Abstract
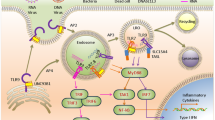
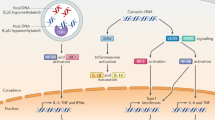
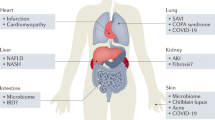
Nucleic acid sensors, primarily TLR and RLR family members, as well as cGAS–STING signalling, play a critical role in the preservation of cellular and organismal homeostasis. Accordingly, deregulated nucleic acid sensing contributes to the origin of a diverse range of disorders, including infectious diseases, as well as cardiovascular, autoimmune and neoplastic conditions. Accumulating evidence indicates that normalizing aberrant nucleic acid sensing can mediate robust therapeutic effects. However, targeting nucleic acid sensors with pharmacological agents, such as STING agonists, presents multiple obstacles, including drug-, target-, disease- and host-related issues. Here, we discuss preclinical and clinical data supporting the potential of this therapeutic paradigm and highlight key limitations and possible strategies to overcome them.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jackson, S. A. et al. CRISPR-Cas: adapting to change. Science 356, eaal5056 (2017).
Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 16, 35–50 (2016).
Vanpouille-Box, C., Demaria, S., Formenti, S. C. & Galluzzi, L. Cytosolic DNA sensing in organismal tumor control. Cancer Cell 34, 361–378 (2018).
Schlee, M. & Hartmann, G. Discriminating self from non-self in nucleic acid sensing. Nat. Rev. Immunol. 16, 566–580 (2016).
Kieser, K. J. & Kagan, J. C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 17, 376–390 (2017).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Roers, A., Hiller, B. & Hornung, V. Recognition of endogenous nucleic acids by the innate immune system. Immunity 44, 739–754 (2016).
Chow, K. T., Gale, M., Jr. & Loo, Y. M. RIG-I and Other RNA sensors in antiviral immunity. Annu. Rev. Immunol. 36, 667–694 (2018).
Ablasser, A. & Chen, Z. J. cGAS in action: expanding roles in immunity and inflammation. Science 363, eaat8657 (2019).
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in drosophila adults. Cell 86, 973–983 (1996).
Hoffmann, J. A. The immune response of Drosophila. Nature 426, 33–38 (2003).
Junt, T. & Barchet, W. Translating nucleic acid-sensing pathways into therapies. Nat. Rev. Immunol. 15, 529–544 (2015).
Zuany-Amorim, C., Hastewell, J. & Walker, C. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat. Rev. Drug Discov. 1, 797–807 (2002).
Smith, M. et al. Trial watch: Toll-like receptor agonists in cancer immunotherapy. Oncoimmunology 7, e1526250 (2018).
Campbell, J. D. Development of the CpG adjuvant 1018: a case study. Methods Mol. Biol. 1494, 15–27 (2017).
Redelman-Sidi, G., Glickman, M. S. & Bochner, B. H. The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat. Rev. Urol. 11, 153–162 (2014).
Hancock, R. E., Nijnik, A. & Philpott, D. J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 10, 243–254 (2012).
Vijayan, D., Young, A., Teng, M. W. L. & Smyth, M. J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 17, 709–724 (2017).
Savva, A. & Roger, T. Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front. Immunol. 4, 387 (2013).
Yu, M. & Levine, S. J. Toll-like receptor, RIG-I-like receptors and the NLRP3 inflammasome: key modulators of innate immune responses to double-stranded RNA viruses. Cytokine Growth Factor Rev. 22, 63–72 (2011).
Yamamoto, M. et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 (2002).
Matsumoto, M., Oshiumi, H. & Seya, T. Antiviral responses induced by the TLR3 pathway. Rev. Med. Virol. 21, 67–77 (2011).
Hennessy, E. J., Parker, A. E. & O’Neill, L. A. Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 9, 293–307 (2010).
Lim, H. K. et al. Severe influenza pneumonitis in children with inherited TLR3 deficiency. J. Exp. Med. 216, 2038–2056 (2019).
Field, A. K., Tytell, A. A., Lampson, G. P. & Hilleman, M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc. Natl Acad. Sci. USA 58, 1004–1010 (1967).
Gresser, I., Maury, C., Bandu, M. T., Tovey, M. & Maunoury, M. T. Role of endogenous interferon in the anti-tumor effect of poly I-C and statolon as demonstrated by the use of anti-mouse interferon serum. Int. J. Cancer 21, 72–77 (1978).
Schulz, O. et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892 (2005).
Cheng, L. et al. Human innate responses and adjuvant activity of TLR ligands in vivo in mice reconstituted with a human immune system. Vaccine 35, 6143–6153 (2017).
Robinson, R. A. et al. A phase I-II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patieonts with leukemia or solid tumors. J. Natl Cancer Inst. 57, 599–602 (1976).
Levy, H. B. et al. A modified polyriboinosinic-polyribocytidylic acid complex that induces interferon in primates. J. Infect. Dis. 132, 434–439 (1975).
Levine, A. S., Sivulich, M., Wiernik, P. H. & Levy, H. B. Initial clinical trials in cancer patients of polyriboinosinic-polyribocytidylic acid stabilized with poly-L-lysine, in carboxymethylcellulose [poly(ICLC)], a highly effective interferon inducer. Cancer Res. 39, 1645–1650 (1979).
Nakamura, O., Shitara, N., Matsutani, M., Takakura, K. & Machida, H. Phase I-II trials of poly(ICLC) in malignant brain tumor patients. J. Interferon Res. 2, 1–4 (1982).
Hammerich, L. et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 25, 814–824 (2019).
Lampkin, B. C., Levine, A. S., Levy, H., Krivit, W. & Hammond, D. Phase II trial of a complex polyriboinosinic-polyribocytidylic acid with poly-L-lysine and carboxymethyl cellulose in the treatment of children with acute leukemia and neuroblastoma: a report from the children’s cancer study group. Cancer Res. 45, 5904–5909 (1985).
Dillon, P. M. et al. A pilot study of the immunogenicity of a 9-peptide breast cancer vaccine plus poly-ICLC in early stage breast cancer. J. Immunother. Cancer 5, 92 (2017).
Mehrotra, S. et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J. Hematol. Oncol. 10, 82 (2017).
Rodriguez-Ruiz, M. E. et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. 29, 1312–1319 (2018).
Okada, H. et al. Induction of robust type-I CD8+ T-cell responses in WHO grade 2 low-grade glioma patients receiving peptide-based vaccines in combination with poly-ICLC. Clin. Cancer Res. 21, 286–294 (2015).
Pollack, I. F. et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J. Clin. Oncol. 32, 2050–2058 (2014).
Saxena, M. et al. Poly-ICLC, a TLR3 agonist, induces transient innate immune responses in patients with treated HIV-infection: a randomized double-blinded placebo controlled trial. Front. Immunol. 10, 725 (2019).
Hubbell, H. R., Kvalnes-Krick, K., Carter, W. A. & Strayer, D. R. Antiproliferative and immunomodulatory actions of beta-interferon and double-stranded RNA, individually and in combination, on human bladder tumor xenografts in nude mice. Cancer Res. 45, 2481–2486 (1985).
Carter, W. A. et al. Clinical, immunological, and virological effects of ampligen, a mismatched double-stranded RNA, in patients with AIDS or AIDS-related complex. Lancet 1, 1286–1292 (1987).
Mitchell, W. M., Montefiori, D. C., Robinson, W. E. Jr., Strayer, D. R. & Carter, W. A. Mismatched double-stranded RNA (Ampligen) reduces concentration of zidovudine (azidothymidine) required for in-vitro inhibition of human immunodeficiency virus. Lancet 1, 890–892 (1987).
Armstrong, J. A. et al. A phase I study of Ampligen in human immunodeficiency virus-infected subjects. J. Infect. Dis. 166, 717–722 (1992).
Thompson, K. A. et al. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:polyC12U in the treatment of HIV infection. Eur. J. Clin. Microbiol. Infect. Dis. 15, 580–587 (1996).
Strayer, D. R. et al. A controlled clinical trial with a specifically configured RNA drug, poly(I).poly(C12U), in chronic fatigue syndrome. Clin. Infect. Dis. 18, S88–S95 (1994).
Strayer, D. R. et al. A double-blind, placebo-controlled, randomized, clinical trial of the TLR-3 agonist rintatolimod in severe cases of chronic fatigue syndrome. PLOS ONE 7, e31334 (2012).
Navabi, H. et al. A clinical grade poly I:C-analogue (Ampligen) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine 27, 107–115 (2009).
Takeda, Y. et al. A TLR3-specific adjuvant relieves innate resistance to PD-L1 blockade without cytokine toxicity in tumor vaccine immunotherapy. Cell Rep. 19, 1874–1887 (2017).
Kawai, T. & Akira, S. TLR signaling. Cell Death Differ. 13, 816–825 (2006).
Ishii, N., Funami, K., Tatematsu, M., Seya, T. & Matsumoto, M. Endosomal localization of TLR8 confers distinctive proteolytic processing on human myeloid cells. J. Immunol. 193, 5118–5128 (2014).
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Heil, F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Hemmi, H. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3, 196–200 (2002).
Thomas, A. et al. Investigating Toll-like receptor agonists for potential to treat hepatitis C virus infection. Antimicrob. Agents Chemother. 51, 2969–2978 (2007).
Gerster, J. F. et al. Synthesis and structure-activity-relationships of 1H-imidazo[4,5-c]quinolines that induce interferon production. J. Med. Chem. 48, 3481–3491 (2005).
Harrison, C. J., Jenski, L., Voychehovski, T. & Bernstein, D. I. Modification of immunological responses and clinical disease during topical R-837 treatment of genital HSV-2 infection. Antivir. Res. 10, 209–223 (1988).
Hanna, E., Abadi, R. & Abbas, O. Imiquimod in dermatology: an overview. Int. J. Dermatol. 55, 831–844 (2016).
Walter, A. et al. Aldara activates TLR7-independent immune defence. Nat. Commun. 4, 1560 (2013). This study reveals that the immunostimulatory effects of Aldara depend on both TLR7-dependent pathways (elicited by imiquimod) and TLR7-independent mechanisms (elicited by the cream vehicle).
Nian, H. et al. R-848 triggers the expression of TLR7/8 and suppresses HIV replication in monocytes. BMC Infect. Dis. 12, 5 (2012).
Mark, K. E. et al. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J. Infect. Dis. 195, 1324–1331 (2007).
Pockros, P. J. et al. Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J. Hepatol. 47, 174–182 (2007).
Sidky, Y. A. et al. Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res. 52, 3528–3533 (1992).
Dewan, M. Z. et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin. Cancer Res. 18, 6668–6678 (2012).
Han, J. H. et al. TLR7 expression is decreased during tumour progression in transgenic adenocarcinoma of mouse prostate mice and its activation inhibits growth of prostate cancer cells. Am J Reprod. Immunol. 70, 317–326 (2013).
Cai, Y. et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 35, 596–610 (2011).
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Salazar, L. G. et al. Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous metastases: a phase 2 clinical trial. JAMA Oncol. 3, 969–973 (2017).
Hurt, C. N. et al. Recurrence of vulval intraepithelial neoplasia following treatment with cidofovir or imiquimod: results from a multicentre, randomised, phase II trial (RT3VIN). BJOG 125, 1171–1177 (2018).
Teulings, H. E. et al. Anti-melanoma immunity and local regression of cutaneous metastases in melanoma patients treated with monobenzone and imiquimod; a phase 2 a trial. Oncoimmunology 7, e1419113 (2018).
Galluzzi, L., Chan, T. A., Kroemer, G., Wolchok, J. D. & Lopez-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl Med. 10, eaat7807 (2018).
Ito, H., Ando, T., Arioka, Y., Saito, K. & Seishima, M. Inhibition of indoleamine 2,3-dioxygenase activity enhances the anti-tumour effects of a Toll-like receptor 7 agonist in an established cancer model. Immunology 144, 621–630 (2015).
Fujimura, T. et al. Successful treatment of nivolumab-resistant multiple in-transit melanomas with ipilimumab and topical imiquimod. Case Rep. Oncol. 11, 1–5 (2018).
Rook, A. H. et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood 126, 1452–1461 (2015).
Sabado, R. L. et al. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high-risk melanoma. Cancer Immunol. Res. 3, 278–287 (2015).
Fahey, L. M., Raff, A. B., Da Silva, D. M. & Kast, W. M. Reversal of human papillomavirus-specific T cell immune suppression through TLR agonist treatment of Langerhans cells exposed to human papillomavirus type 16. J. Immunol. 182, 2919–2928 (2009).
Lu, H. et al. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin. Cancer Res. 18, 499–509 (2012).
Stephenson, R. M. et al. TLR8 stimulation enhances cetuximab-mediated natural killer cell lysis of head and neck cancer cells and dendritic cell cross-priming of EGFR-specific CD8+ T cells. Cancer Immunol. Immunother. 62, 1347–1357 (2013).
Doener, F. et al. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine 37, 1819–1826 (2019). This article reports safety and activity results from the first-in-concept human trial of the RNA-based agonist CV8102 (an agonist of TLR7, TLR8 and RIG-I) administered alone or in combination with fractional doses of a rabies vaccine.
Moisan, J. et al. TLR7 ligand prevents allergen-induced airway hyperresponsiveness and eosinophilia in allergic asthma by a MYD88-dependent and MK2-independent pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L987–L995 (2006).
Ellis, A. K., Tsitoura, D. C., Quint, D., Powley, W. & Lee, L. A. Safety and pharmacodynamics of intranasal GSK2245035, a TLR7 agonist for allergic rhinitis: a randomized trial. Clin. Exp. Allergy 47, 1193–1203 (2017).
Lee, C. C., Avalos, A. M. & Ploegh, H. L. Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 12, 168–179 (2012).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 (2001).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Xu, R. H. et al. Sequential activation of two pathogen-sensing pathways required for type I interferon expression and resistance to an acute DNA virus infection. Immunity 43, 1148–1159 (2015).
Ives, A. et al. MyD88 and TLR9 dependent immune responses mediate resistance to Leishmania guyanensis infections, irrespective of Leishmania RNA virus burden. PLOS ONE 9, e96766 (2014).
Klinman, D. M., Verthelyi, D., Takeshita, F. & Ishii, K. J. Immune recognition of foreign DNA: a cure for bioterrorism? Immunity 11, 123–129 (1999).
Kandimalla, E. R. et al. Immunomodulatory oligonucleotides containing a cytosine-phosphate-2′-deoxy-7-deazaguanosine motif as potent toll-like receptor 9 agonists. Proc. Natl Acad. Sci. USA 102, 6925–6930 (2005).
Makowska, Z. et al. Sequential induction of type I and II interferons mediates a long-lasting gene induction in the liver in response to a novel toll-like receptor 9 agonist. J. Hepatol. 58, 743–749 (2013).
Sarasin-Filipowicz, M. et al. Interferon signaling and treatment outcome in chronic hepatitis C. Proc. Natl Acad. Sci. USA 105, 7034–7039 (2008).
Olbrich, A. R. et al. Effective postexposure treatment of retrovirus-induced disease with immunostimulatory DNA containing CpG motifs. J. Virol. 76, 11397–11404 (2002).
Wang, Y. et al. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J. Virol. 79, 14355–14370 (2005).
Equils, O. et al. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J. Immunol. 170, 5159–5164 (2003).
Sundstrom, J. B., Little, D. M., Villinger, F., Ellis, J. E. & Ansari, A. A. Signaling through Toll-like receptors triggers HIV-1 replication in latently infected mast cells. J. Immunol. 172, 4391–4401 (2004).
Brichacek, B. et al. Contrasting roles for TLR ligands in HIV-1 pathogenesis. PLOS ONE 5, e12831 (2010).
Vibholm, L. K. et al. Effects of 24 week Toll-like receptor 9 agonist treatment in HIV-1+ individuals: a single-arm, phase 1B/2A trial. AIDS 33, 1315–1325 (2019).
Krarup, A. R. et al. The TLR9 agonist MGN1703 triggers a potent type I interferon response in the sigmoid colon. Mucosal Immunol. 11, 449–461 (2018).
Cooper, C. L., Angel, J. B., Seguin, I., Davis, H. L. & Cameron, D. W. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin. Infect. Dis. 46, 1310–1314 (2008).
Ito, H. et al. Induction of humoral and cellular immune response to hepatitis B virus (HBV) vaccine can be upregulated by CpG oligonucleotides complexed with dectin-1 ligand. J. Viral Hepat. 24, 155–162 (2017).
Li, J. et al. Hepatitis B surface antigen (HBsAg) and core antigen (HBcAg) combine CpG oligodeoxynucletides as a novel therapeutic vaccine for chronic hepatitis B infection. Vaccine 33, 4247–4254 (2015).
Brignole, C. et al. Therapeutic targeting of TLR9 inhibits cell growth and induces apoptosis in neuroblastoma. Cancer Res. 70, 9816–9826 (2010).
Damiano, V. et al. TLR9 agonist acts by different mechanisms synergizing with bevacizumab in sensitive and cetuximab-resistant colon cancer xenografts. Proc. Natl Acad. Sci. USA 104, 12468–12473 (2007).
Zoglmeier, C. et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin. Cancer Res. 17, 1765–1775 (2011).
Heckelsmiller, K. et al. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J. Immunol. 169, 3892–3899 (2002).
Houot, R. & Levy, R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood 113, 3546–3552 (2009).
Lonsdorf, A. S. et al. Intratumor CpG-oligodeoxynucleotide injection induces protective antitumor T cell immunity. J. Immunol. 171, 3941–3946 (2003).
Leonard, J. P. et al. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin’s lymphoma. Clin. Cancer Res. 13, 6168–6174 (2007).
Zent, C. S. et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk. Lymphoma 53, 211–217 (2012).
Carpentier, A. et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro. Oncol. 12, 401–408 (2010).
Pashenkov, M. et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J. Clin. Oncol. 24, 5716–5724 (2006).
Thompson, J. A., Kuzel, T., Drucker, B. J., Urba, W. J. & Bukowski, R. M. Safety and efficacy of PF-3512676 for the treatment of stage IV renal cell carcinoma: an open-label, multicenter phase I/II study. Clin. Genitourin. Cancer 7, E58–E65 (2009).
Weber, J. S. et al. Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF-3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer 115, 3944–3954 (2009).
Hirsh, V. et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J. Clin. Oncol. 29, 2667–2674 (2011).
Manegold, C. et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann. Oncol. 23, 72–77 (2012).
Manegold, C. et al. Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 26, 3979–3986 (2008).
Koster, B. D. et al. Local adjuvant treatment with low-dose CpG-B offers durable protection against disease recurrence in clinical stage I-II melanoma: data from two randomized phase ii trials. Clin. Cancer Res. 23, 5679–5686 (2017).
[No authors listed] Warming “cold” melanoma with TLR9 agonists. Cancer Discov. 8, 670 (2018).
Ribas, A. et al. SD-101 in Combination with pembrolizumab in advanced melanoma: results of a phase Ib, multicenter study. Cancer Discov. 8, 1250–1257 (2018).
Frank, M. J. et al. In Situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 8, 1258–1269 (2018).
Rodriguez-Ruiz, M. E., Vanpouille-Box, C., Melero, I., Formenti, S. C. & Demaria, S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 39, 644–655 (2018).
Kline, J. N. & Krieg, A. M. Toll-like receptor 9 activation with CpG oligodeoxynucleotides for asthma therapy. Drug News Perspect. 21, 434–439 (2008).
Li-Ping Thio, C., Chuan-Ying Lai, A., Chi, P. Y., Webster, G. & Chang, Y. J. Toll-like receptor 9-dependent interferon production prevents group 2 innate lymphoid cell-driven airway hyperreactivity. J. Allergy Clin. Immunol. 144, 682–697.e9 (2019).
Reikine, S., Nguyen, J. B. & Modis, Y. Pattern recognition and signaling mechanisms of RIG-I and MDA5. Front. Immunol. 5, 342 (2014).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Blander, J. M. A long-awaited merger of the pathways mediating host defence and programmed cell death. Nat. Rev. Immunol. 14, 601–618 (2014).
Korolowicz, K. E. et al. Antiviral efficacy and host innate immunity associated with SB 9200 treatment in the woodchuck model of chronic hepatitis B. PLOS ONE 11, e0161313 (2016).
Travassos, L. H. et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11, 55–62 (2010).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Suresh, M. et al. Antiviral efficacy and host immune response induction during sequential treatment with sb 9200 followed by entecavir in woodchucks. PLOS ONE 12, e0169631 (2017).
Jones, M. et al. SB 9200, a novel agonist of innate immunity, shows potent antiviral activity against resistant HCV variants. J. Med. Virol. 89, 1620–1628 (2017).
Hornung, V. et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Olagnier, D. et al. Inhibition of dengue and chikungunya virus infections by RIG-I-mediated type I interferon-independent stimulation of the innate antiviral response. J. Virol. 88, 4180–4194 (2014).
Goulet, M. L. et al. Systems analysis of a RIG-I agonist inducing broad spectrum inhibition of virus infectivity. PLOS Pathog. 9, e1003298 (2013).
Linehan, M. M. et al. A minimal RNA ligand for potent RIG-I activation in living mice. Sci. Adv. 4, e1701854 (2018).
Poeck, H. et al. 5′-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat. Med. 14, 1256–1263 (2008).
Li, D. et al. 5′-Triphosphate siRNA targeting MDR1 reverses multi-drug resistance and activates RIG-I-induced immune-stimulatory and apoptotic effects against human myeloid leukaemia cells. Leuk. Res. 58, 23–30 (2017).
Ranoa, D. R. et al. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs. Oncotarget 7, 26496–26515 (2016).
Claudepierre, M. C. et al. Yeast virus-derived stimulator of the innate immune system augments the efficacy of virus vector-based immunotherapy. J. Virol. 88, 5242–5255 (2014).
Oberson, A. et al. NAB2 is a novel immune stimulator of MDA-5 that promotes a strong type I interferon response. Oncotarget 9, 5641–5651 (2018).
Tormo, D. et al. Targeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cells. Cancer Cell 16, 103–114 (2009).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Aznar, M. A. et al. Immunotherapeutic effects of intratumoral nanoplexed poly I:C. J. Immunother. Cancer 7, 116 (2019).
Lamborn, I. T. et al. Recurrent rhinovirus infections in a child with inherited MDA5 deficiency. J. Exp. Med. 214, 1949–1972 (2017). This is the first report of a homozygous missense mutation in IFIH1 in humans, resulting in increased susceptibility to viral infection.
Ishizuka, J. J. et al. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature 565, 43–48 (2019). This study identifies in ADAR a therapeutic target for circumventing the resistance of cancer patients to ICBs, reflecting the ability of ADAR to limit the availability of dsRNA for PKR and MDA5 activation.
Donnelly, N., Gorman, A. M., Gupta, S. & Samali, A. The eIF2alpha kinases: their structures and functions. Cell Mol. Life Sci. 70, 3493–3511 (2013).
Liddicoat, B. J. et al. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science 349, 1115–1120 (2015).
Gannon, H. S. et al. Identification of ADAR1 adenosine deaminase dependency in a subset of cancer cells. Nat. Commun. 9, 5450 (2018).
Dhir, A. et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 560, 238–242 (2018).
Probst, P. et al. A small-molecule IRF3 agonist functions as an influenza vaccine adjuvant by modulating the antiviral immune response. Vaccine 35, 1964–1971 (2017).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384 (2013).
Ablasser, A. et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503, 530–534 (2013).
Gao, P. et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153, 1094–1107 (2013).
Civril, F. et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498, 332–337 (2013).
Gao, D. et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341, 903–906 (2013).
Li, X. D. et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341, 1390–1394 (2013).
Woo, S. R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Vanpouille-Box, C. et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618 (2017). This article demonstrates that the cytosolic exonuclease TREX1 is upregulated by radiation doses greater than 12–14 Gy in most cancer cells, resulting in limited availability of dsDNA for cGAS activation and hence reduced secretion of type I interferon.
Sivick, K. E. et al. Magnitude of therapeutic sting activation determines CD8+ T cell-mediated anti-tumor immunity. Cell Rep. 25, 3074–3085 e3075 (2018).
Gulen, M. F. et al. Signalling strength determines proapoptotic functions of STING. Nat. Commun. 8, 427 (2017). This article and reference 162 demonstrate that robust STING agonism mediates cytostatic and cytotoxic effects in T cells, hence being detrimental to (rather than beneficial for) anticancer immune responses.
Larkin, B. et al. Cutting edge: activation of sting in T cells induces type I IFN responses and cell death. J. Immunol. 199, 397–402 (2017).
Wu, T. Y. Strategies for designing synthetic immune agonists. Immunology 148, 315–325 (2016).
Prantner, D. et al. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J. Biol. Chem. 287, 39776–39788 (2012).
Kanwar, J. R., Kanwar, R. K., Pandey, S., Ching, L. M. & Krissansen, G. W. Vascular attack by 5,6-dimethylxanthenone-4-acetic acid combined with B7.1 (CD80)-mediated immunotherapy overcomes immune resistance and leads to the eradication of large tumors and multiple tumor foci. Cancer Res. 61, 1948–1956 (2001).
Perera, P. Y., Barber, S. A., Ching, L. M. & Vogel, S. N. Activation of LPS-inducible genes by the antitumor agent 5,6-dimethylxanthenone-4-acetic acid in primary murine macrophages. Dissection of signaling pathways leading to gene induction and tyrosine phosphorylation. J. Immunol. 153, 4684–4693 (1994).
Guo, F. et al. STING agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrob. Agents Chemother. 59, 1273–1281 (2015).
Ceron, S., North, B. J., Taylor, S. A. & Leib, D. A. The STING agonist 5,6-dimethylxanthenone-4-acetic acid (DMXAA) stimulates an antiviral state and protects mice against herpes simplex virus-induced neurological disease. Virology 529, 23–28 (2019).
Curran, E. et al. STING Pathway activation stimulates potent immunity against acute myeloid leukemia. Cell Rep. 15, 2357–2366 (2016).
Weiss, J. M. et al. The STING agonist DMXAA triggers a cooperation between T lymphocytes and myeloid cells that leads to tumor regression. Oncoimmunology 6, e1346765 (2017).
McKeage, M. J. et al. 5,6-Dimethylxanthenone-4-acetic acid in the treatment of refractory tumors: a phase I safety study of a vascular disrupting agent. Clin. Cancer Res. 12, 1776–1784 (2006).
Pili, R. et al. Phase II study on the addition of ASA404 (vadimezan; 5,6-dimethylxanthenone-4-acetic acid) to docetaxel in CRMPC. Clin. Cancer Res. 16, 2906–2914 (2010).
Lara, P. N., Jr. et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J. Clin. Oncol. 29, 2965–2971 (2011).
Gao, P. et al. Structure-function analysis of STING activation by c[G(2′,5′)pA(3′,5′)p] and targeting by antiviral DMXAA. Cell 154, 748–762 (2013).
Conlon, J. et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J. Immunol. 190, 5216–5225 (2013).
Burdette, D. L. et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518 (2011).
Ohkuri, T. et al. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol. Res. 2, 1199–1208 (2014).
Chandra, D. et al. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol. Res. 2, 901–910 (2014).
Yamamoto, T. et al. STING agonists activate latently infected cells and enhance SIV-specific responses ex vivo in naturally SIV controlled cynomolgus macaques. Sci. Rep. 9, 5917 (2019).
Li, L. et al. Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat. Chem. Biol. 10, 1043–1048 (2014).
Yi, G. et al. Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLOS ONE 8, e77846 (2013).
Rodriguez-Garcia, E. et al. TMEM173 alternative spliced isoforms modulate viral replication through the STING pathway. Immunohorizons 2, 363–376 (2018).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Ager, C. R. et al. Intratumoral STING activation with T-cell checkpoint modulation generates systemic antitumor immunity. Cancer Immunol. Res. 5, 676–684 (2017).
Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl Med. 7, 283ra252 (2015).
Tang, C. H. et al. Agonist-mediated activation of sting induces apoptosis in malignant b cells. Cancer Res. 76, 2137–2152 (2016).
Ramanjulu, J. M. et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 564, 439–443 (2018). This is the first report of a STING agonist that is not a CDN, is amenable to systemic delivery and mediates robust antineoplastic effects as stand-alone therapeutic intervention.
Croft, M. & Siegel, R. M. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat. Rev. Rheumatol. 13, 217–233 (2017).
Galluzzi, L., Yamazaki, T. & Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 19, 731–745 (2018).
Wang, C. M. et al. Genetic variations in Toll-like receptors (TLRs 3/7/8) are associated with systemic lupus erythematosus in a Taiwanese population. Sci. Rep. 4, 3792 (2014).
Patole, P. S. et al. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J. Am. Soc. Nephrol. 16, 1326–1338 (2005).
Annable, T. et al. Using poly I:C as an adjuvant does not induce or exacerbate models of systemic lupus erythematosus. Autoimmunity 48, 29–39 (2015).
Christensen, S. R. et al. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J. Exp. Med. 202, 321–331 (2005).
Tran, N. L., Manzin-Lorenzi, C. & Santiago-Raber, M. L. Toll-like receptor 8 deletion accelerates autoimmunity in a mouse model of lupus through a Toll-like receptor 7-dependent mechanism. Immunology 145, 60–70 (2015).
Nickerson, K. M. et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 184, 1840–1848 (2010).
Vollmer, J. et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 202, 1575–1585 (2005).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Papadimitraki, E. D. et al. Expansion of toll-like receptor 9-expressing B cells in active systemic lupus erythematosus: implications for the induction and maintenance of the autoimmune process. Arthritis Rheum. 54, 3601–3611 (2006).
Migita, K. et al. Toll-like receptor expression in lupus peripheral blood mononuclear cells. J. Rheumatol. 34, 493–500 (2007).
Hasan, M. et al. Cutting edge: inhibiting TBK1 by compound ii ameliorates autoimmune disease in mice. J. Immunol. 195, 4573–4577 (2015).
Kono, D. H. et al. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc. Natl Acad. Sci. USA 106, 12061–12066 (2009).
Berland, R. et al. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity 25, 429–440 (2006).
Subramanian, S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad. Sci. USA 103, 9970–9975 (2006).
Deane, J. A. et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007).
Lenert, P. et al. DNA-like class R inhibitory oligonucleotides (INH-ODNs) preferentially block autoantigen-induced B-cell and dendritic cell activation in vitro and autoantibody production in lupus-prone MRL-Faslpr/lpr mice in vivo. Arthritis Res. Ther. 11, R79 (2009).
Hamm, S. et al. Alternating 2′-O-ribose methylation is a universal approach for generating non-stimulatory siRNA by acting as TLR7 antagonist. Immunobiology 215, 559–569 (2010).
Alzabin, S. et al. Investigation of the role of endosomal Toll-like receptors in murine collagen-induced arthritis reveals a potential role for TLR7 in disease maintenance. Arthritis Res. Ther. 14, R142 (2012).
Duffau, P. et al. Promotion of inflammatory arthritis by interferon regulatory factor 5 in a mouse model. Arthritis Rheumatol. 67, 3146–3157 (2015).
Hasham, M. G. et al. Systemic autoimmunity induced by the TLR7/8 agonist resiquimod causes myocarditis and dilated cardiomyopathy in a new mouse model of autoimmune heart disease. Dis. Model. Mech. 10, 259–270 (2017).
Roelofs, M. F. et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 52, 2313–2322 (2005).
Ospelt, C. et al. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 58, 3684–3692 (2008).
Sacre, S. et al. Oligodeoxynucleotide inhibition of Toll-like receptors 3, 7, 8, and 9 suppresses cytokine production in a human rheumatoid arthritis model. Eur. J. Immunol. 46, 772–781 (2016).
Lai, C. Y., Su, Y. W., Lin, K. I., Hsu, L. C. & Chuang, T. H. Natural modulators of endosomal toll-like receptor-mediated psoriatic skin inflammation. J. Immunol. Res. 2017, 7807313 (2017).
Prinz, M. et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Invest. 116, 456–464 (2006).
Hu, X. et al. RGS1 silencing inhibits the inflammatory response and angiogenesis in rheumatoid arthritis rats through the inactivation of Toll-like receptor signaling pathway. J. Cell. Physiol. 234, 20432–20442 (2019).
Balak, D. M. et al. IMO-8400, a toll-like receptor 7, 8, and 9 antagonist, demonstrates clinical activity in a phase 2a, randomized, placebo-controlled trial in patients with moderate-to-severe plaque psoriasis. Clin. Immunol. 174, 63–72 (2017).
Kearns, A., Gordon, J., Burdo, T. H. & Qin, X. HIV-1-Associated atherosclerosis: unraveling the missing link. J. Am. Coll. Cardiol. 69, 3084–3098 (2017).
Bassendine, M. F. et al. Hepatitis c virus and atherosclerosis: a legacy after virologic cure? Clin. Res. Hepatol. Gastroenterol. 41, 25–30 (2017).
Sessa, R. et al. Chlamydia pneumoniae and atherosclerosis: current state and future prospectives. Int. J. Immunopathol. Pharmacol. 22, 9–14 (2009).
Bjorkbacka, H. et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat. Med. 10, 416–421 (2004).
Michelsen, K. S. et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl Acad. Sci. USA 101, 10679–10684 (2004).
Schoneveld, A. H. et al. Toll-like receptor 2 stimulation induces intimal hyperplasia and atherosclerotic lesion development. Cardiovasc. Res. 66, 162–169 (2005).
Vink, A. et al. In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation 106, 1985–1990 (2002).
Ma, C. et al. Toll-like receptor 9 inactivation alleviated atherosclerotic progression and inhibited macrophage polarized to M1 phenotype in ApoE−/− mice. Dis. Markers 2015, 909572 (2015).
Karper, J. C. et al. Blocking toll-like receptors 7 and 9 reduces postinterventional remodeling via reduced macrophage activation, foam cell formation, and migration. Arterioscler. Thromb. Vasc. Biol. 32, e72–e80 (2012).
Salagianni, M. et al. Toll-like receptor 7 protects from atherosclerosis by constraining “inflammatory” macrophage activation. Circulation 126, 952–962 (2012).
Oka, T. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251–255 (2012). This is the first demonstration that autophagy prevents dsDNA released by damaged mitochondria from initiating a TLR9-dependent pathway with cardiotoxic effects.
Lim, J. E. et al. MyD88 deficiency ameliorates beta-amyloidosis in an animal model of Alzheimer’s disease. Am. J. Pathol. 179, 1095–1103 (2011).
Lehmann, S. M. et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 15, 827–835 (2012).
Maatouk, L. et al. TLR9 activation via microglial glucocorticoid receptors contributes to degeneration of midbrain dopamine neurons. Nat. Commun. 9, 2450 (2018).
Ros-Bernal, F. et al. Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc. Natl Acad. Sci. USA 108, 6632–6637 (2011).
Scholtzova, H. et al. Innate immunity stimulation via Toll-like receptor 9 ameliorates vascular amyloid pathology in Tg-SwDI mice with associated cognitive benefits. J. Neurosci. 37, 936–959 (2017).
Sheridan, C. Drug developers switch gears to inhibit STING. Nat. Biotechnol. 37, 199–201 (2019).
Pestal, K. et al. Isoforms of RNA-editing enzyme ADAR1 independently control nucleic acid sensor MDA5-driven autoimmunity and multi-organ development. Immunity 43, 933–944 (2015).
Nakahama, T. et al. ADAR1-mediated RNA editing is required for thymic self-tolerance and inhibition of autoimmunity. EMBO Rep. 19 (2018).
Laxminarayana, D., Khan, I. U. & Kammer, G. Transcript mutations of the alpha regulatory subunit of protein kinase A and up-regulation of the RNA-editing gene transcript in lupus T lymphocytes. Lancet 360, 842–849 (2002).
Hadjadj, J. et al. Pediatric Evans syndrome is associated with a high frequency of potentially damaging variants in immune genes. Blood 134, 9–21 (2019).
Kono, M. et al. Dyschromatosis symmetrica hereditaria and Aicardi-Goutieres syndrome 6 are phenotypic variants caused by ADAR1 mutations. J. Invest. Dermatol. 136, 875–878 (2016).
Crow, Y. J. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat. Genet. 38, 917–920 (2006).
Lee-Kirsch, M. A. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat. Genet. 39, 1065–1067 (2007).
Kerur, N. et al. cGAS drives noncanonical-inflammasome activation in age-related macular degeneration. Nat. Med. 24, 50–61 (2018).
King, K. R. et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 23, 1481–1487 (2017). This is the first report proving that abnormal secretion of type I interferon downstream of deregulated CGAS–STING signalling contributes to the long-term detrimental consequences of myocardial infarction.
Hu, Q. et al. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. EBioMedicine 41, 497–508 (2019).
Luo, X. et al. Expression of sting is increased in liver tissues from patients with NAFLD and promotes macrophage-mediated hepatic inflammation and fibrosis in mice. Gastroenterology 155, 1971–1984.e1974 (2018).
Yu, Y. et al. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J. Clin. Invest. 129, 546–555 (2019).
Ahn, J. et al. Inflammation-driven carcinogenesis is mediated through STING. Nat. Commun. 5, 5166 (2014).
Papinska, J. et al. Activation of stimulator of interferon genes (STING) and Sjogren syndrome. J. Dent. Res. 97, 893–900 (2018).
Haag, S. M. et al. Targeting STING with covalent small-molecule inhibitors. Nature 559, 269–273 (2018). This article and reference 249 independently report the identification of small molecules and a cyclopeptide that potently inhibit STING.
Li, S. et al. The cyclopeptide astin C specifically inhibits the innate immune CDN sensor sting. Cell Rep. 25, 3405–3421.e3407 (2018).
An, J. et al. Inhibition of cyclic GMP-AMP synthase using a novel antimalarial drug derivative in Trex1-deficient mice. Arthritis Rheumatol. 70, 1807–1819 (2018).
Dai, J. et al. Acetylation blocks cGA activity and inhibits self-DNA-induced autoimmunity. Cell 176, 1447–1460.e1414 (2019). This report reveals that aspirin promotes the non-enzymatic inhibitory acetylation of CGAS, unveiling a novel mechanism whereby aspirin mediates anti-inflammatory effects.
Wang, T., Larcher, L. M., Ma, L. & Veedu, R. N. Systematic screening of commonly used commercial transfection reagents towards efficient transfection of single-stranded oligonucleotides. Molecules 23, 2564 (2018).
Bocanegra Gondan, A. I. et al. Effective cancer immunotherapy in mice by polyIC-imiquimod complexes and engineered magnetic nanoparticles. Biomaterials 170, 95–115 (2018).
Salaun, B., Coste, I., Rissoan, M. C., Lebecque, S. J. & Renno, T. TLR3 can directly trigger apoptosis in human cancer cells. J. Immunol. 176, 4894–4901 (2006).
Tsitoura, D. et al. Early clinical evaluation of the intranasal TLR7 agonist GSK2245035: use of translational biomarkers to guide dosing and confirm target engagement. Clin. Pharmacol. Ther. 98, 369–380 (2015).
Felten, R., Scher, F., Sagez, F., Chasset, F. & Arnaud, L. Spotlight on anifrolumab and its potential for the treatment of moderate-to-severe systemic lupus erythematosus: evidence to date. Drug Des. Devel. Ther. 13, 1535–1543 (2019).
Lai, Y. F. et al. Functional polymorphisms of the TLR7 and TLR8 genes contribute to Mycobacterium tuberculosis infection. Tuberculosis 98, 125–131 (2016).
Mollaki, V. et al. Polymorphisms and haplotypes in TLR9 and MYD88 are associated with the development of Hodgkin’s lymphoma: a candidate-gene association study. J. Hum. Genet. 54, 655–659 (2009).
Bharti, D. et al. The role of TLR9 polymorphism in susceptibility to pulmonary tuberculosis. Immunogenetics 66, 675–681 (2014).
Forsbach, A. et al. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J. Immunol. 180, 3729–3738 (2008).
Sistigu, A. et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Chan, Y. K. & Gack, M. U. Viral evasion of intracellular DNA and RNA sensing. Nat. Rev. Microbiol. 14, 360–373 (2016).
Bidwell, B. N. et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 18, 1224–1231 (2012).
Brand, F. J., 3rd, de Rivero Vaccari, J. C., Mejias, N. H., Alonso, O. F. & de Rivero Vaccari, J. P. RIG-I contributes to the innate immune response after cerebral ischemia. J. Inflamm. 12, 52 (2015).
Galluzzi, L., Bravo-San Pedro, J. M., Levine, B., Green, D. R. & Kroemer, G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 16, 487–511 (2017).
Theisen, D. J. et al. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 362, 694–699 (2018).
Yang, W. et al. Genome-wide association study in Asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLOS Genet. 6, e1000841 (2010).
Kochi, Y. et al. Splicing variant of WDFY4 augments MDA5 signalling and the risk of clinically amyopathic dermatomyositis. Ann. Rheum. Dis. 77, 602–611 (2018).
Echem, C. et al. Mitochondrial DNA: a new driver for sex differences in spontaneous hypertension. Pharmacol. Res. 144, 142–150 (2019).
Xin, G. et al. Sex hormone affects the severity of non-alcoholic steatohepatitis through the MyD88-dependent IL-6 signaling pathway. Exp. Biol. Med. 240, 1279–1286 (2015).
Cillo, A. R. & Mellors, J. W. Which therapeutic strategy will achieve a cure for HIV-1? Curr. Opin. Virol. 18, 14–19 (2016).
Bartsch, K. et al. Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Hum. Mol. Genet. 26, 3960–3972 (2017).
Banerjee, S. et al. OAS-RNase L innate immune pathway mediates the cytotoxicity of a DNA-demethylating drug. Proc. Natl Acad. Sci. USA 116, 5071–5076 (2019).
Chiu, Y. H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Ferguson, B. J., Mansur, D. S., Peters, N. E., Ren, H. & Smith, G. L. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. eLife 1, e00047 (2012).
Dutta, D. et al. BRCA1 Regulates IFI16 mediated nuclear innate sensing of herpes viral DNA and subsequent induction of the innate inflammasome and interferon-beta responses. PLOS Pathog. 11, e1005030 (2015).
Kondo, T. et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc. Natl Acad. Sci. USA 110, 2969–2974 (2013).
Parvatiyar, K. et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat. Immunol. 13, 1155–1161 (2012).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Almine, J. F. et al. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes. Nat. Commun. 8, 14392 (2017).
Shannon, J. L. et al. Polyglutamine binding protein 1 (PQBP1) inhibits innate immune responses to cytosolic DNA. Mol. Immunol. 99, 182–190 (2018).
Tan, Y. & Kagan, J. C. Innate immune signaling organelles display natural and programmable signaling flexibility. Cell 177, 384–398.e311 (2019). This study demonstrates that supramolecular platforms commonly used to drive cytokine secretion in response to nucleic acid sensing, such as the myddosome, can be engineered to mediate user-defined biological outcomes.
Lama, L. et al. Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nat. Commun. 10, 2261 (2019).
Chung, H. et al. Human ADAR1 prevents endogenous RNA from triggering translational shutdown. Cell 172, 811–824.e14 (2018).
Isaacs, A., Lindenmann, J. & Valentine, R. C. Virus interference. II. Some properties of interferon. Proc. R. Soc. Lond. B Biol. Sci. 147, 268–273 (1957).
Isaacs, A. & Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147, 258–267 (1957).
Jensen, K. E., Neal, A. L., Owens, R. E. & Warren, J. Interferon responses of chick embryo fibroblasts to nucleic acids and related compounds. Nature 200, 433–434 (1963).
Rotem, Z., Cox, R. A. & Isaacs, A. Inhibition of virus multiplication by foreign nucleic acid. Nature 197, 564–566 (1963).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. Jr. A human homologue of the drosophila toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Ferrandon, D., Imler, J. L., Hetru, C. & Hoffmann, J. A. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 7, 862–874 (2007).
Gay, N. J., Symmons, M. F., Gangloff, M. & Bryant, C. E. Assembly and localization of Toll-like receptor signalling complexes. Nat. Rev. Immunol. 14, 546–558 (2014).
Galluzzi, L., Vanpouille-Box, C., Bakhoum, S. F. & Demaria, S. SnapShot: CGAS-STING signaling. Cell 173, 276–276 e271 (2018).
Acknowledgements
The authors apologize to the authors of multiple preclinical and clinical studies on pharmacological modulators of the nucleic acid sensing machinery that could not be cited owing to space limitations. C.V.B. is supported by a start-up grant from the Department of Radiation Oncology at Weill Cornell Medicine (New York, NY, USA) and a Career Development Award from the Radiation Research Foundation (Kansas City, MI, USA). L.G. is supported by a Breakthrough Level 2 grant from the US Department of Defense, Breast Cancer Research Program (BC180476P1), by a start-up grant from the Department of Radiation Oncology at Weill Cornell Medicine (New York, NY, USA), by industrial collaborations with Lytix (Oslo, Norway) and Phosplatin (New York, NY, USA) and by donations from Phosplatin (New York, NY, USA), the Luke Heller TECPR2 Foundation (Boston, MA, USA) and Sotio (Prague, Czech Republic).
Author information
Authors and Affiliations
Contributions
C.V.B. and L.G. conceived this Review and wrote the first version of the manuscript, with constructive input from J.A.H. C.V.B. and L.G. prepared display items. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.V.B. and J.H. have no relevant conflicts of interest. L.G. provides remunerated consulting to OmniSEQ (Buffalo, NY, USA), Astra Zeneca (Gaithersburg, MD, USA), VL47 (New York, NY, USA) and the Luke Heller TECPR2 Foundation (Boston, MA, USA), and is a member of the Scientific Advisory Committee of OmniSEQ (Buffalo, NY, USA).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Bristol-Myers Squibb (In the Pipeline): https://www.bms.com/researchers-and-partners/in-the-pipeline.html
ClinicalTrials.gov: http://www.clinicaltrials.gov
Glossary
- Pattern recognition receptors
-
(PRRs). Members of an evolutionarily ancient group of receptors that initiate innate immunity on detection of conserved microbial products or endogenous damage-associated molecular patterns.
- Damage-associated molecular patterns
-
Endogenous molecules with immunomodulatory activity that are exposed or secreted upon cellular damage or death, culminating in the activation of pattern recognition receptors on neighbouring cells.
- CpG regions
-
Portions of a nucleic acid rich in unmethylated cytosine–guanine dimers.
- Dendritic cell
-
(DC). Haematopoietic cell of myeloid derivation specialized in the uptake of antigens from the microenvironment and their presentation to T cells.
- Cross-priming
-
Process whereby specific dendritic cell subsets initiate CD8+ (rather than CD4+) cell responses against antigens taken up from the microenvironment.
- Immune checkpoint blocker
-
(ICB). Member of a class of drugs that mediate anticancer effects by inhibiting the molecular systems involved in the natural extinction of immune responses.
- Plasmacytoid DCs
-
Particular variant of dendritic cells (DCs) that morphologically resemble plasma cells and secrete high levels of type I interferon in response to activatory signals.
- Monocyte-derived DCs
-
Specific variant of dendritic cells (DCs) that originate from circulating monocytes on recruitment to sites of inflammation.
- Viral interference
-
Process whereby virally infected cells establish resistance to infection locally via paracrine circuitries depending on type I interferon.
- Imidazoquinolines
-
Organic molecules characterized by a tricyclic structure that has been frequently used as a lead for the development of TLR7 agonists.
- CD4+ T helper cells
-
Lymphocytes that support and direct the activity of other immune cells, including CD8+ T cells, by releasing cytokines.
- γδ T cells
-
Relatively rare variant of T cells that express a γδ (rather than the common αβ) T cell receptor, and hence are endowed with major histocompatibility complex-independent activity.
- Monobenzone
-
Monobenzyl ether of hydroquinone, often used for medical depigmentation.
- Nivolumab
-
Clinically used monoclonal antibody that acts by inhibiting programmed cell death 1 (PD-1), hence unleashing the effector function of T cells.
- CD8+ cytotoxic T lymphocyte
-
(CTL). Lymphoid cell capable of mediating cytotoxic functions in response to a specific antigenic epitope presented on major histocompatibility complex class I molecules.
- Natural killer cells
-
(NK cells). Lymphoid cells capable of mediating cytotoxic functions in an antigen-independent manner.
- Cetuximab
-
Monoclonal antibody specific for epidermal growth factor receptor, commonly used in the clinic for the treatment of colorectal cancer and some forms of lung cancer.
- Durvalumab
-
Clinically used monoclonal antibody that acts by inhibiting PD-L1, hence unleashing the effector function of T cells.
- Oligodeoxynucleotides
-
Short nucleic acids based on a deoxyribose backbone.
- Interferon-stimulated genes
-
(ISGs). Members of a large gene set that is upregulated in response to type I interferon signalling, such as MX1 or CXCL10.
- Ribavirin
-
Commonly used antiviral medication that acts as a guanosine analogue, hence inhibiting viral replication and mRNA processing
- TH1 cell cytokine
-
Cytokine that favours the polarization of the immune response towards a type 1 T helper cell (TH1 cell) profile, which is generally associated with robust antiviral and anticancer functions (for example, interferon-γ, tumour necrosis factor).
- Regulated cell death
-
Variant of cell death that is mediated by a genetically encoded machinery, and hence can be modulated by pharmacological or genetic interventions.
- Neoangiogenesis
-
Process whereby cancer cells initiate the vascularization of developing tumours, which is instrumental for disease progression and metastatic dissemination.
- Myeloid-derived suppressor cells
-
Immature myeloid cells that mediate robust immunosuppressive effects, hence favouring cancer progression and resistance to therapy.
- M2-like tumour-associated macrophages
-
Tumour-infiltrating macrophages that have immunosuppressive and proangiogenic function.
- M1-like tumour-associated macrophages
-
Tumour-infiltrating macrophages that mediate immunostimulatory and inflammatory effects.
- Pembrolizumab
-
Clinically used monoclonal antibody that acts by inhibiting programmed cell death 1 (PD-1), hence unleashing the effector function of T cells.
- Abscopal response
-
In radiation oncology, the relatively rare phenomenon whereby irradiation of one malignant lesion results in objective responses at a non-irradiated disease site.
- Inflammasome
-
Supramolecular platform that initiates the proteolytic maturation of proinflammatory cytokines such as IL-1β and IL-18.
- Entecavir
-
Orally available antiviral medication that acts as a guanosine analogue, commonly used for the treatment of hepatitis B virus infection.
- Tenofovir
-
Antiviral medication that acts as a reverse transcriptase inhibitor, commonly used for the treatment of hepatitis B virus infection and pre-exposure and post-exposure HIV-1 prophylaxis.
- Small interfering RNAs
-
Short RNA duplexes extensively used for temporary gene silencing on transfection.
- A-to-I editing
-
Post-transcriptional modification of RNA molecules that involves the enzymatic deamination of adenosine to inosine.
- Non-oncogene addition
-
Concept identifying the dependency of some cancer cells on molecular functions that are not involved in oncogenesis.
- Cyclic dinucleotides
-
(CDNs). Single-phosphate nucleotides with a cyclic bond arrangement between the sugar and phosphate groups, largely used by both prokaryotes and eukaryotes as a second messenger.
- Systemic lupus erythematosus
-
(SLE). Systemic autoimmune disease in which the immune system mistakenly attacks healthy tissues, including the skin, joints, kidneys, brain and other organs.
- Rheumatoid arthritis
-
Long-term autoimmune disorder that primarily affects joints, resulting in warmness, swelling and pain and ultimately leading to permanent joint destruction.
- Synovial lining
-
The lining of the joints, normally only one or two cell layers thick, that is responsible for the production of the joint fluid.
- Dermatomyositis
-
Acquired muscle disease that is characterized by chronic muscle inflammation accompanied by weakness.
- Evans syndrome
-
Very rare autoimmune disorder in which the immune system destroys red blood cells, white blood cells and/or platelets.
- Aicardi–Goutières syndrome
-
Inherited encephalopathy that affects newborns and is characterized by dysregulated type I interferon production.
- Sjögren syndrome
-
Autoimmune disorder affecting salivary and tear glands that generally manifests itself with dry mouth and dry eyes.
- Tumour-draining lymph nodes
-
Lymph nodes that collect extracellular fluids from the anatomical district where a malignant lesion is located.
- Cytokine release syndrome
-
Potentially lethal side effect of some immunotherapies (including some nucleic acid sensor agonists) characterized by the widespread activation of immune cells and consequent release of large amounts of cytokines in the bloodstream.
- Autophagy
-
Evolutionarily old homeostatic process culminating in the lysosomal degradation of superfluous, ectopic or potentially dangerous cytosolic entities.
Rights and permissions
About this article
Cite this article
Vanpouille-Box, C., Hoffmann, J.A. & Galluzzi, L. Pharmacological modulation of nucleic acid sensors — therapeutic potential and persisting obstacles. Nat Rev Drug Discov 18, 845–867 (2019). https://doi.org/10.1038/s41573-019-0043-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-019-0043-2
This article is cited by
-
Drp1: Focus on Diseases Triggered by the Mitochondrial Pathway
Cell Biochemistry and Biophysics (2024)
-
Mitochondrial control of inflammation
Nature Reviews Immunology (2023)
-
Type I interferon signaling in malignant blasts contributes to treatment efficacy in AML patients
Cell Death & Disease (2023)
-
The RIG-I agonist M8 triggers cell death and natural killer cell activation in human papillomavirus-associated cancer and potentiates cisplatin cytotoxicity
Cancer Immunology, Immunotherapy (2023)
-
T cell-independent eradication of experimental glioma by intravenous TLR7/8-agonist-loaded nanoparticles
Nature Communications (2023)