Med. 2, 1167–1176 (2005).
Ji, H. et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell 9, 485–495 (2006).
Starrett, J. H. et al. Drug sensitivity and allele specificity of first-line osimertinib resistance EGFR mutations. Cancer Res. 80, 2017–2030 (2020).
Politi, K., Fan, P. D., Shen, R., Zakowski, M. & Varmus, H. Erlotinib resistance in mouse models of epidermal growth factor receptor-induced lung adenocarcinoma. Dis. Model Mech. 3, 111–119 (2010).
Hata, A. N. et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 22, 262–269 (2016).
Dungo, R. T. & Keating, G. M. Afatinib: first global approval. Drugs 73, 1503–1515 (2013).
Wu, Y. L. et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label. Lancet Oncol. 18, 1454–1466 (2017).
Pao, W. et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2, e73 (2005).
Crystal, A. S. et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 346, 1480–1486 (2014).
Ogino, A. et al. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non-small cell lung cancer cell line. Cancer Res. 67, 7807–7814 (2007).
Regales, L. et al. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PLoS ONE 2, e810 (2007).
Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018).
Leonetti, A. et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 121, 725–737 (2019).
Schoenfeld, A. J. et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin. Cancer Res. 26, 2654–2663 (2020).
Marranci, A. et al. The landscape of BRAF transcript and protein variants in human cancer. Mol. Cancer 16, 85 (2017).
Wan, P. T. C. et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004).
Hoeflich, K. P. et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 66, 999–1006 (2006).
Dankort, D. et al. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 21, 379–384 (2007).
Dhomen, N. et al. Oncogenic braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15, 294–303 (2009).
Charles, R. P., Silva, J., Iezza, G., Phillips, W. A. & McMahon, M. Activating BRAF and PIK3CA mutations cooperate to promote anaplastic thyroid carcinogenesis. Mol. Cancer Res. 12, 979–986 (2014).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Heidorn, S. J. et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221 (2010).
Poulikakos, P. I., Zhang, C., Bollag, G., Shokat, K. M. & Rosen, N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464, 427–430 (2010).
Hatzivassiliou, G. et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 464, 431–435 (2010).
Cox, A. D. & Der, C. J. The raf inhibitor paradox: unexpected consequences of targeted drugs. Cancer Cell 17, 221–223 (2010).
Oberholzer, P. A. et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J. Clin. Oncol. 30, 316–321 (2012).
Quintanilla, M., Brown, K., Ramsden, M. & Balmain, A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature 322, 78–80 (1986).
Su, F. et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 366, 207–215 (2012).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Sosman, J. A. et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 366, 707–714 (2012).
Nazarian, R. et al. Melanomas acquire resistance to B-RAF (V600E) inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 (2010).
Moriceau, G. et al. Tunable-combinatorial mechanisms of acquired resistance limit the efficacy of BRAF/MEK cotargeting but result in melanoma drug addiction. Cancer Cell 27, 240–256 (2015).
Shi, H. et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 4, 80–93 (2014).
Poulikakos, P. I. et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF (V600E). Nature 480, 387–390 (2011).
Wagle, N. et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 29, 3085–3096 (2011).
Sullivan, R. J. & Flaherty, K. T. Resistance to BRAF-targeted therapy in melanoma. Eur. J. Cancer 49, 1297–1304 (2013).
Johnson, D. B. et al. Acquired BRAF inhibitor resistance: a multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of. Eur. J. Cancer 51, 2792–2799 (2015).
Amaral, T. et al. MAPK pathway in melanoma part II — secondary and adaptive resistance mechanisms to BRAF inhibition. Eur. J. Cancer 73, 93–101 (2017).
Ji, H. et al. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res. 10, 4933–4939 (2007).
Emery, C. M. et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc. Natl Acad. Sci. USA 106, 20411–20416 (2009).
Villanueva, J. et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 18, 683–695 (2010).
Larkin, J. et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 371, 1867–1876 (2014).
Long, G. V. et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 377, 1813–1823 (2017).
Dummer, R. et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 19, 603–615 (2018).
Heinzerling, L. et al. Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management. ESMO Open 4, 491 (2019).
Su, F. et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 72, 969–978 (2012).
Kopetz, S. et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J. Clin. Oncol. 33, 4032–4038 (2015).
Hyman, D. M. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 373, 726–736 (2015).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–104 (2012).
Corcoran, R. B. et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition. Cancer Discov. 23, 227–235 (2012).
Corcoran, R. B. et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 8, 428–443 (2018).
Mauri, G. et al. The evolutionary landscape of treatment for BRAFV600E mutant metastatic colorectal cancer. Cancers 13, 137 (2021).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
Kummar, S. & Lassen, U. N. TRK inhibition: a new tumor-agnostic treatment strategy. Target. Oncol. 13, 545–556 (2018).
Marquart, J., Chen, E. Y. & Prasad, V. Estimation of the percentage of US patients with cancer who benefit from genome-driven oncology. JAMA Oncol. 4, 1093–1098 (2018).
Lee, W. C. et al. Multiregion gene expression profiling reveals heterogeneity in molecular subtypes and immunotherapy response signatures in lung cancer. Mod. Pathol. 31, 947–955 (2018).
Wu, D. et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Semin. Cancer Biol. 42, 13–19 (2017).
Jarnuczak, A. F. et al. An integrated landscape of protein expression in human cancer. Preprint at biorxiv https://doi.org/10.1101/665968v1.abstract (2019).
Ben-David, U. et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 49, 1567–1575 (2017).
Ling, A., Gruener, R. F., Fessler, J. & Huang, R. S. More than fishing for a cure: The promises and pitfalls of high throughput cancer cell line screens. Pharmacol. Ther. 191, 178–189 (2018).
McDonald, E. R. et al. Project DRIVE: a compendium of cancer dependencies and synthetic lethal relationships uncovered by large-scale, deep RNAi screening. Cell 170, 577–592.e10 (2017).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576.e16 (2017).
Domcke, S., Sinha, R., Levine, D. A., Sander, C. & Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 4, 1–10 (2013).
Yu, K. et al. Comprehensive transcriptomic analysis of cell lines as models of primary tumors across 22 tumor types. Nat. Commun. 10, 1–11 (2019).
Chen, B., Sirota, M., Fan-Minogue, H., Hadley, D. & Butte, A. J. Relating hepatocellular carcinoma tumor samples and cell lines using gene expression data in translational research. BMC Med. Genomics 8, S5 (2015).
Lin, A., Giuliano, C. J., Sayles, N. M. & Sheltzer, J. M. CRISPR/Cas9 mutagenesis invalidates a putative cancer dependency targeted in on-going clinical trials. eLife 6, e24179 (2017).
Lin, A. & Sheltzer, J. M. Discovering and validating cancer genetic dependencies: approaches and pitfalls. Nat. Rev. Genet. 21, 671–682 (2020).
Ben-David, U., Beroukhim, R. & Golub, T. R. Genomic evolution of cancer models: perils and opportunities. Nat. Rev. Cancer 19, 97–109 (2019).
Buqué, A. & Galluzzi, L. Modeling tumor immunology and immunotherapy in mice. Trends Cancer 4, 599–601 (2018).
Combest, A. J. et al. Genetically engineered cancer models, but not xenografts, faithfully predict anticancer drug exposure in melanoma tumors. Oncologist 17, 1303–1316 (2012).
Westcott, P. M. K. et al. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature 517, 489–492 (2015).
Mcfadden, D. G. et al. Mutational landscape of EGFR-, MYC-, and Kras-driven genetically engineered mouse models of lung adenocarcinoma. Proc. Natl Acad. Sci. USA 113, 6409–6417 (2016).
Chung, W.-J. et al. Kras mutant genetically engineered mouse models of human cancers are genomically heterogeneous. Proc. Natl Acad. Sci. USA 114, 10947–10955 (2017).
Weber, J. & Rad, R. Engineering CRISPR mouse models of cancer. Curr. Opin. Genet. Dev. 54, 88–96 (2019).
Rogers, Z. N. et al. Mapping the in vivo fitness landscape of lung adenocarcinoma tumor suppression in mice. Nat. Genet. 50, 483–486 (2018).
Weber, J. et al. CRISPR/Cas9 somatic multiplex-mutagenesis for high-Throughput functional cancer genomics in mice. Proc. Natl Acad. Sci. USA 112, 13982–13987 (2015).
Winters, I. P., Murray, C. W. & Winslow, M. M. Towards quantitative and multiplexed in vivo functional cancer genomics. Nat. Rev. Genet. 19, 741–755 (2018).
Köhler, C. et al. Mouse cutaneous melanoma induced by mutant BRaf arises from expansion and dedifferentiation of mature pigmented melanocytes. Cell Stem Cell 21, 679–693.e6 (2017).
Schittek, B., Jel, M. D. & Tüting, T. in Melanoma Development: Molecular Biology, Genetics and Clinical Application (ed. Bosserhoff, A. K.) 369–398 (Springer International Publishing, 2017).
Evangelista, M., Tian, H. & de Sauvage, F. J. The Hedgehog signaling pathway in cancer. Clin. Cancer Res. 12, 5924–5928 (2006).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Infinity reports update from phase 2 study of saridegib plus gemcitabine in patients with metastatic pancreatic cancer (Businesswire, 2021); https://www.businesswire.com/news/home/20120127005146/en/Infinity-Reports-Update-Phase-2-Study-Saridegib.
Catenacci, D. V. T. et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J. Clin. Oncol. 33, 4284–4292 (2015).
Jesus-Acosta, A. D. et al. Phase 2 study of vismodegib, a hedgehog inhibitor, combined with gemcitabine and Nab-paclitaxel in patients with untreated metastatic pancreatic adenocarcinoma. Br. J. Cancer 122, 498–505 (2020).
Rhim, A. D. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014).
Hidalgo, M. et al. Patient-derived Xenograft models: an emerging platform for translational cancer research. Cancer Discov. 4, 998–1013 (2014).
Jung, J., Seol, H. S. & Chang, S. The generation and application of patient-derived xenograft model for cancer research. Cancer Res. Treat. 50, 1–10 (2018).
Shi, J., Tong, S. J., Li, Y., Jia, R. & Fan, X. The fidelity of cancer cells in PDX models: characteristics, mechanism and clinical significance. Int. J. Cancer 146, 2078–2088 (2020).
Derose, Y. S. et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 17, 1514–1520 (2011).
Bertotti, A. et al. A molecularly annotated platform of patient- derived xenografts (‘xenopatients’) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 1, 508–523 (2011).
Long, J. E. et al. Therapeutic resistance and susceptibility is shaped by cooperative multi-compartment tumor adaptation. Cell Death Differ. 26, 2416–2429 (2019).
Wegner, C. S. et al. Increasing aggressiveness of patient-derived xenograft models of cervix carcinoma during serial transplantation. Oncotarget 9, 21036–21051 (2018).
Sato, K. et al. Multiregion genomic analysis of serially transplanted patient-derived xenograft tumors. Cancer Genomics Proteom. 16, 21–27 (2019).
Fan, H., Demirci, U. & Chen, P. Emerging organoid models: leaping forward in cancer research. J. Hematol. Oncol. 12, 142 (2019).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
Cantrell, M. A. & Kuo, C. J. Organoid modeling for cancer precision medicine. Genome Med. 7, 32 (2015).
Maru, Y., Tanaka, N., Itami, M. & Hippo, Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gnyecol. Oncol. 154, 189–198 (2019).
Takeda, H. et al. CRISPR-Cas9-mediated gene knockout in intestinal tumor organoids provides functional validation for colorectal cancer driver genes. Proc. Natl Acad. Sci. USA 116, 15635–15644 (2019).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Kolahi, K. S., Nakano, M. & Kuo, C. J. Organoids as oracles for precision medicine in rectal cancer. Cell Stem Cell 26, 4–6 (2020).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988 (2018).
Tran, F. et al. Stem cells and organoid technology in precision medicine in inflammation: Are we there yet? Front. Immunol. 11, 573562 (2020).
Powley, I. R. et al. Patient-derived explants (PDEs) as a powerful preclinical platform for anti-cancer drug and biomarker discovery. Br. J. Cancer 122, 735–744 (2020).
Stebbing, J. et al. Patient-derived xenografts for individualized care in advanced sarcoma. Cancer 120, 2006–2015 (2014).
Katayama, R. et al. The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat. Commun. 10, 1–12 (2019).
Krepler, C. et al. A comprehensive patient-derived xenograft collection representing the heterogeneity of melanoma. Cell Rep. 21, 1953–1967 (2017).
Bock, C. et al. The organoid cell atlas. Nat. Biotechnol. 39, 13–17 (2020).
Gao, H. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 21, 1318–1325 (2015).
Sartore-Bianchi, A. et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 17, 738–746 (2016).
Parikh, A., Atreya, C., Korn, W. M. & Venook, A. P. Prolonged response to HER2-directed therapy in a patient With HER2-amplified, rapidly progressive metastatic colorectal cancer. J. Natl Compr. Canc. Netw. 15, 3–8 (2017).
Schwaederle, M. et al. Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J. Clin. Oncol. 33, 3817–3825 (2015).
Daemen, A. et al. Transcriptional subtypes resolve tumor heterogeneity and identify vulnerabilities to MEK inhibition in lung adenocarcinoma. Clin. Cancer Res. 27, 1162–1173 (2021).
Gould, S. E., Junttila, M. R. & Sauvage, F. J. D. Translational value of mouse models in oncology drug development. Nat. Med. 21, 431–439 (2015).
Wong, H. et al. Antitumor activity of targeted and cytotoxic agents in murine subcutaneous tumor models correlates with clinical response. Clin. Cancer Res. 18, 3846–3855 (2012).
Peters, S. A., Petersson, C., Blaukat, A., Halle, J. P. & Dolgos, H. Prediction of active human dose: learnings from 20 years of Merck KGaA experience, illustrated by case studies. Drug Discov. Today 25, 909–919 (2020).
Miller, N. A., Reddy, M. B., Heikkinen, A. T., Lukacova, V. & Parrott, N. Physiologically based pharmacokinetic modelling for first-in-human predictions: an updated model building strategy illustrated with challenging industry case studies. Clin. Pharmacokinet. 58, 727–746 (2019).
Garnett, M. J. et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483, 570–575 (2012).
Yang, W. et al. Genomics of Drug Sensitivity in Cancer (GDSC): A resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 41, D955–D961 (2013).
Acar, A. et al. Exploiting evolutionary steering to induce collateral drug sensitivity in cancer. Nat. Commun. 11, 1–14 (2020).
Punzi, S. et al. Development of personalized therapeutic strategies by targeting actionable vulnerabilities in metastatic and chemotherapy-resistant breast cancer PDXs. Cells 8, 605 (2019).
Holohan, C., Schaeybroeck, S. V., Longley, D. B. & Johnston, P. G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013).
Boumahdi, S. & de Sauvage, F. J. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 19, 39–56 (2020).
Massagué, J., Batlle, E. & Gomis, R. R. Understanding the molecular mechanisms driving metastasis. Mol. Oncol. 11, 3–4 (2017).
Ben-David, U. et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature 560, 325–330 (2018).
Zhong, W. et al. Comparison of the molecular and cellular phenotypes of common mouse syngeneic models with human tumors. BMC Genomics 21, 2 (2020).
Pénzváltó, Z. et al. A syngeneic ErbB2 mammary cancer model for preclinical immunotherapy trials. J. Mammary Gland. Biol. Neoplasia 24, 149–162 (2019).
Larsson, S. et al. Cell line-based xenograft mouse model of paediatric glioma stem cells mirrors the clinical course of the patient. Carcinogenesis 39, 1304–1309 (2018).
Borowsky, A. D. et al. Syngeneic mouse mammary carcinoma cell lines: Two closely related cell lines with divergent metastatic behavior. Clin. Exp. Metastasis 22, 47–59 (2005).
Radhakrishnan, P. et al. Predicting tumor-immune response to checkpoint inhibitors using a novel patient-derived live tumor explant model. J. Clin. Oncol. 35, e20035–e20035 (2017).
Broutier, L. et al. Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435 (2017).
Rosenbluth, J. M. et al. Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nat. Commun. 11, 1–14 (2020).
Tsai, S. et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer 18, 335 (2018).
O’Rourke, K. P. et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 35, 577–582 (2017).
Melo, F. D. S. E. et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
Murphy, J. F. Patient-derived xenograft (PDX) models: an emerging platform for cancer drug development and translational research. MOJ Immunol. 3, 00094 (2016).
Okada, S., Vaeteewoottacharn, K. & Kariya, R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells 8, 889 (2019).
Stecklum, M., Wulf-Goldenberg, A., Brzezicha, B. & Fichtner, I., Hoffmann, J. Humanized immune-oncology mouse models. AACR 1697, abstr. (2017).
Aparicio, S., Hidalgo, M. & Kung, A. L. Examining the utility of patient-derived xenograft mouse models. Nat. Rev. Cancer 15, 311–316 (2015).
Arriaga, J. M. & Abate-Shin, C. Genetically engineered mouse models of prostate cancer in the postgenomic era. Cold Spring Harb. Perspect. Med. 9, a030528 (2019).
Stuckelberger, S. & Drapkin, R. Precious GEMMs: emergence of faithful models for ovarian cancer research. J. Pathol. 245, 129–131 (2018).
DuPage, M. & Jacks, T. Genetically engineered mouse models of cancer reveal new insights about the antitumor immune response. Curr. Opin. Immunol. 25, 192–199 (2013).
Kersten, K., Visser, K. E., Miltenburg, M. H. & Jonkers, J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 9, 137–153 (2017).
Acknowledgements
The authors dedicate this work to the memory of Dr Georgia Hatzivassiliou. They also express gratitude to clinical trial patients and their families for their selfless contributions to research that is crucial for translation research and paves the way for improved patient treatments.
Author information
Authors and Affiliations
Contributions
All authors researched data, wrote, contributed to discussions of the content and edited and reviewed the article before submission.
Corresponding authors
Ethics declarations
Competing interests
S.K. is a consultant and member of the advisory boards of Boehringer Ingelheim, Springworks Therapeutics, Bayer, Genome & Company, HarbourBiomed, Seattle Genetics, Mundibiopharma and Gilead. She is also cofounder of PathomIQ, and her spouse is a member of the advisory board of Cadila Pharmaceuticals and cofounder of Arxeon. A.H. is a founder and shareholder of Arxeon. S.V.M. is founder and shareholder of Arxeon and scientific adviser for Cadila Pharmaceutical. M.R.J. is an employee of and owns stocks from ORIC Pharmaceuticals.
Additional information
Peer review information
Nature Reviews Drug Discovery thanks Uri Ben-David, Sara Colombetti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Cancer Therapeutics Response Portal: A CTD2 Network Resource for Mining Candidate Cancer Dependencies: https://ocg.cancer.gov/e-newsletter-issue/issue-11/cancer-therapeutics-response-portal-ctd%C2%B2-network
DepMap: The Cancer Dependency Map Project at Broad Institute: https://depmap.org/portal/ccle/
Office of Cancer Genomics: https://ocg.cancer.gov/node/300
Rights and permissions
About this article
Cite this article
Honkala, A., Malhotra, S.V., Kummar, S. et al. Harnessing the predictive power of preclinical models for oncology drug development. Nat Rev Drug Discov 21, 99–114 (2022). https://doi.org/10.1038/s41573-021-00301-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-021-00301-6
This article is cited by
-
Homologous Recombination Deficiency Unrelated to Platinum and PARP Inhibitor Response in Cell Line Libraries
Scientific Data (2024)
-
Investigating the potential of oncolytic viruses for cancer treatment via MSC delivery
Cell Communication and Signaling (2023)
-
Resurgence of syphilis: focusing on emerging clinical strategies and preclinical models
Journal of Translational Medicine (2023)
-
Patient-derived xenograft models in cancer therapy: technologies and applications
Signal Transduction and Targeted Therapy (2023)
-
Recommendations for robust and reproducible preclinical research in personalised medicine
BMC Medicine (2023)