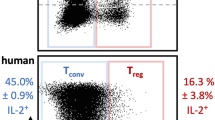
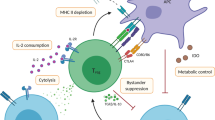
Abstract
Regulatory T cells (Treg cells) are a small subset of immune cells that are dedicated to curbing excessive immune activation and maintaining immune homeostasis. Accordingly, deficiencies in Treg cell development or function result in uncontrolled immune responses and tissue destruction and can lead to inflammatory disorders such as graft-versus-host disease, transplant rejection and autoimmune diseases. As Treg cells deploy more than a dozen molecular mechanisms to suppress immune responses, they have potential as multifaceted adaptable smart therapeutics for treating inflammatory disorders. Indeed, early-phase clinical trials of Treg cell therapy have shown feasibility, tolerability and potential efficacy in these disease settings. In the meantime, progress in the development of chimeric antigen receptors and in genome editing (including the application of CRISPR–Cas9) over the past two decades has facilitated the genetic optimization of primary T cell therapy for cancer. These technologies are now being used to enhance the specificity and functionality of Treg cells. In this Review, we describe the key advances and prospects in designing and implementing Treg cell-based therapy in autoimmunity and transplantation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gershon, R. K. & Kondo, K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 18, 723–737 (1970).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995).
Husebye, E. S., Anderson, M. S. & Kampe, O. Autoimmune polyendocrine syndromes. N. Engl. J. Med. 378, 1132–1141 (2018).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Zhou, X. et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009). This study reveals that T reg cells can become unstable, losing FOXP3 expression and converting into pathogenic T cells (‘ex-T reg cells’) in vivo.
Abbas, A. K., Trotta, E., D, R. S., Marson, A. & Bluestone, J. A. Revisiting IL-2: biology and therapeutic prospects. Sci. Immunol. 3, eaat1482 (2018). A comprehensive review discussing the discovery, biology and therapeutic potential of the cytokine IL-2.
Fan, M. Y. et al. Differential roles of IL-2 signaling in developing versus mature Tregs. Cell Rep. 25, 1204–1213.e1204 (2018).
Liu, W. et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203, 1701–1711 (2006).
Seddiki, N. et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203, 1693–1700 (2006). Together with Liu et al. (2006) this article describes the use of CD127 (IL-7 receptor subunit-α) as a surface marker, the exclusion of which dramatically enhances peripheral blood human T reg cell purity after FACS.
Ohkura, N., Kitagawa, Y. & Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 38, 414–423 (2013).
Miller, A., Lider, O. & Weiner, H. L. Antigen-driven bystander suppression after oral administration of antigens. J. Exp. Med. 174, 791–798 (1991).
Gershon, R. K. & Kondo, K. Infectious immunological tolerance. Immunology 21, 903–914 (1971). Seminal experimental article establishing one of the basic tenets of dominant immune tolerance: infectious tolerance.
Qin, S. et al. “Infectious” transplantation tolerance. Science 259, 974–977 (1993).
Esensten, J. H., Muller, Y. D., Bluestone, J. A. & Tang, Q. Regulatory T cell therapy for autoimmune and autoinflammatory diseases: the next frontier. J. Allergy Clin. Immunol. (2018).
Koch, M. A. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 (2009).
Duhen, T., Duhen, R., Lanzavecchia, A., Sallusto, F. & Campbell, D. J. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 119, 4430–4440 (2012).
Chaudhry, A. et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326, 986–991 (2009).
Zheng, Y. et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 458, 351–356 (2009). Together with Koch et al. (2009) and Chaudhry et al. (2009) this article reveals that T reg cells can adopt the expression of master transcription factors of T helper cell subsets (T H 1, T H 17 and T H 2) and this results in enhanced T reg cell migration and suppression specifically of the mimicked T helper cells.
Sharma, A. & Rudra, D. Emerging functions of regulatory T cells in tissue homeostasis. Front. Immunol. 9, 883 (2018).
Levine, A. G., Arvey, A., Jin, W. & Rudensky, A. Y. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 15, 1070–1078 (2014).
Takahashi, T. et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10, 1969–1980 (1998).
Sprouse, M. L. et al. Cutting edge: low-affinity TCRs support regulatory T cell function in autoimmunity. J. Immunol. 200, 909–914 (2018).
Yeh, W. I. et al. Avidity and bystander suppressive capacity of human regulatory T cells expressing de novo autoreactive T-cell receptors in type 1 diabetes. Front. Immunol. 8, 1313 (2017).
Plesa, G. et al. TCR affinity and specificity requirements for human regulatory T-cell function. Blood 119, 3420–3430 (2012).
Tang, Q. & Bluestone, J. A. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9, 239–244 (2008).
Zheng, Y. et al. Acquisition of suppressive function by activated human CD4+ CD25− T cells is associated with the expression of CTLA-4 not FoxP3. J. Immunol. 181, 1683–1691 (2008).
Oberle, N., Eberhardt, N., Falk, C. S., Krammer, P. H. & Suri-Payer, E. Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J. Immunol. 179, 3578–3587 (2007).
Marek-Trzonkowska, N. et al. Administration of CD4+CD25highCD127- regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care 35, 1817–1820 (2012).
Singh, K. et al. Superiority of rapamycin over tacrolimus in preserving nonhuman primate Treg half-life and phenotype after adoptive transfer. Am. J. Transpl. 14, 2691–2703 (2014).
Brunstein, C. G. et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117, 1061–1070 (2011).
Bluestone, J. A. et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl Med. 7, 315ra189 (2015). First clinical trial demonstrating up to 1 year survival of infused T reg cells in patients with T1D.
Gratz, I. K. et al. Cutting edge: memory regulatory T cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 190, 4483–4487 (2013).
Vasanthakumar, A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16, 276–285 (2015).
Bluestone, J. A. FOXP3, the transcription factor at the heart of the rebirth of immune tolerance. J. Immunol. 198, 979–980 (2017).
Van Gool, F. et al. A mutation in the transcription factor Foxp3 drives T helper 2 effector function in regulatory T cells. Immunity 50, 362–377 (2019).
Passerini, L. et al. CD4+ T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci. Transl Med. 5, 215ra174 (2013).
Takatori, H. et al. Helios enhances Treg cell function in cooperation with FoxP3. Arthritis Rheumatol. 67, 1491–1502 (2015).
Roychoudhuri, R. et al. BACH2 represses effector programs to stabilize Treg-mediated immune homeostasis. Nature 498, 506–510 (2013).
He, X. et al. Targeting PKC in human T cells using sotrastaurin (AEB071) preserves regulatory T cells and prevents IL-17 production. J. Invest. Dermatol. 134, 975–983 (2014).
Kwon, M. J., Ma, J., Ding, Y., Wang, R. & Sun, Z. Protein kinase C-theta promotes Th17 differentiation via upregulation of Stat3. J. Immunol. 188, 5887–5897 (2012).
Chen, Z. et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 (2013).
Gao, Y. et al. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc. Natl Acad. Sci. USA 112, E3246–E3254 (2015).
Tang, Q. et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 199, 1455–1465 (2004). Seminal study showing that antigen-specific T reg cells can be expanded ex vivo and cure T1D in mice.
Masteller, E. L. et al. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J. Immunol. 175, 3053–3059 (2005).
Hull, C. M. et al. Generation of human islet-specific regulatory T cells by TCR gene transfer. J. Autoimmun. 79, 63–73 (2017).
Kim, Y. C. et al. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood 125, 1107–1115 (2015).
Brusko, T. M. et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLOS ONE 5, e11726 (2010).
MacDonald, K. G. et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 126, 1413–1424 (2016).
Mekala, D. J. & Geiger, T. L. Immunotherapy of autoimmune encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Blood 105, 2090–2092 (2005). First study redirecting T reg cells using a chimeric receptor, designed to recognize autoreactive T cells in EAE.
Fuchs, A. et al. Minimum information about T regulatory cells: a step toward reproducibility and standardization. Front. Immunol. 8, 1844 (2017). This article provides guidelines on the minimum information required about T reg cells for therapy, in an effort to help standardize T reg cell manufacture.
Brunstein, C. G. et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 127, 1044–1051 (2016).
Seay, H. R. et al. Expansion of human Tregs from cryopreserved umbilical cord blood for GMP-compliant autologous adoptive cell transfer therapy. Mol. Ther. Methods Clin. Dev. 4, 178–191 (2017).
Dijke, I. E. et al. Discarded human thymus is a novel source of stable and long-lived therapeutic regulatory T cells. Am. J. Transpl. 16, 58–71 (2016).
Miyara, M. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 (2009).
Cuadrado, E. et al. Proteomic analyses of human regulatory T cells reveal adaptations in signaling pathways that protect cellular identity. Immunity 48, 1046–1059 (2018). A large study using transcriptomic and proteomic analyses of human T reg cells and T eff cells to derive a signature that uniquely identifies T reg cells that found that FOXP3 is the only molecule enriched in T reg cells at both the transcript level and the protein level.
Miragaia, R. J. et al. Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity 50, 493–504.e497 (2019).
Tang, Q. & Lee, K. Regulatory T-cell therapy for transplantation: how many cells do we need? Curr. Opin. Organ. Transpl. 17, 349–354 (2012).
Putnam, A. L. et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 58, 652–662 (2009). This article describes the good manufacturing practice manufacture of T reg cells isolated from patients with T1D for T reg cell therapy.
Trzonkowski, P. et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin. Immunol. 133, 22–26 (2009).
Mathew, J. M. et al. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci. Rep. 8, 7428 (2018).
Coenen, J. J., Koenen, H. J., van Rijssen, E., Hilbrands, L. B. & Joosten, I. Rapamycin, and not cyclosporin a, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood 107, 1018–1023 (2006).
Haxhinasto, S., Mathis, D. & Benoist, C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 (2008).
Battaglia, M., Stabilini, A. & Roncarolo, M. G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105, 4743–4748 (2005).
Lee, K., Nguyen, V., Lee, K. M., Kang, S. M. & Tang, Q. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am. J. Transpl. 14, 27–38 (2014).
Putnam, A. L. et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am. J. Transpl. 13, 3010–3020 (2013).
Banerjee, D. K., Dhodapkar, M. V., Matayeva, E., Steinman, R. M. & Dhodapkar, K. M. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood 108, 2655–2661 (2006).
Golovina, T. N. et al. CD28 costimulation is essential for human T regulatory expansion and function. J. Immunol. 181, 2855–2868 (2008).
Afzali, B. et al. Comparison of regulatory T cells in hemodialysis patients and healthy controls: implications for cell therapy in transplantation. Clin. J. Am. Soc. Nephrol. 8, 1396–1405 (2013).
Canavan, J. B. et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut 65, 584–594 (2016).
Hoffmann, P. et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood 108, 4260–4267 (2006).
Smigiel, K. S. et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 211, 121–136 (2014).
Santner-Nanan, B. et al. Accelerated age-dependent transition of human regulatory T cells to effector memory phenotype. Int. Immunol. 20, 375–383 (2008).
Miyao, T. et al. Plasticity of Foxp3+ T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 36, 262–275 (2012).
Ohkura, N. et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012).
Sehouli, J. et al. Epigenetic quantification of tumor-infiltrating T-lymphocytes. Epigenetics 6, 236–246 (2011).
Cohen, J. L., Trenado, A., Vasey, D., Klatzmann, D. & Salomon, B. L. CD4+CD25+ immunoregulatory T cells: new therapeutics for graft-versus-host disease. J. Exp. Med. 196, 401–406 (2002).
Edinger, M. et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 9, 1144–1150 (2003).
Martelli, M. F. et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 124, 638–644 (2014).
Theil, A. et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy 17, 473–486 (2015).
Di Ianni, M. et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928 (2011).
Joffre, O. et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat. Med. 14, 88–92 (2008).
Tang, Q. & Bluestone, J. A. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb. Perspect. Med. 3 (2013).
Safinia, N. et al. Cell therapy in organ transplantation: our experience on the clinical translation of regulatory T cells. Front. Immunol. 9, 354 (2018).
Chandran, S. et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am. J. Transpl. 17, 2945–2954 (2017).
Tang, Q. et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28, 687–697 (2008).
D’Alise, A. M. et al. The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors. Proc. Natl Acad. Sci. USA 105, 19857–19862 (2008).
Dall’Era, M. et al. Adoptive Treg cell therapy in a patient with systemic lupus erythematosus. Arthritis Rheumatol. 71, 431–440 (2018).
Thonhoff, J. R. et al. Expanded autologous regulatory T-lymphocyte infusions in ALS: A phase I, first-in-human study. Neurol. Neuroimmunol. Neuroinflamm. 5, e465 (2018).
Sadlack, B. et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 25, 3053–3059 (1995).
Furtado, G. C., Curotto de Lafaille, M. A., Kutchukhidze, N. & Lafaille, J. J. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J. Exp. Med. 196, 851–857 (2002).
Tang, Q. Therapeutic window of interleukin-2 for autoimmune diseases. Diabetes 64, 1912–1913 (2015).
Saadoun, D. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011).
Koreth, J. et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011).
He, J. et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 22, 991–993 (2016).
Todd, J. A. et al. Regulatory T cell responses in participants with type 1 diabetes after a single dose of interleukin-2: a non-randomised, open label, adaptive dose-finding trial. PLOS Med. 13, e1002139 (2016).
Rosenzwajg, M. et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J. Autoimmun. 58, 48–58 (2015).
Hartemann, A. et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 1, 295–305 (2013).
Trotta, E. et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat. Med. 24, 1005–1014 (2018).
Sockolosky, J. T. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 359, 1037–1042 (2018). This study reports the generation of an orthogonal IL-2–IL-2 receptor pair that could be used to trigger IL-2 signalling specifically in infused engineered T reg cells.
Furukawa, A., Wisel, S. A. & Tang, Q. Impact of immune-modulatory drugs on regulatory T cell. Transplantation 100, 2288–2300 (2016).
Tang, Q. & Vincenti, F. Transplant trials with Tregs: perils and promises. J. Clin. Invest. 127, 2505–2512 (2017).
Landwehr-Kenzel, S. et al. Ex vivo expanded natural regulatory T cells from patients with end-stage renal disease or kidney transplantation are useful for autologous cell therapy. Kidney Int. 93, 1452–1464 (2018).
Green, E. A., Choi, Y. & Flavell, R. A. Pancreatic lymph node-derived CD4+CD25+ Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity 16, 183–191 (2002). This article demonstrates that small numbers (2,000 cells) of pancreatic antigen-specific T reg cells exposed to TNF receptor signalling in vivo can prevent autoimmune diabetes in mice.
Huang, J. et al. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4+ T cells. Immunity 39, 846–857 (2013).
Bluhm, J. et al. CAR T Cells with enhanced sensitivity to B cell maturation antigen for the targeting of B cell non-Hodgkin’s lymphoma and multiple myeloma. Mol. Ther. 26, 1906–1920 (2018).
Elhanati, Y., Sethna, Z., Callan, C. G. Jr., Mora, T. & Walczak, A. M. Predicting the spectrum of TCR repertoire sharing with a data-driven model of recombination. Immunol. Rev. 284, 167–179 (2018).
Klein, L., Kyewski, B., Allen, P. M. & Hogquist, K. A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat. Rev. Immunol. 14, 377–391 (2014).
Jordan, M. S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 301–306 (2001).
Malchow, S. et al. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity 44, 1102–1113 (2016).
Anderson, M. S. et al. Projection of an immunological self shadow within the thymus by the Aire protein. Science 298, 1395–1401 (2002).
Hsieh, C. S., Zheng, Y., Liang, Y., Fontenot, J. D. & Rudensky, A. Y. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7, 401–410 (2006).
Leonard, J. D. et al. Identification of natural regulatory T cell epitopes reveals convergence on a dominant autoantigen. Immunity 47, 107–117 e108 (2017).
Yang, M. et al. Cutting edge: processing of oxidized peptides in macrophages regulates T cell activation and development of autoimmune arthritis. J. Immunol. 199, 3937–3942 (2017).
Moss, C. X., Matthews, S. P., Lamont, D. J. & Watts, C. Asparagine deamidation perturbs antigen presentation on class II major histocompatibility complex molecules. J. Biol. Chem. 280, 18498–18503 (2005).
Valesini, G. et al. Citrullination and autoimmunity. Autoimmun. Rev. 14, 490–497 (2015).
Patterson, H., Nibbs, R., McInnes, I. & Siebert, S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin. Exp. Immunol. 176, 1–10 (2014).
Delong, T. et al. Diabetogenic T-cell clones recognize an altered peptide of chromogranin A. Diabetes 61, 3239–3246 (2012).
McGinty, J. W., Marre, M. L., Bajzik, V., Piganelli, J. D. & James, E. A. T cell epitopes and post-translationally modified epitopes in type 1 diabetes. Curr. Diab. Rep. 15, 90 (2015).
Jin, N. et al. N-terminal additions to the WE14 peptide of chromogranin A create strong autoantigen agonists in type 1 diabetes. Proc. Natl Acad. Sci. USA 112, 13318–13323 (2015).
Delong, T. et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351, 711–714 (2016). This article shows that some diabetogenic T cells recognize epitopes formed by fusion of proinsulin peptides to other peptides that are found in the secretory vesicles of beta cells.
Liu, Z. et al. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature 528, 225–230 (2015).
Moran, A. E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Spence, A. et al. Revealing the specificity of regulatory T cells in murine autoimmune diabetes. Proc. Natl Acad. Sci. USA 115, 5265–5270 (2018).
Wei, X. et al. Reciprocal expression of IL-35 and IL-10 defines two distinct effector Treg subsets that are required for maintenance of immune tolerance. Cell Rep. 21, 1853–1869 (2017).
Seay, H. R. et al. Tissue distribution and clonal diversity of the T and B cell repertoire in type 1 diabetes. JCI Insight 1, e88242 (2016).
Howie, B. et al. High-throughput pairing of T cell receptor alpha and beta sequences. Sci. Transl Med. 7, 301ra131 (2015).
Glanville, J. et al. Identifying specificity groups in the T cell receptor repertoire. Nature 547, 94–98 (2017).
Dash, P. et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature 547, 89–93 (2017).
Dembic, Z. et al. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature 320, 232–238 (1986).
Tsang, J. Y. et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J. Clin. Invest. 118, 3619–3628 (2008).
Wright, G. P. et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc. Natl Acad. Sci. USA 106, 19078–19083 (2009).
Cohen, C. J. et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–3903 (2007).
Kuball, J. et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 109, 2331–2338 (2007).
Sommermeyer, D. & Uckert, W. Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J. Immunol. 184, 6223–6231 (2010).
Bialer, G., Horovitz-Fried, M., Ya’acobi, S., Morgan, R. A. & Cohen, C. J. Selected murine residues endow human TCR with enhanced tumor recognition. J. Immunol. 184, 6232–6241 (2010).
Uckert, W. & Schumacher, T. N. TCR transgenes and transgene cassettes for TCR gene therapy: status in 2008. Cancer Immunol. Immunother. 58, 809–822 (2009).
Torikai, H. et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119, 5697–5705 (2012).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018).
Govers, C. et al. TCRs genetically linked to CD28 and CD3epsilon do not mispair with endogenous TCR chains and mediate enhanced T cell persistence and anti-melanoma activity. J. Immunol. 193, 5315–5326 (2014).
Walseng, E. et al. A TCR-based chimeric antigen receptor. Sci. Rep. 7, 10713 (2017).
Elahi, R., Khosh, E., Tahmasebi, S. & Esmaeilzadeh, A. Immune cell hacking: challenges and clinical approaches to create smarter generations of chimeric antigen receptor T cells. Front. Immunol. 9, 1717 (2018).
Maher, J., Brentjens, R. J., Gunset, G., Riviere, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Deeks, S. G. et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol. Ther. 5, 788–797 (2002).
Walker, R. E. et al. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood 96, 467–474 (2000).
Leibman, R. S. et al. Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLOS Pathog. 13, e1006613 (2017).
Irving, B. A. & Weiss, A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64, 891–901 (1991). Seminal study reporting the first CAR.
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Carpenito, C. et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl Acad. Sci. USA 106, 3360–3365 (2009).
Hombach, A. A., Heiders, J., Foppe, M., Chmielewski, M. & Abken, H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4+ T cells. Oncoimmunology 1, 458–466 (2012).
Mekala, D. J., Alli, R. S. & Geiger, T. L. IL-10-dependent infectious tolerance after the treatment of experimental allergic encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Proc. Natl Acad. Sci. USA 102, 11817–11822 (2005).
Elinav, E., Adam, N., Waks, T. & Eshhar, Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology 136, 1721–1731 (2009).
Elinav, E., Waks, T. & Eshhar, Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology 134, 2014–2024 (2008).
Blat, D., Zigmond, E., Alteber, Z., Waks, T. & Eshhar, Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol. Ther. 22, 1018–1028 (2014).
Skuljec, J. et al. Chimeric antigen receptor-redirected regulatory T cells suppress experimental allergic airway inflammation, a model of asthma. Front. Immunol. 8, 1125 (2017).
Smithson, J. E., Warren, B. F., Young, S., Pigott, R. & Jewell, D. P. Heterogeneous expression of carcinoembryonic antigen in the normal colon and upregulation in active ulcerative colitis. J. Pathol. 180, 146–151 (1996).
Hombach, A. A., Kofler, D., Rappl, G. & Abken, H. Redirecting human CD4+CD25+ regulatory T cells from the peripheral blood with pre-defined target specificity. Gene Ther. 16, 1088–1096 (2009).
Lee, J. C. et al. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 71, 2871–2881 (2011).
Ferreira, L. M. & Tang, Q. Generating antigen-specific regulatory T cells in the fast lane. Am. J. Transpl. 17, 851–853 (2017).
Noyan, F. et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am. J. Transpl. 17, 917–930 (2017).
Boardman, D. A. et al. Expression of a chimeric antigen receptor specific for donor HLA Class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am. J. Transpl. 17, 931–943 (2017).
Semple, K., Yu, Y., Wang, D., Anasetti, C. & Yu, X. Z. Efficient and selective prevention of GVHD by antigen-specific induced Tregs via linked-suppression in mice. Biol. Blood Marrow Transpl. 17, 309–318 (2011).
Dawson, N. A. et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight 4, 123672 (2019).
Peyvandi, F., Garagiola, I. & Young, G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet 388, 187–197 (2016).
Yoon, J. et al. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood 129, 238–245 (2017).
Zhang, A. H., Yoon, J., Kim, Y. C. & Scott, D. W. Targeting antigen-specific B cells using antigen-expressing transduced regulatory T cells. J. Immunol. 201, 1434–1441 (2018).
Mathsson, L. et al. Antibodies against citrullinated vimentin in rheumatoid arthritis: higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 58, 36–45 (2008).
Raffin, C. et al. Development of citrullinated-vimentin-specific CAR for targeting Tregs to treat autoimmune rheumatoid arthritis. J. Immunol. 196, 210.19 (2016).
Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl Med. 5, 178ra140 (2013).
Loskog, A. et al. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia 20, 1819–1828 (2006).
Kofler, D. M. et al. CD28 costimulation impairs the efficacy of a redirected T-cell antitumor attack in the presence of regulatory T cells which can be overcome by preventing Lck activation. Mol. Ther. 19, 760–767 (2011).
van der Stegen, S. J., Hamieh, M. & Sadelain, M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 14, 499–509 (2015).
Nowak, A. et al. CD137+CD154- expression as a regulatory T cell (Treg)-specific activation signature for identification and sorting of stable human Tregs from in vitro expansion cultures. Front. Immunol. 9, 199 (2018).
Koristka, S. et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J. Autoimmun. 90, 116–131 (2018).
Boroughs, A. C. et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight 5, 126194 (2019).
Ferreira, L. M. et al. Tailoring a new generation of chimeric antigen receptors for regulatory T cells. J. Immunol. 200, 176.115 (2018).
Hudecek, M. et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 19, 3153–3164 (2013).
Helsen, C. W. et al. The chimeric TAC receptor co-opts the T cell receptor yielding robust anti-tumor activity without toxicity. Nat. Commun. 9, 3049 (2018).
Chang, Z. L. et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 14, 317–324 (2018).
Cho, J. H., Collins, J. J. & Wong, W. W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438 (2018).
Roybal, K. T. et al. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 167, 419–432 .e416, (2016).This article describes the development of synthetic Notch signalling to control gene circuits in human T cells.
Leen, A. M. et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 22, 1211–1220 (2014).
Chaudhry, A. et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34, 566–578 (2011).
Schukur, L., Geering, B., Charpin-El Hamri, G. & Fussenegger, M. Implantable synthetic cytokine converter cells with AND-gate logic treat experimental psoriasis. Sci. Transl Med. 7, 318ra201 (2015).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Mandal, P. K. et al. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15, 643–652 (2014). First report of CRISPR–Cas9 genome editing in human T cells.
Schumann, K. et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl Acad. Sci. USA 112, 10437–10442 (2015).
Gomes-Silva, D. et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 130, 285–296 (2017).
Rupp, L. J. et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 7, 737 (2017).
Liu, X. et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 27, 154–157 (2017).
Kosicki, M., Tomberg, K. & Bradley, A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 36, 765–771 (2018).
Konermann, S. et al. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 173, 665–676 (2018).
Liu, X. S. et al. Editing DNA methylation in the mammalian genome. Cell 167, 233–247.e217, (2016).
Matharu, N. et al. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 363, eaau0629 (2019).
Milone, M. C. & O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 32, 1529–1541 (2018).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017). This study shows that knocking the CAR gene cassette into the endogenous TCR locus in T cells leads to CAR T cells with physiologically regulated CAR expression, less variability in CAR expression levels across patients and reduced exhaustion upon repeated antigen stimulation.
Naso, M. F., Tomkowicz, B., Perry, W. L. 3rd & Strohl, W. R. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 31, 317–334 (2017).
Wang, J. et al. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 44, e30 (2016).
Paulk, N. K. et al. Bioengineered AAV capsids with combined high human liver transduction in vivo and unique humoral seroreactivity. Mol. Ther. 26, 289–303 (2018).
Deverman, B. E., Ravina, B. M., Bankiewicz, K. S., Paul, S. M. & Sah, D. W. Y. Gene therapy for neurological disorders: progress and prospects. Nat. Rev. Drug Discov. 17, 641–659 (2018).
Bak, R. O., Dever, D. P. & Porteus, M. H. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat. Protoc. 13, 358–376 (2018).
Tripathi, D. et al. c-Jun N-terminal kinase 1 defective CD4+CD25+FoxP3+ cells prolong islet allograft survival in diabetic mice. Sci. Rep. 8, 3310 (2018).
Hoffmann, P. et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 39, 1088–1097 (2009).
Rudra, D. et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat. Immunol. 13, 1010–1019 (2012).
Chinen, T. et al. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 17, 1322–1333 (2016).
Zhang, C. et al. ‘Repair’ Treg cells in tissue injury. Cell Physiol. Biochem. 43, 2155–2169 (2017).
Arpaia, N. et al. A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019).
Han, X. et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl Acad. Sci. USA 116, 10441–10446 (2019). This article describes the development of gene-edited hypoimmunogenic human pluripotent stem cells, which could in the future be used as an inexhaustible source for universally compatible T reg cells.
Haque, R. et al. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J. Immunol. 189, 1228–1236 (2012).
Booth, N. J. et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J. Immunol. 184, 4317–4326 (2010).
Gavin, M. A., Clarke, S. R., Negrou, E., Gallegos, A. & Rudensky, A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat. Immunol. 3, 33–41 (2002).
Walker, L. S., Chodos, A., Eggena, M., Dooms, H. & Abbas, A. K. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198, 249–258 (2003).
Gregori, S. & Roncarolo, M. G. Engineered t regulatory type 1 cells for clinical application. Front. Immunol. 9, 233 (2018).
Gagliani, N. et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19, 739–746 (2013).
Barrat, F. J. et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 195, 603–616 (2002).
Gregori, S., Roncarolo, M. G. & Bacchetta, R. Methods for in vitro generation of human type 1 regulatory T cells. Methods Mol Biol 677, 31–46 (2011).
Desreumaux, P. et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 143, 1207–1217.e1202 (2012).
Bacchetta, R. et al. Immunological outcome in haploidentical-HSC transplanted patients treated with IL-10-anergized donor T cells. Front. Immunol. 5, 16 (2014).
Gershon, R. K., Cohen, P., Hencin, R. & Liebhaber, S. A. Suppressor T cells. J. Immunol. 108, 586–590 (1972).
Cantor, H., Shen, F. W. & Boyse, E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J. Exp. Med. 143, 1391–1340 (1976).
Bezie, S. et al. Ex vivo expanded human non-cytotoxic CD8+CD45RClow/- Tregs efficiently delay skin graft rejection and GVHD in humanized mice. Front. Immunol. 8, 2014 (2017).
Ablamunits, V., Bisikirska, B. & Herold, K. C. Acquisition of regulatory function by human CD8+ T cells treated with anti-CD3 antibody requires TNF. Eur J. Immunol. 40, 2891–2901 (2010).
Kim, H. J. et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 350, 334–339 (2015).
Poussier, P., Ning, T., Banerjee, D. & Julius, M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J. Exp. Med. 195, 1491–1497 (2002).
Tang, X. et al. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha+TCRalphabeta+ T cells. J. Immunol. 177, 7645–7655 (2006).
Chen, Y., Kuchroo, V. K., Inobe, J., Hafler, D. A. & Weiner, H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265, 1237–1240 (1994).
Carrier, Y., Yuan, J., Kuchroo, V. K. & Weiner, H. L. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J. Immunol. 178, 179–185 (2007).
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015).
Katz, S. I., Parker, D. & Turk, J. L. B-cell suppression of delayed hypersensitivity reactions. Nature 251, 550–551 (1974).
Wolf, S. D., Dittel, B. N., Hardardottir, F. & Janeway, C. A. Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 184, 2271–2278 (1996).
Fillatreau, S., Sweenie, C. H., McGeachy, M. J., Gray, D. & Anderton, S. M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3, 944–950 (2002).
Mizoguchi, A., Mizoguchi, E., Takedatsu, H., Blumberg, R. S. & Bhan, A. K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16, 219–230 (2002).
Nathan, C. & Ding, A. Nonresolving inflammation. Cell 140, 871–882 (2010).
Carter, N. A. et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J. Immunol. 186, 5569–5579 (2011).
Flores-Borja, F. et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl Med. 5, 173ra123 (2013).
Ray, A., Basu, S., Williams, C. B., Salzman, N. H. & Dittel, B. N. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J. Immunol. 188, 3188–3198 (2012).
Lee, K. M. et al. TGF-beta-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J. Immunol. 44, 1728–1736 (2014).
Kuwana, Y. et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 149, 960–968 (1987).
Gross, G., Waks, T. & Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10024–10028 (1989).
Thornton, A. M. & Shevach, E. M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188, 287–296 (1998).
Levings, M. K., Sangregorio, R. & Roncarolo, M. G. Human Cd25+Cd4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193, 1295–1302 (2001).
Jonuleit, H. et al. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193, 1285–1294 (2001).
Dieckmann, D., Plottner, H., Berchtold, S., Berger, T. & Schuler, G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193, 1303–1310 (2001).
Ng, W. F. et al. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood 98, 2736–2744 (2001).
Stephens, L. A., Mottet, C., Mason, D. & Powrie, F. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31, 1247–1254 (2001).
Baecher-Allan, C., Brown, J. A., Freeman, G. J. & Hafler, D. A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167, 1245–1253 (2001). Together with Levings et al. (2001), Jonuleit et al. (2001), Dieckmann et al. (2001), Ng et al. (2001) and Stephens et al. (2001), this article is a seminal study, the first to describe T reg cells in humans.
Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Hoffmann, P., Eder, R., Kunz-Schughart, L. A., Andreesen, R. & Edinger, M. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood 104, 895–903 (2004).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Stone, J. D., Chervin, A. S. & Kranz, D. M. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology 126, 165–176 (2009).
Liu, X. et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 75, 3596–3607 (2015).
Irvine, D. J., Purbhoo, M. A., Krogsgaard, M. & Davis, M. M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Watanabe, K. et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J. Immunol. 194, 911–920 (2015).
Stone, J. D., Aggen, D. H., Schietinger, A., Schreiber, H. & Kranz, D. M. A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell engagers (BiTEs). Oncoimmunology 1, 863–873 (2012).
Walker, A. J. et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol. Ther. 25, 2189–2201 (2017).
Acknowledgements
L.M.R.F. is the Jeffrey G. Klein Family Diabetes Fellow. Y.D.M. was supported by the Swiss National Science Foundation (Advanced Postdoctoral Mobility grant no. P300PB_174500). J.A.B. acknowledges support of the Sean N. Parker Autoimmune Laboratory. The authors apologize to all of the researchers whose work they could not cite owing to reference number limitations.
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the article. L.M.R.F. and Y.D.M. contributed equally.
Corresponding authors
Ethics declarations
Competing interests
J.A.B. and Q.T. acknowledge support from the Juvenile Diabetes Research Foundation, Caladrius Biosciences, Becton, Dickinson and Company (BD), Juno Therapeutics and Pfizer. L.M.R.F. has patents and pending patents on genome editing of T cells, haematopoietic stem cells and pluripotent stem cells. J.A.B. and Q.T. have patents and pending patents on polyclonal and antigen-specific regulatory T cells and consult for Third Rock Ventures and Juno Therapeutics in this area. J.A.B. serves on the board of Pfizer. Y.D.M. has no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Caladrius Biosciences Treg cell phase II trial: https://www.evaluate.com/vantage/articles/interviews/caladrius-refuses-give-tregs-diabetes
ClinicalTrials.gov: https://clinicaltrials.gov/
CRISPR Therapeutics press release (25 February 2019): http://ir.crisprtx.com/news-releases/news-release-details/crispr-therapeutics-and-vertex-announce-progress-clinical
IMGT: http://www.imgt.org
Glossary
- Anergy
-
Peripheral mechanism for tolerizing T cells in which they are blocked at the G1 phase of the cell cycle and unable to proliferate.
- Systemic lupus erythematosus
-
A group of chronic autoimmune disorders defined by inflammation affecting various connective tissues in various organs, including the skin, joints, kidney, lung, nervous system or haematopoietic system.
- Autoimmune polyendocrine syndromes
-
A group of diseases characterized by loss of tolerance and inflammation in endocrine glands, including the thyroid, parathyroid and adrenal glands or the pancreas. They are frequently associated with alopecia, vitiligo, coeliac disease and autoimmune gastritis.
- Immunodysregulation polyendocrinopathy enteropathy X-linked syndrome
-
Specific form of an inherited autoimmune polyendocrine syndrome characterized by a mutation or mutations in the master transcription factor forkhead box protein P3 gene (FOXP3), leading to regulatory T cell dysfunction.
- Type 1 T helper cell
-
(TH1 cell). A type of CD4+ T helper cell expressing TBET as a key transcription factor and defined by its ability to preferentially secrete interferon-γ and induce CD8+ T cell and macrophage activation.
- Type 2 T helper cell
-
(TH2 cell). A type of CD4+ T helper cell expressing GATA3 as a key transcription factor and defined by its ability to preferentially secrete Il-4, IL-5 and IL-13 and promote B cell expansion and antibody class switching.
- IL-17-producing T helper cell
-
(TH17 cell). A type of CD4+ T helper cell expressing RORɣt as a key transcription factor and defined by the production of IL-17, a cytokine important for maintaining mucosal barrier integrity and clearing helminth infections.
- Mixed lymphocyte reactions
-
In vitro tests consisting of mixing different subsets of T cells together in the presence of antigen-presenting cells.
- K562 cell-based artificial APCs
-
K562 cells are an immortalized human leukocyte antigen (HLA)-deficient cell line initially isolated from a chronic myelogenous leukaemia. Artificial antigen-presenting cells (APCs) are K562 cells that have been gene edited to express CD80 and/or CD86 and specific HLA alleles to function as APCs.
- C-peptide
-
Short polypeptide connecting the A chain of proinsulin to the B chain. After packaging in vesicles in pancreatic beta cells, C-peptide is removed from proinsulin, leaving the A chain and the B chain linked by a disulfide bridge. Blood C-peptide levels are used to monitor endogenous insulin expression in patients with diabetes.
- Amyotrophic lateral sclerosis
-
Neurodegenerative disorder characterized by the progressive loss of motor neurons, causing muscle weakness, atrophy and eventually death.
- Pemphigus vulgaris
-
Group of skin disorders characterized by the formation of blisters induced by autoantibodies targeting intercellular adhesion molecules on keratinocytes.
- Guillain–Barré syndrome
-
Immune-mediated polyneuropathy, usually started after an infection, sharing cross-reactive epitopes with peripheral nerves (myelin or axonal membrane).
- Alzheimer’s disease
-
Neurogenerative disorder leading to progressive dementia associated with the accumulation of amyloid-β plaques in nervous cells of the central nervous system.
- Marginal zone
-
High-transit area constituted by B cells, macrophages, dendritic cells and granulocytes, interposed between lymphoid tissues and the circulation, serving as a sentinel for blood-borne antigens.
- HLA restriction
-
The presentation of a peptide by a specific human leukocyte antigen (HLA) to a T cell’s receptor.
- Complementarity-determining regions
-
Parts of the variable chain of the T cell receptor or of an antibody that determine specificity to their cognate antigen.
- Tonic signalling
-
Low level of signalling independent of activating antigen in resting T cells.
Rights and permissions
About this article
Cite this article
Ferreira, L.M.R., Muller, Y.D., Bluestone, J.A. et al. Next-generation regulatory T cell therapy. Nat Rev Drug Discov 18, 749–769 (2019). https://doi.org/10.1038/s41573-019-0041-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-019-0041-4
This article is cited by
-
Multiply restimulated human cord blood-derived Tregs maintain stabilized phenotype and suppressive function and predict their therapeutic effects on autoimmune diabetes
Diabetology & Metabolic Syndrome (2024)
-
GMP-manufactured CRISPR/Cas9 technology as an advantageous tool to support cancer immunotherapy
Journal of Experimental & Clinical Cancer Research (2024)
-
Biomaterials to enhance adoptive cell therapy
Nature Reviews Bioengineering (2024)
-
Disulfiram treatment suppresses antibody-producing reactions by inhibiting macrophage activation and B cell pyrimidine metabolism
Communications Biology (2024)
-
The immunology of type 1 diabetes
Nature Reviews Immunology (2024)