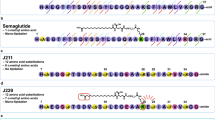
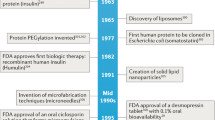
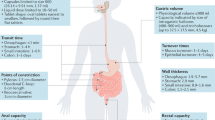
Abstract
Protein and peptide therapeutics require parenteral administration, which can be a deterrent to medication adherence. For this reason, there have been extensive efforts to develop alternative delivery strategies, particularly for peptides such as insulin that are used to treat endocrine disorders. Oral delivery is especially desirable, but it faces substantial barriers related to the structural organization and physiological function of the gastrointestinal tract. This article highlights strategies designed to overcome these barriers, including permeation enhancers, inhibitors of gut enzymes, and mucus-penetrating and cell-penetrating peptides. It then focuses on the experience with oral peptides that have reached clinical trials, including insulin, calcitonin, parathyroid hormone and vasopressin, with an emphasis on the advances that have recently led to the landmark approval of an oral formulation of the glucagon-like peptide 1 receptor agonist semaglutide for the treatment of type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Spain, C. V., Wright, J. J., Hahn, R. M., Wivel, A. & Martin, A. A. Self-reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clin. Ther. 38, 1653–1664.e1 (2016).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Anselmo, A. C., Gokarn, Y. & Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 18, 19–40 (2019).
Maher, S., Mrsny, R. J. & Brayden, D. J. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev. 106, 277–319 (2016).
Di, L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 17, 134–143 (2015).
Doak, B. C., Over, B., Giordanetto, F. & Kihlberg, J. Oral druggable space beyond the rule of 5: insights from drugs and clinical candidates. Chem. Biol. 21, 1115–1142 (2014).
Wong, C. Y., Al-Salami, H. & Dass, C. R. Microparticles, microcapsules and microspheres: a review of recent developments and prospects for oral delivery of insulin. Int. J. Pharm. 537, 223–244 (2018).
Viggiano, D. et al. Gut barrier in health and disease: focus on childhood. Eur. Rev. Med. Pharmacol. Sci. 19, 1077–1085 (2015).
Groschwitz, K. R. & Hogan, S. P. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 124, 3–20; quiz 21–22 (2009).
Lundquist, P. & Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 106, 256–276 (2016).
Kisser, B. et al. The Ussing chamber assay to study drug metabolism and transport in the human intestine. Curr. Protoc. Pharmacol. 77, 7.17.1–7.17.19 (2017).
Wang, J., Yadav, V., Smart, A. L., Tajiri, S. & Basit, A. W. Toward oral delivery of biopharmaceuticals: an assessment of the gastrointestinal stability of 17 peptide drugs. Mol. Pharm. 12, 966–973 (2015).
Zimmermann, M., Zimmermann-Kogadeeva, M., Wegmann, R. & Goodman, A. L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 363, eaat9931 (2019).
Johansson, M. E., Sjovall, H. & Hansson, G. C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 (2013).
Ensign, L. M., Cone, R. & Hanes, J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 64, 557–570 (2012).
Antoni, L. et al. Human colonic mucus is a reservoir for antimicrobial peptides. J. Crohns Colitis 7, e652–e664 (2013).
Shan, M. et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013).
Boegh, M., Garcia-Diaz, M., Mullertz, A. & Nielsen, H. M. Steric and interactive barrier properties of intestinal mucus elucidated by particle diffusion and peptide permeation. Eur. J. Pharm. Biopharm. 95, 136–143 (2015).
Lemmer, H. J. & Hamman, J. H. Paracellular drug absorption enhancement through tight junction modulation. Expert Opin. Drug Deliv. 10, 103–114 (2013).
Garcia, M. A., Nelson, W. J. & Chavez, N. Cell–cell junctions organize structural and signaling networks. Cold Spring Harb. Perspect. Biol. 10, a029181 (2018).
Fanning, A. S., Van Itallie, C. M. & Anderson, J. M. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol. Biol. Cell 23, 577–590 (2012).
Han, X., Fink, M. P., Yang, R. & Delude, R. L. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock 21, 261–270 (2004).
Hamman, J. H., Demana, P. H. & Olivier, E. I. Targeting receptors, transporters and site of absorption to improve oral drug delivery. Drug Target Insights 2, 71–81 (2007).
Terada, T. & Hira, D. Intestinal and hepatic drug transporters: pharmacokinetic, pathophysiological, and pharmacogenetic roles. J. Gastroenterol. 50, 508–519 (2015).
Bissa, B., Beedle, A. M. & Govindarajan, R. Lysosomal solute carrier transporters gain momentum in research. Clin. Pharmacol. Therap. 100, 431–436 (2016).
Tyagi, P., Pechenov, S. & Anand Subramony, J. Oral peptide delivery: translational challenges due to physiological effects. J. Control. Release 287, 167–176 (2018).
Boronikolos, G. C. et al. Upper gastrointestinal motility and symptoms in individuals with diabetes, prediabetes and normal glucose tolerance. Diabetologia 58, 1175–1182 (2015).
Bharucha, A. E., Kudva, Y. C. & Prichard, D. O. Diabetic gastroparesis. Endocrine Rev. 40, 1318–1352 (2019).
Sugihara, M. et al. Analysis of intra- and intersubject variability in oral drug absorption in human bioequivalence studies of 113 generic products. Mol. Pharm. 12, 4405–4413 (2015).
Artursson, P. & Magnusson, C. Epithelial transport of drugs in cell culture. II: effect of extracellular calcium concentration on the paracellular transport of drugs of different lipophilicities across monolayers of intestinal epithelial (Caco-2) cells. J. Pharm. Sci. 79, 595–600 (1990).
Whitehead, K., Karr, N. & Mitragotri, S. Safe and effective permeation enhancers for oral drug delivery. Pharm. Res. 25, 1782–1788 (2008).
Whitehead, K. & Mitragotri, S. Mechanistic analysis of chemical permeation enhancers for oral drug delivery. Pharm. Res. 25, 1412–1419 (2008).
Whitehead, K., Karr, N. & Mitragotri, S. Discovery of synergistic permeation enhancers for oral drug delivery. J. Control. Release 128, 128–133 (2008).
Madden, L. R. et al. Bioprinted 3D primary human intestinal tissues model aspects of native physiology and ADME/Tox functions. iScience 2, 156–167 (2018).
Aguirre, T. A. et al. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trials. Adv. Drug Deliv. Rev. 106, 223–241 (2016).
Twarog, C. et al. Intestinal permeation enhancers for oral delivery of macromolecules: a comparison between salcaprozate sodium (SNAC) and sodium caprate (C10). Pharmaceutics 11, E78 (2019).
Leone-Bay, A. et al. N-acylated alpha-amino acids as novel oral delivery agents for proteins. J. Med. Chem. 38, 4263–4269 (1995).
Buckley, S. T. et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 10, eaar7047 (2018).
Gschwind, H. P. et al. Metabolism and disposition of the oral absorption enhancer 14C-radiolabeled 8-(N-2-hydroxy-5-chlorobenzoyl)-amino-caprylic acid (5-CNAC) in healthy postmenopausal women and supplementary investigations in vitro. Eur. J. Pharm. Sci. 47, 44–55 (2012).
McCartney, F., Gleeson, J. P. & Brayden, D. J. Safety concerns over the use of intestinal permeation enhancers: a mini-review. Tissue Barriers 4, e1176822 (2016).
Halberg, I. B. et al. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. Lancet Diabetes Endocrinol. 7, 179–188 (2019).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Binkley, N. et al. A phase 3 trial of the efficacy and safety of oral recombinant calcitonin: the Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL) trial. J. Bone Miner. Res. 27, 1821–1829 (2012).
Lee, Y. H. et al. Impact of regional intestinal pH modulation on absorption of peptide drugs: oral absorption studies of salmon calcitonin in beagle dogs. Pharm. Res. 16, 1233–1239 (1999).
Liu, H., Tang, R., Pan, W. S., Zhang, Y. & Liu, H. Potential utility of various protease inhibitors for improving the intestinal absorption of insulin in rats. J. Pharm. Pharmacol. 55, 1523–1529 (2003).
Arbit, E. & Kidron, M. Oral insulin delivery in a physiologic context: review. J. Diabetes Sci. Technol. 11, 825–832 (2017).
Barone, G. et al. The pharmacokinetics of a microemulsion formulation of cyclosporine in primary renal allograft recipients. The Neoral Study Group. Transplantation 61, 875–880 (1996).
Matsui, K. et al. Resistance of 1-deamino-[8-D-arginine]-vasopressin to in vitro degradation as compared with arginine vasopressin. Endocrinol. Jpn 32, 547–557 (1985).
Nielsen, D. S. et al. Orally absorbed cyclic peptides. Chem. Rev. 117, 8094–8128 (2017).
Shan, W. et al. Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS Nano 9, 2345–2356 (2015).
Karamanidou, T. et al. Effective incorporation of insulin in mucus permeating self-nanoemulsifying drug delivery systems. Eur. J. Pharm. Biopharm. 97, 223–229 (2015).
Sheng, J. et al. Enhancing insulin oral absorption by using mucoadhesive nanoparticles loaded with LMWP-linked insulin conjugates. J. Control. Release 233, 181–190 (2016).
Boegh, M. & Nielsen, H. M. Mucus as a barrier to drug delivery—understanding and mimicking the barrier properties. Basic Clin. Pharmacol. Toxicol. 116, 179–186 (2015).
Rehmani, S. & Dixon, J. E. Oral delivery of anti-diabetes therapeutics using cell penetrating and transcytosing peptide strategies. Peptides 100, 24–35 (2018).
Niu, Z. et al. Rational design of polyarginine nanocapsules intended to help peptides overcoming intestinal barriers. J. Control. Release 263, 4–17 (2017).
Gupta, V. et al. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J. Control. Release 172, 753–762 (2013).
Banerjee, A., Chen, R., Arafin, S. & Mitragotri, S. Intestinal iontophoresis from mucoadhesive patches: a strategy for oral delivery. J. Control. Release 297, 71–78 (2019).
Fukuoka, Y. et al. Combination strategy with complexation hydrogels and cell-penetrating peptides for oral delivery of insulin. Biol. Pharm. Bull. 41, 811–814 (2018).
Kamei, N. et al. Complexation hydrogels for intestinal delivery of interferon beta and calcitonin. J. Control. Release 134, 98–102 (2009).
Ahmad, N., Mohd Amin, M. C., Ismail, I. & Buang, F. Enhancement of oral insulin bioavailability: in vitro and in vivo assessment of nanoporous stimuli-responsive hydrogel microparticles. Expert Opin. Drug Deliv. 13, 621–632 (2016).
Hashim, M. et al. Jejunal wall delivery of insulin via an ingestible capsule in anesthetized swine—a pharmacokinetic and pharmacodynamic study. Pharmacol. Res. Perspect. 7, e00522 (2019).
Abramson, A. et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 25, 1512–1518 (2019).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019).
Qureshi, S., Galiveeti, S., Bichet, D. G. & Roth, J. Diabetes insipidus: celebrating a century of vasopressin therapy. Endocrinology 155, 4605–4621 (2014).
Manning, M., Balaspiri, L., Moehring, J., Haldar, J. & Sawyer, W. H. Synthesis and some pharmacological properties of deamino(4-threonine,8-D-arginine)vasopressin and deamino(8-D-arginine)vasopressin, highly potent and specific antidiuretic peptides, and (8-D-arginine)vasopressin and deamino-arginine-vasopressin. J. Med. Chem. 19, 842–845 (1976).
Vavra, I. et al. Effect of a synthetic analogue of vasopressin in animals and in patients with diabetes insipidus. Lancet 1, 948–952 (1968).
Hammer, M. & Vilhardt, H. Peroral treatment of diabetes insipidus with a polypeptide hormone analog, desmopressin. J. Pharmacol. Exp. Ther. 234, 754–760 (1985).
Mannucci, P. M. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years. Blood 90, 2515–2521 (1997).
Heinemann, L. & Jacques, Y. Oral insulin and buccal insulin: a critical reappraisal. J. Diabetes Sci. Technol. 3, 568–584 (2009).
Genser, L. et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 246, 217–230 (2018).
Thaiss, C. A. et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359, 1376–1383 (2018).
Gedawy, A., Martinez, J., Al-Salami, H. & Dass, C. R. Oral insulin delivery: existing barriers and current counter-strategies. J. Pharm. Pharmacol. 70, 197–213 (2018).
Kidron, M. et al. A novel per-oral insulin formulation: proof of concept study in non-diabetic subjects. Diabet. Med. 21, 354–357 (2004).
Khedkar, A. et al. Impact of insulin tregopil and its permeation enhancer on pharmacokinetics of metformin in healthy volunteers: randomized, open-label, placebo-controlled, crossover study. Clin. Transl. Sci. 12, 276–282 (2019).
Khedkar, A. et al. A dose range finding study of novel oral insulin (IN-105) under fed conditions in type 2 diabetes mellitus subjects. Diabetes Obes. Metab. 12, 659–664 (2010).
Gregory, J. M. et al. Enterically delivered insulin tregopil exhibits rapid absorption characteristics and a pharmacodynamic effect similar to human insulin in conscious dogs. Diabetes Obes. Metab. 21, 160–169 (2019).
Eldor, R., Arbit, E., Corcos, A. & Kidron, M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLOS ONE 8, e59524 (2013).
Eldor, R., Neutel, J., Homer, K. & Kidron, M. Multiple oral insulin (ORMD-0801) doses elicit a cumulative effect on glucose control in T2DM patients. Diabetes 67 (Suppl. 1), 982-P (2018).
Geho, W. B., Geho, H. C., Lau, J. R. & Gana, T. J. Hepatic-directed vesicle insulin: a review of formulation development and preclinical evaluation. J. Diabetes Sci. Technol. 3, 1451–1459 (2009).
Geho, W. B., Rosenberg, L. N., Schwartz, S. L., Lau, J. R. & Gana, T. J. A single-blind, placebo-controlled, dose-ranging trial of oral hepatic-directed vesicle insulin add-on to oral antidiabetic treatment in patients with type 2 diabetes mellitus. J. Diabetes Sci. Technol. 8, 551–559 (2014).
Zheng, Y. et al. Multifunctional nanoparticles enable efficient oral delivery of biomacromolecules via improving payload stability and regulating the transcytosis pathway. ACS Appl. Mater. Interfaces 10, 34039–34049 (2018).
Lin, P. Y. et al. Safety and efficacy of self-assembling bubble carriers stabilized with sodium dodecyl sulfate for oral delivery of therapeutic proteins. J. Control. Release 259, 168–175 (2017).
Banerjee, A. et al. Ionic liquids for oral insulin delivery. Proc. Natl Acad. Sci. USA 115, 7296–7301 (2018).
Wu, S. et al. A delivery system for oral administration of proteins/peptides through bile acid transport channels. J. Pharm. Sci. 108, 2143–2152 (2019).
Guo, F. et al. Enhanced oral absorption of insulin using colon-specific nanoparticles co-modified with amphiphilic chitosan derivatives and cell-penetrating peptides. Biomater. Sci. 7, 1493–1506 (2019).
Cefalu, W. T. et al. Insulin Access and Affordability Working Group: conclusions and recommendations. Diabetes Care 41, 1299–1311 (2018).
Drucker, D. J., Habener, J. F. & Holst, J. J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Investig. 127, 4217–4227 (2017).
Eng, J., Kleinman, W. A., Singh, L., Singh, G. & Raufman, J. P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem. 267, 7402–7405 (1992).
Drucker, D. J. et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372, 1240–1250 (2008).
Meier, J. J. et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care 38, 1263–1273 (2015).
Drucker, D. J., Dritselis, A. & Kirkpatrick, P. Liraglutide. Nat. Rev. Drug Discov. 9, 267–268 (2010).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Glaesner, W. et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab. Res. Rev. 26, 287–296 (2010).
Lau, J. et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 58, 7370–7380 (2015).
Pratley, R. E. et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 6, 275–286 (2018).
Suzuki, K., Kim, K. S. & Bae, Y. H. Long-term oral administration of exendin-4 to control type 2 diabetes in a rat model. J. Control. Release 294, 259–267 (2019).
Xu, Y. et al. Novel strategy for oral peptide delivery in incretin-based diabetes treatment. Gut https://doi.org/10.1136/gutjnl-2019-319146 (2019).
Song, Y. et al. Synthesis of CSK-DEX-PLGA nanoparticles for the oral delivery of exenatide to improve its mucus penetration and intestinal absorption. Mol. Pharm. 16, 518–532 (2019).
Zhang, L. et al. The use of low molecular weight protamine to enhance oral absorption of exenatide. Int. J. Pharm. 547, 265–273 (2018).
Soudry-Kochavi, L., Naraykin, N., Nassar, T. & Benita, S. Improved oral absorption of exenatide using an original nanoencapsulation and microencapsulation approach. J. Control. Release 217, 202–210 (2015).
Kapitza, C. et al. Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J. Clin. Pharmacol. 55, 497–504 (2015).
Granhall, C., Sondergaard, F. L., Thomsen, M. & Anderson, T. W. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin. Pharmacokinet. 57, 1571–1580 (2018).
Davies, M. et al. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. J. Am. Med. Assoc. 318, 1460–1470 (2017).
Baekdal, T. A., Thomsen, M., Kupcova, V., Hansen, C. W. & Anderson, T. W. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J. Clin. Pharmacol. 58, 1314–1323 (2018).
Baekdal, T. A., Borregaard, J., Hansen, C. W., Thomsen, M. & Anderson, T. W. Effect of oral semaglutide on the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin in healthy subjects. Clin. Pharmacokinet. 58, 1193–1203 (2019).
Aroda, V. R. et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care 42, 1724–1732 (2019).
Montanya, E. et al. 54-OR: oral semaglutide vs. empagliflozin added on to metformin monotherapy in uncontrolled type 2 diabetes: PIONEER 2. Diabetes 68 (Suppl. 1), 54-OR (2019).
Mosenzon, O. et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 7, 515–527 (2019).
Pratley, R. et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 394, 39–50 (2019).
Rosenstock, J. et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. J. Am. Med. Assoc. 321, 1466–1480 (2019).
Zinman, B. et al. Efficacy, safety and tolerability of oral semaglutide versus placebo added to insulin ± metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care 42, 2262–2271 (2019).
Rodbard, H. W. et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care 42, 2272–2281 (2019).
Naot, D., Musson, D. S. & Cornish, J. The activity of peptides of the calcitonin family in bone. Physiol. Rev. 99, 781–805 (2019).
Chesnut, C. H. III et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am. J. Med. 109, 267–276 (2000).
Bandeira, L., Lewiecki, E. M. & Bilezikian, J. P. Pharmacodynamics and pharmacokinetics of oral salmon calcitonin in the treatment of osteoporosis. Expert Opin. Drug Metab. Toxicol. 12, 681–689 (2016).
Buclin, T., Cosma Rochat, M., Burckhardt, P., Azria, M. & Attinger, M. Bioavailability and biological efficacy of a new oral formulation of salmon calcitonin in healthy volunteers. J. Bone Miner. Res. 17, 1478–1485 (2002).
Henriksen, K. et al. A randomized, double-blind, multicenter, placebo-controlled study to evaluate the efficacy and safety of oral salmon calcitonin in the treatment of osteoporosis in postmenopausal women taking calcium and vitamin D. Bone 91, 122–129 (2016).
Compston, J. E., McClung, M. R. & Leslie, W. D. Osteoporosis. Lancet 393, 364–376 (2019).
Mannstadt, M. et al. Efficacy and safety of recombinant human parathyroid hormone (1–84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 1, 275–283 (2013).
Hodsman, A. B. et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr. Rev. 26, 688–703 (2005).
Hammerle, S. P. et al. The single dose pharmacokinetic profile of a novel oral human parathyroid hormone formulation in healthy postmenopausal women. Bone 50, 965–973 (2012).
Sturmer, A. et al. Pharmacokinetics of oral recombinant human parathyroid hormone [rhPTH(1–31)NH(2)] in postmenopausal women with osteoporosis. Clin. Pharmacokinet. 52, 995–1004 (2013).
Henriksen, K. et al. Evaluation of the efficacy, safety and pharmacokinetic profile of oral recombinant human parathyroid hormone [rhPTH(1–31)NH(2)] in postmenopausal women with osteoporosis. Bone 53, 160–166 (2013).
Hwang, S. R., Seo, D. H., Byun, Y. & Park, J. W. Preparation and in vivo evaluation of an orally available enteric-microencapsulated parathyroid hormone (1–34)-deoxycholic acid nanocomplex. Int. J. Nanomed. 11, 4231–4246 (2016).
Lamberts, S. W., Van der Lely, A. J., De Herder, W. W. & Hofland, L. J. Octreotide. N. Engl. J. Med. 334, 246–254 (1996).
Tuvia, S. et al. A novel suspension formulation enhances intestinal absorption of macromolecules via transient and reversible transport mechanisms. Pharm. Res. 31, 2010–2021 (2014).
Tuvia, S. et al. Oral octreotide absorption in human subjects: comparable pharmacokinetics to parenteral octreotide and effective growth hormone suppression. J. Clin. Endocrinol. Metab. 97, 2362–2369 (2012).
Melmed, S. et al. Safety and efficacy of oral octreotide in acromegaly: results of a multicenter phase III trial. J. Clin. Endocrinol. Metab. 100, 1699–1708 (2015).
Zijlstra, E., Heinemann, L. & Plum-Morschel, L. Oral insulin reloaded: a structured approach. J. Diabetes Sci. Technol. 8, 458–465 (2014).
Buse, J. B. et al. Randomized clinical trial comparing basal insulin peglispro and insulin glargine in patients with type 2 diabetes previously treated with basal insulin: IMAGINE 5. Diabetes Care 39, 92–100 (2016).
Acknowledgements
D.J.D. is supported in part by a Banting and Best Diabetes Centre Novo Nordisk Chair in Incretin Biology, by CIHR Foundation Grant 154321 and by investigator-initiated operating grants for preclinical GLP1 science from Novo Nordisk.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.J.D. has served as an adviser or consultant or speaker within the past 12 months to Forkhead Biotherapeutics, Heliome Inc., Intarcia Therapeutics, Kallyope, Merck Research Laboratories, Novo Nordisk Inc., Pfizer Inc. and Sanofi Inc. Neither D.J.D. nor his family members hold stock directly or indirectly in any of these companies. GLP2 is the subject of a patent licence agreement between Shire Inc. and the University of Toronto, Toronto General Hospital (UHN) and D.J.D.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Chiasma press release: http://ir.chiasmapharma.com/node/8156/pdf
Entera Bio press release: https://www.globenewswire.com/news-release/2019/09/23/1919104/0/en/Entera-Bio-Reports-Positive-Results-from-a-Phase-2-PK-PD-Study-of-Oral-PTH-1-34-in-Patients-with-Hypoparathyroidism.html
Rani Therapeutics press release: https://res.cloudinary.com/vwp/v1551478356/First_Human_Study_of_RaniPill_Capsule_to_Replace_Injections_Announced_by_Rani_Therapeutics_pbzlhb.pdf.
Rights and permissions
About this article
Cite this article
Drucker, D.J. Advances in oral peptide therapeutics. Nat Rev Drug Discov 19, 277–289 (2020). https://doi.org/10.1038/s41573-019-0053-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-019-0053-0
This article is cited by
-
Oral nanotherapeutic formulation of insulin with reduced episodes of hypoglycaemia
Nature Nanotechnology (2024)
-
Modular synthesis of clickable peptides via late-stage maleimidation on C(7)-H tryptophan
Nature Communications (2023)
-
De novo development of small cyclic peptides that are orally bioavailable
Nature Chemical Biology (2023)
-
Smart nanoparticles for cancer therapy
Signal Transduction and Targeted Therapy (2023)
-
Material design for oral insulin delivery
Med-X (2023)