Abstract
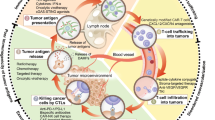
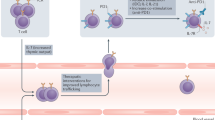
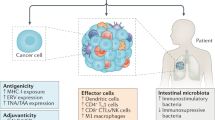
Immunotherapies are the most rapidly growing drug class and have a major impact in oncology and on human health. It is increasingly clear that the effectiveness of immunomodulatory strategies depends on the presence of a baseline immune response and on unleashing of pre-existing immunity. Therefore, a general consensus emerged on the central part played by effector T cells in the antitumour responses. Recent technological, analytical and mechanistic advances in immunology have enabled the identification of patients who are more likely to respond to immunotherapy. In this Review, we focus on defining hot, altered and cold tumours, the complexity of the tumour microenvironment, the Immunoscore and immune contexture of tumours, and we describe approaches to treat such tumours with combination immunotherapies, including checkpoint inhibitors. In the upcoming era of combination immunotherapy, it is becoming critical to understand the mechanisms responsible for hot, altered or cold immune tumours in order to boost a weak antitumour immunity. The impact of combination therapy on the immune response to convert an immune cold into a hot tumour will be discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shankaran, V. et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006). This article is the first demonstration of the dependence of tumour progression and invasion on the intratumoural adaptive immunity. T cell infiltrates and IFNγ signatures have predictive value superior to TNM with respect to the natural history of primary cancers.
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014). This article shows that the baseline density and location at the invasive margin of T cells in metastatic melanomas predicts the treatment outcome of patients receiving PD-1-targeting therapies.
Van Allen, E. M. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015). This article is the first to show that patients with metastatic melanoma with high mutational burden, neoantigen load and expression of cytolytic markers in their tumours are more likely to respond to anti-CTLA4 immunotherapy.
Huang, A. C. et al. T cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017).
Mlecnik, B. et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 29, 610–618 (2011).
Galon, J., Angell, H. K., Bedognetti, D. & Marincola, F. M. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39, 11–26 (2013).
Angell, H. & Galon, J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr. Opin. Immunol. 25, 261–267 (2013).
Galluzzi, L. et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology 1, 1111–1134 (2012).
Galon, J., Fridman, W. H. & Pages, F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 67, 1883–1886 (2007).
Pages, F. et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 27, 5944–5951 (2009).
Galon, J. et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 232, 199–209 (2014).
Pages, F. et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139 (2018). This article validates Immunoscore as a consensus and standardized cytotoxic T cell assay that defines immune hot, altered and cold tumours and has a greater prognostic value in CRC than T stage, N stage, lymphovascular invasion, tumour differentiation and MSI status.
Camus, M. et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 69, 2685–2693 (2009). This paper presents the first description of the immune hot (optimal), altered-excluded, altered-immunosuppressed and cold (absent) tumours.
Mlecnik, B. et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci. Transl Med. 6, 228ra37 (2014).
Mlecnik, B. et al. Integrative analyses of colorectal cancer show Immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 44, 698–711 (2016).
Boland, C. R. & Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 138, 2073–2087 (2010).
Gajewski, T. F. et al. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Adv. Exp. Med. Biol. 1036, 19–31 (2017).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874 (2016).
Angelova, M. et al. Evolution of metastases in space and time under immune selection. Cell 175, 601 (2018).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013). This article is the first to describe the immunome from immune signatures of purified immune cell subpopulations applied to human tumours. Immune infiltrate composition changes at each tumour stage and T, B and T FH cells have a major impact on survival.
Gu-Trantien, C. et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2, 91487 (2017).
Chew, V. et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 61, 427–438 (2012).
Gajewski, T. F., Schreiber, H. & Fu, Y. X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022 (2013).
Goc, J. et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+T cells. Cancer Res. 74, 705–715 (2014).
Ingels, A. et al. T-helper 1 immunoreaction influences survival in muscle-invasive bladder cancer: proof of concept. Ecancermedicalscience 8, 486 (2014).
Mulligan, A. M., Pinnaduwage, D., Tchatchou, S., Bull, S. B. & Andrulis, I. L. Validation of intratumoral T-bet+lymphoid cells as predictors of disease-free survival in breast cancer. Cancer Immunol. Res. 4, 41–48 (2016).
Mulligan, A. M. et al. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin. Cancer Res. 19, 336–346 (2013).
Cristescu, R. et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362, eaar3593 (2018).
Cheon, H., Borden, E. C. & Stark, G. R. Interferons and their stimulated genes in the tumor microenvironment. Semin. Oncol. 41, 156–173 (2014).
Lin, C. F. et al. Escape from IFN-gamma-dependent immunosurveillance in tumorigenesis. J. Biomed. Sci. 24, 10 (2017).
Zaidi, M. R. & Merlino, G. The two faces of interferon-gamma in cancer. Clin. Cancer Res. 17, 6118–6124 (2011).
Mandai, M. et al. Dual faces of IFNgamma in cancer progression: a role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin. Cancer Res. 22, 2329–2334 (2016).
Snell, L. M., McGaha, T. L. & Brooks, D. G. Type I interferon in chronic virus infection and cancer. Trends Immunol. 38, 542–557 (2017).
Minn, A. J. & Wherry, E. J. Combination cancer therapies with immune checkpoint blockade: convergence on interferon signaling. Cell 165, 272–275 (2016).
Benci, J. L. et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 167, 1540–1554 (2016).
Sun, T. et al. Inhibition of tumor angiogenesis by interferon-gamma by suppression of tumor-associated macrophage differentiation. Oncol. Res. 21, 227–235 (2014).
Kim, H. J. & Cantor, H. CD4 T cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer Immunol. Res. 2, 91–98 (2014).
Placek, K., Coffre, M., Maiella, S., Bianchi, E. & Rogge, L. Genetic and epigenetic networks controlling T helper 1 cell differentiation. Immunology 127, 155–162 (2009).
Kato, D. et al. Prospects for personalized combination immunotherapy for solid tumors based on adoptive cell therapies and immune checkpoint blockade therapies. Nihon Rinsho Meneki Gakkai Kaishi 40, 68–77 (2017).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015). This article shows that a melanoma cell-intrinsic oncogenic pathway (active β-catenin signalling) contributes to a lack of T cell infiltration in tumour sites and resistance to anti-PD-L1 and/or anti-CTLA4 mAb therapy.
Yaguchi, T. et al. Immune suppression and resistance mediated by constitutive activation of Wnt/beta-catenin signaling in human melanoma cells. J. Immunol. 189, 2110–2117 (2012).
Sumimoto, H., Imabayashi, F., Iwata, T. & Kawakami, Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 203, 1651–1656 (2006).
Sumimoto, H. et al. Inhibition of growth and invasive ability of melanoma by inactivation of mutated BRAF with lentivirus-mediated RNA interference. Oncogene 23, 6031–6039 (2004).
Iwata-Kajihara, T. et al. Enhanced cancer immunotherapy using STAT3-depleted dendritic cells with high Th1-inducing ability and resistance to cancer cell-derived inhibitory factors. J. Immunol. 187, 27–36 (2011).
Nishio, H. et al. Immunosuppression through constitutively activated NF-kappaB signalling in human ovarian cancer and its reversal by an NF-kappaB inhibitor. Br. J. Cancer 110, 2965–2974 (2014).
Mlecnik, B. et al. Comprehensive intrametastatic immune quantification and major impact of immunoscore on survival. J. Natl Cancer Inst. 110, 97–108 (2018).
Zhang, A. W. et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell 173, 1755–1769 (2018).
Yoshida, M. et al. Modification of the tumor microenvironment in KRAS or c-MYC-induced ovarian cancer-associated peritonitis. PLOS ONE 11, e0160330 (2016).
McFarland, C. D. et al. The damaging effect of passenger mutations on cancer progression. Cancer Res. 77, 4763–4772 (2017).
Tauriello, D. V. F. et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Zitvogel, L. & Kroemer, G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 1, 1223–1225 (2012).
Mlecnik, B. et al. Biomolecular network reconstruction identifies T cell homing factors associated with survival in colorectal cancer. Gastroenterology 138, 1429–1440 (2010).
van der Woude, L. L., Gorris, M. A. J., Halilovic, A., Figdor, C. G. & de Vries, I. J. M. Migrating into the tumor: a roadmap for T cells. Trends Cancer 3, 797–808 (2017).
Ahmadzadeh, M. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009).
Gros, A. et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014).
Ji, R. R. et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol. Immunother. 61, 1019–1031 (2012).
Eroglu, Z. et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553, 347–350 (2018).
Blank, C. U., Haanen, J. B., Ribas, A. & Schumacher, T. N. The “cancer immunogram”. Science 352, 658–660 (2016).
Wieland, A. et al. T cell receptor sequencing of activated CD8 T cells in the blood identifies tumor-infiltrating clones that expand after PD-1 therapy and radiation in a melanoma patient. Cancer Immunol. Immunother. 67, 1767–1776 (2018).
Simon, S. et al. Emergence of high-avidity Melan-A-specific clonotypes as a reflection of anti-PD-1 clinical efficacy. Cancer Res. 77, 7083–7093 (2017).
Riaz, N. et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171, 934–949 (2017).
Fife, B. T. et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1–PD-L1 pathway. J. Exp. Med. 203, 2737–2747 (2006).
Whiteside, T. L., Demaria, S., Rodriguez-Ruiz, M. E., Zarour, H. M. & Melero, I. Emerging opportunities and challenges in cancer immunotherapy. Clin. Cancer Res. 22, 1845–1855 (2016).
Weng, N. P., Araki, Y. & Subedi, K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat. Rev. Immunol. 12, 306–315 (2012).
Durgeau, A., Virk, Y., Corgnac, S. & Mami-Chouaib, F. Recent advances in targeting CD8 T-Cell immunity for more effective cancer immunotherapy. Front. Immunol. 9, 14 (2018).
Mlecnik, B. et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl Med. 8, 327ra26 (2016).
Pages, F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353, 2654–2666 (2005).
Church, S. E. & Galon, J. Tumor microenvironment and immunotherapy: the whole picture is better than a glimpse. Immunity 43, 631–633 (2015).
Demaria, S., Coleman, C. N. & Formenti, S. C. Radiotherapy: changing the game in immunotherapy. Trends Cancer 2, 286–294 (2016).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Koyama, S. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 7, 10501 (2016).
Shayan, G. et al. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology 6, e1261779 (2017).
Granier, C. et al. Tim-3 expression on tumor-infiltrating PD-1(+)CD8(+) T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. 77, 1075–1082 (2017).
Hellmann, M. D., Friedman, C. F. & Wolchok, J. D. Combinatorial cancer immunotherapies. Adv. Immunol. 130, 251–277 (2016).
Eggermont, A. M. M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 378, 1789–1801 (2018).
Buchbinder, E. I. & Desai, A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 39, 98–106 (2016).
Taube, J. M. Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade. Oncoimmunology 3, e963413 (2014).
Taube, J. M. et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod. Pathol. 31, 214–234 (2018).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378, 2093–2104 (2018).
Du, W. et al. TIM-3 as a target for cancer immunotherapy and mechanisms of action. Int. J. Mol. Sci. 18, E645 (2017).
Manieri, N. A., Chiang, E. Y. & Grogan, J. L. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol. 38, 20–28 (2017).
Sedy, J. R. et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 6, 90–98 (2005).
Gao, J. et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 23, 551–555 (2017).
Stanczak, M. A. et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J. Clin. Invest. https://doi.org/10.1172/JCI120612 (2018).
Buchan, S. L., Rogel, A. & Al-Shamkhani, A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 131, 39–48 (2018).
Esensten, J. H., Helou, Y. A., Chopra, G., Weiss, A. & Bluestone, J. A. CD28 costimulation: from mechanism to therapy. Immunity 44, 973–988 (2016).
Hui, E. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017).
Kamphorst, A. O. et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355, 1423–1427 (2017).
Sanmamed, M. F. et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin. Oncol. 42, 640–655 (2015).
Hunig, T. The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nat. Rev. Immunol. 12, 317–318 (2012).
Elpek, K. et al. Efficacy of anti-ICOS agonist monoclonal antibodies in preclinical tumor models provides a rationale for clinical development as cancer immunotherapeutics. Cancer Immunol. Res. 4, A059 (2016).
Chaudhary, B. & Elkord, E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines 4, E28 (2016).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2017).
Snyder, A., Pamer, E. & Wolchok, J. Could microbial therapy boost cancer immunotherapy? Science 350, 1031–1032 (2015).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2017).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Nolz, J. C. Molecular mechanisms of CD8(+) T cell trafficking and localization. Cell. Mol. Life Sci. 72, 2461–2473 (2015).
Nagarsheth, N., Wicha, M. S. & Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 17, 559–572 (2017).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015).
Nagarsheth, N. et al. PRC2 epigenetically silences Th1-type chemokines to suppress effector T-cell trafficking in colon cancer. Cancer Res. 76, 275–282 (2016).
Huang, Y. et al. CD4+and CD8+T cells have opposing roles in breast cancer progression and outcome. Oncotarget 6, 17462–17478 (2015).
Sweis, R. F. et al. Molecular drivers of the non-T cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol. Res. 4, 563–568 (2016).
Tang, H. et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 29, 285–296 (2016).
Venning, F. A., Wullkopf, L. & Erler, J. T. Targeting ECM disrupts cancer progression. Front. Oncol. 5, 224 (2015).
Huang, Y., Goel, S., Duda, D. G., Fukumura, D. & Jain, R. K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 73, 2943–2948 (2013).
Carmeliet, P. & Jain, R. K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 10, 417–427 (2011).
Lanitis, E., Irving, M. & Coukos, G. Targeting the tumor vasculature to enhance T cell activity. Curr. Opin. Immunol. 33, 55–63 (2015).
Tan, L. Y. et al. Control of immune cell entry through the tumour vasculature: a missing link in optimising melanoma immunotherapy? Clin. Transl Immunol. 6, e134 (2017).
Wigerup, C., Pahlman, S. & Bexell, D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 164, 152–169 (2016).
Serra, S. et al. Adenosine signaling mediates hypoxic responses in the chronic lymphocytic leukemia microenvironment. Blood Adv. 1, 47–61 (2016).
Stagg, J. & Smyth, M. J. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 29, 5346–5358 (2010).
Antonioli, L., Blandizzi, C., Pacher, P. & Hasko, G. Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer 13, 842–857 (2013).
Stagg, J. et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 71, 2892–2900 (2011).
Antonioli, L., Yegutkin, G. G., Pacher, P., Blandizzi, C. & Hasko, G. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer 2, 95–109 (2016).
Hayes, G. M. et al. CD39 is a promising therapeutic antibody target for the treatment of soft tissue sarcoma. Am. J. Transl Res. 7, 1181–1188 (2015).
Sun, X. et al. Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/ENTPD1 null mice. Hepatology 57, 205–216 (2013).
Leone, R. D. & Emens, L. A. Targeting adenosine for cancer immunotherapy. J. Immunother. Cancer 6, 57 (2018).
Yu, T., Tang, B. & Sun, X. Development of inhibitors targeting hypoxia-inducible factor 1 and 2 for cancer therapy. Yonsei Med. J. 58, 489–496 (2017).
Leone, R. D., Lo, Y. C. & Powell, J. D. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput. Struct. Biotechnol. J. 13, 265–272 (2015).
Cardones, A. R. & Banez, L. L. VEGF inhibitors in cancer therapy. Curr. Pharm. Des. 12, 387–394 (2006).
Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Rajabi, M. & Mousa, S. A. The role of angiogenesis in cancer treatment. Biomedicines 5, 34 (2017).
Ebos, J. M. et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15, 232–239 (2009).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Goel, S., Wong, A. H. & Jain, R. K. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb. Perspect. Med. 2, a006486 (2012).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017).
Sabat, R. et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 21, 331–344 (2010).
Yang, L. TGFbeta a potent regulator of tumor microenvironment and host immune response, implication for therapy. Curr. Mol. Med. 10, 374–380 (2010).
Zhang, H., Wang, Y., Hwang, E. S. & He, Y. W. Interleukin-10: an immune-activating cytokine in cancer immunotherapy. J. Clin. Oncol. 34, 3576–3578 (2016).
Neuzillet, C. et al. Targeting the TGFbeta pathway for cancer therapy. Pharmacol. Ther. 147, 22–31 (2015).
Lan, Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-beta. Sci. Transl Med. 10, eaan5488 (2018).
Shimabukuro-Vornhagen, A. et al. The immunosuppressive factors IL-10, TGF-beta, and VEGF do not affect the antigen-presenting function of CD40-activated B cells. J. Exp. Clin. Cancer Res. 31, 47 (2012).
Gabrilovich, D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat. Rev. Immunol. 4, 941–952 (2004).
Eil, R. et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 537, 539–543 (2016).
Richards, C. H., Mohammed, Z., Qayyum, T., Horgan, P. G. & McMillan, D. C. The prognostic value of histological tumor necrosis in solid organ malignant disease: a systematic review. Future Oncol. 7, 1223–1235 (2011).
Holmgaard, R. B. et al. Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep. 13, 412–424 (2015).
Timosenko, E., Hadjinicolaou, A. V. & Cerundolo, V. Modulation of cancer-specific immune responses by amino acid degrading enzymes. Immunotherapy 9, 83–97 (2017).
Kumar, V., Patel, S., Tcyganov, E. & Gabrilovich, D. I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 37, 208–220 (2016).
Raber, P. L. et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int. J. Cancer 134, 2853–2864 (2014).
Long, G. V. et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: results of the phase 3 ECHO-301/KEYNOTE-252 study. J. Clin. Oncol. 36, 108 (2018).
De Henau, O. et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature 539, 443–447 (2016).
Cannarile, M. A. et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 5, 53 (2017).
Moore, E. et al. Established T cell-inflamed tumors rejected after adaptive resistance was reversed by combination STING activation and PD-1 pathway blockade. Cancer Immunol. Res. 4, 1061–1071 (2016).
Clavijo, P. E. et al. Resistance to CTLA-4 checkpoint inhibition reversed through selective elimination of granulocytic myeloid cells. Oncotarget 8, 55804–55820 (2017).
Harlin, H. et al. Chemokine expression in melanoma metastases associated with CD8+T cell recruitment. Cancer Res. 69, 3077–3085 (2009).
Ulloa-Montoya, F. et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J. Clin. Oncol. 31, 2388–2395 (2013).
Sistigu, A. et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Cai, X., Chiu, Y. H. & Chen, Z. J. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell 54, 289–296 (2014).
Corrales, L., McWhirter, S. M., Dubensky, T. W. Jr & Gajewski, T. F. The host STING pathway at the interface of cancer and immunity. J. Clin. Invest. 126, 2404–2411 (2016).
Sanchez-Paulete, A. R. et al. Antigen cross-presentation and T cell cross-priming in cancer immunology and immunotherapy. Ann. Oncol. 28, xii44–xii55 (2017).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Huck, B. R., Kotzner, L. & Urbahns, K. Small molecules drive big improvements in immuno-oncology therapies. Angew. Chem. Int. Ed. 57, 4412–4428 (2018).
Foote, J. B. et al. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in tolerized mice. Cancer Immunol. Res. 5, 468–479 (2017).
Shekarian, T. et al. Pattern recognition receptors: immune targets to enhance cancer immunotherapy. Ann. Oncol. 28, 1756–1766 (2017).
Elion, D. L. & Cook, R. S. Harnessing RIG-I and intrinsic immunity in the tumor microenvironment for therapeutic cancer treatment. Oncotarget 9, 29007–29017 (2018).
Le Mercier, I. et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 73, 4629–4640 (2013).
Kim, Y. H. et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood 119, 355–363 (2012).
Li, J. et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J. Immunol. 179, 2493–2500 (2007).
Wang, S. et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc. Natl Acad. Sci. USA 113, E7240–E7249 (2016).
Sato-Kaneko, F. et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2, 93397 (2017).
Sagiv-Barfi, I. et al. Eradication of spontaneous malignancy by local immunotherapy. Sci. Transl Med. 10, eaan4488 (2018).
Marin-Acevedo, J. A., Soyano, A. E., Dholaria, B., Knutson, K. L. & Lou, Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J. Hematol. Oncol. 11, 8 (2018).
Wilkinson, R. W. & Leishman, A. J. Further advances in cancer immunotherapy: going beyond checkpoint blockade. Front. Immunol. 9, 1082 (2018).
Frank, M. J. et al. In situ vaccination with a TLR 9 agonist and local low dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-18-0743 (2018).
Chow, L. Q. M. et al. Phase Ib trial of the toll-like receptor 8 agonist, motolimod (VTX-2337), combined with cetuximab in patients with recurrent or metastatic SCCHN. Clin. Cancer Res. 23, 2442–2450 (2017).
Marabelle, A., Kohrt, H., Caux, C. & Levy, R. Intratumoral immunization: a new paradigm for cancer therapy. Clin. Cancer Res. 20, 1747–1756 (2014).
Ridnour, L. A. et al. Molecular pathways: toll-like receptors in the tumor microenvironment—poor prognosis or new therapeutic opportunity. Clin. Cancer Res. 19, 1340–1346 (2013).
Huang, L., Xu, H. & Peng, G. TLR-mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cell. Mol. Immunol. 15, 428–437 (2018).
Li, J. K., Balic, J. J., Yu, L. & Jenkins, B. TLR agonists as adjuvants for cancer vaccines. Adv. Exp. Med. Biol. 1024, 195–212 (2017).
Iribarren, K. et al. Trial watch: immunostimulation with toll-like receptor agonists in cancer therapy. Oncoimmunology 5, e1088631 (2016).
Vonderheide, R. H. & Glennie, M. J. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 19, 1035–1043 (2013).
Tutt, A. L. et al. T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J. Immunol. 168, 2720–2728 (2002).
Mangsbo, S. M. et al. The human agonistic CD40 antibody ADC-1013 eradicates bladder tumors and generates T cell-dependent tumor immunity. Clin. Cancer Res. 21, 1115–1126 (2015).
Beatty, G. L., Li, Y. & Long, K. B. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev. Anticancer Ther. 17, 175–186 (2017).
Ohta, T. et al. Crucial roles of XCR1-expressing dendritic cells and the XCR1-XCL1 chemokine axis in intestinal immune homeostasis. Sci. Rep. 6, 23505 (2016).
Krummel, M. F., Bartumeus, F. & Gerard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 16, 193–201 (2016).
Bottcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037 (2018).
Villadangos, J. A. & Shortman, K. Found in translation: the human equivalent of mouse CD8+dendritic cells. J. Exp. Med. 207, 1131–1134 (2010).
Woo, S. R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014). This article shows that innate immune sensing of cancer occurs via the host STING pathway and subsequent type I interferon production. Spontaneous CD8 + T cell priming against tumours depends on STING.
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Harding, S. M. et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470 (2017).
Zheng, W. et al. Combination of radiotherapy and vaccination overcomes checkpoint blockade resistance. Oncotarget 7, 43039–43051 (2016).
Bonvalot, S. et al. First-in-human study testing a new radioenhancer using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin. Cancer Res. 23, 908–917 (2017).
Beatty, G. L. & Gladney, W. L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 21, 687–692 (2015).
Zitvogel, L., Kepp, O. & Kroemer, G. Decoding cell death signals in inflammation and immunity. Cell 140, 798–804 (2010).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016). This article demonstrates that a relationship exists between clonal neoantigen burden and overall survival in primary lung adenocarcinomas. Sensitivity to PD-1 and CTLA4 blockade in patients with advanced NSCLC and melanoma was enhanced in tumours enriched for clonal neoantigens.
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Vacchelli, E. et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 350, 972–978 (2015).
Ruffell, B. et al. Leukocyte composition of human breast cancer. Proc. Natl Acad. Sci. USA 109, 2796–2801 (2012).
Park, J. H. et al. Clonal expansion of antitumor T cells in breast cancer correlates with response to neoadjuvant chemotherapy. Int. J. Oncol. 49, 471–478 (2016).
Ladoire, S. et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy 11, 1878–1890 (2015).
Ladoire, S. et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy 12, 864–875 (2016).
Fucikova, J. et al. Calreticulin expression in human non-small cell lung cancers correlates with increased accumulation of antitumor immune cells and favorable prognosis. Cancer Res. 76, 1746–1756 (2016).
Stoll, G. et al. Calreticulin expression: interaction with the immune infiltrate and impact on survival in patients with ovarian and non-small cell lung cancer. Oncoimmunology 5, e1177692 (2016).
Wemeau, M. et al. Calreticulin exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. Cell Death Dis. 1, e104 (2010).
Fucikova, J. et al. Calreticulin exposure by malignant blasts correlates with robust anticancer immunity and improved clinical outcome in AML patients. Blood 128, 3113–3124 (2016).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28, 690–714 (2015).
Vincent, J. et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 (2010).
Ghiringhelli, F. et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 56, 641–648 (2007).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Wang, W. et al. Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell 165, 1092–1105 (2016).
Anitei, M. G. et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin. Cancer Res. 20, 1891–1899 (2014).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Brea, E. J. et al. Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol. Res. 4, 936–947 (2016).
Gang, A. O. et al. 5-Azacytidine treatment sensitizes tumor cells to T cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies. Blood Cancer J. 4, e197 (2014).
de Charette, M., Marabelle, A. & Houot, R. Turning tumour cells into antigen presenting cells: the next step to improve cancer immunotherapy? Eur. J. Cancer 68, 134–147 (2016).
Chiappinelli, K. B. et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 162, 974–986 (2015).
Stengel, S., Fiebig, U., Kurth, R. & Denner, J. Regulation of human endogenous retrovirus-K expression in melanomas by CpG methylation. Genes Chromosomes Cancer 49, 401–411 (2010).
Strissel, P. L. et al. Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: emergence of new molecular targets. Oncotarget 3, 1204–1219 (2012).
Sharma, S., Kaufmann, T. & Biswas, S. Impact of inhibitor of apoptosis proteins on immune modulation and inflammation. Immunol. Cell Biol. 95, 236–243 (2017).
Kotschy, A. et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538, 477–482 (2016).
Yea, S. S. & Fruman, D. A. Achieving cancer cell death with PI3K/mTOR-targeted therapies. Ann. NY Acad. Sci. 1280, 15–18 (2013).
Kumari, N., Dwarakanath, B. S., Das, A. & Bhatt, A. N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 37, 11553–11572 (2016).
Liepe, J. et al. A large fraction of HLA class I ligands are proteasome-generated spliced peptides. Science 354, 354–358 (2016).
Lauss, M. et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat. Commun. 8, 1738 (2017).
Germano, G. et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 552, 116–120 (2017). This article shows that inactivation of DNA MMR mechanisms increased mutational load, promoted continuous renewal of neoantigens in human CRCs and triggered immune surveillance in mouse models.
Wieringa, H. W., van der Zee, A. G., de Vries, E. G. & van Vugt, M. A. Breaking the DNA damage response to improve cervical cancer treatment. Cancer Treat. Rev. 42, 30–40 (2016).
Hemann, M. T. From breaking bad to worse: exploiting homologous DNA repair deficiency in cancer. Cancer Discov. 4, 516–518 (2014).
Yang, Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J. Clin. Invest. 125, 3335–3337 (2015).
Rosenbaum, L. Tragedy, perseverance, and chance — the story of CAR-T therapy. N. Engl. J. Med. 377, 1313–1315 (2017).
Jensen, M. C. & Riddell, S. R. Designing chimeric antigen receptors to effectively and safely target tumors. Curr. Opin. Immunol. 33, 9–15 (2015).
Gomes-Silva, D. & Ramos, C. A. Cancer immunotherapy using CAR-T cells: from the research bench to the assembly line. Biotechnol. J. 13, 1700097 (2018).
Wang, X. & Riviere, I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol. Ther. Oncolyt. 3, 16015 (2016).
von Kalle, C., Deichmann, A. & Schmidt, M. Vector integration and tumorigenesis. Hum. Gene Ther. 25, 475–481 (2014).
Wright, A. V., Nunez, J. K. & Doudna, J. A. Biology and applications of CRISPR systems: harnessing nature’s toolbox for genome engineering. Cell 164, 29–44 (2016).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Hervas-Stubbs, S. et al. CD8 T cell priming in the presence of IFN-alpha renders CTLs with improved responsiveness to homeostatic cytokines and recall antigens: important traits for adoptive T cell therapy. J. Immunol. 189, 3299–3310 (2012).
Mishto, M. & Liepe, J. Post-translational peptide splicing and T cell responses. Trends Immunol. 38, 904–915 (2017).
Verdegaal, E. M. et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature 536, 91–95 (2016).
Spranger, S., Dai, D., Horton, B. & Gajewski, T. F. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31, 711–723 (2017).
Galon, J. et al. Characterization of anti-CD19 chimeric antigen receptor (CAR) T cell-mediated tumor microenvironment immune gene profile in a multicenter trial (ZUMA-1) with axicabtagene ciloleucel (axi-cel, KTE-C19). J. Clin. Oncol. 35, 3025 (2017).
Kaufman, H. L., Kohlhapp, F. J. & Zloza, A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 14, 642–662 (2015).
Pataer, A., Swisher, S. G., Roth, J. A., Logothetis, C. J. & Corn, P. G. Inhibition of RNA-dependent protein kinase (PKR) leads to cancer cell death and increases chemosensitivity. Cancer Biol. Ther. 8, 245–252 (2009).
Chesney, J. et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 36, 1658–1667 (2018). This paper presents the first randomized trial evaluating the combination of an oncolytic virus with a checkpoint inhibitor and showing a significantly higher objective response rate for T-VEC plus ipilimumab versus ipilimumab alone.
Ribas, A. et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119 (2017). This article shows that oncolytic virotherapy with T-VEC in patients with advanced melanoma increased the cytotoxic T cell infiltration and therapeutic efficacy of an anti-PD-1 antibody.
Haanen, J. Converting cold into hot tumors by combining immunotherapies. Cell 170, 1055–1056 (2017).
Nestvold, J. et al. Oncolytic peptide LTX-315 induces an immune-mediated abscopal effect in a rat sarcoma model. Oncoimmunology 6, e1338236 (2017).
Patel, A., Kaufman, H. L. & Disis, M. L. Next generation approaches for tumor vaccination. Chin. Clin. Oncol. 6, 19 (2017).
Gibney, G. T., Weiner, L. M. & Atkins, M. B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 17, e542–e551 (2016).
Vigneron, N. Human tumor antigens and cancer immunotherapy. Biomed. Res. Int. 2015, 948501 (2015).
Mitchell, D. A. et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 519, 366–369 (2015).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Liau, L. M. et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl Med. 16, 142 (2018).
Luksza, M. et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517–520 (2017).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017). This paper presents the development and successful application of a personalized vaccine-based immunotherapy exploiting the concept of an individualized mutanome and computational prediction of neo-epitopes.
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017). This article provides a strong rationale for further development of neo-epitope vaccines, alone and in combination with checkpoint blockade or other immunotherapies.
Garcia-Martinez, E. et al. Trial watch: immunostimulation with recombinant cytokines for cancer therapy. Oncoimmunology 7, e1433982 (2018).
Jiang, T., Zhou, C. & Ren, S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 5, e1163462 (2016).
Sockolosky, J. T. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 359, 1037–1042 (2018).
Ochoa, M. C. et al. Interleukin-15 in gene therapy of cancer. Curr. Gene Ther. 13, 15–30 (2013).
Romee, R. et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 131, 2515–2527 (2018).
Mazzucchelli, R. & Durum, S. K. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 7, 144–154 (2007).
Deiser, K., Stoycheva, D., Bank, U., Blankenstein, T. & Schuler, T. Interleukin-7 modulates anti-tumor CD8+T cell responses via its action on host cells. PLOS ONE 11, e0159690 (2016).
Xu, X., Sun, Q., Mei, Y., Liu, Y. & Zhao, L. Newcastle disease virus co-expressing interleukin 7 and interleukin 15 modified tumor cells as a vaccine for cancer immunotherapy. Cancer Sci. 109, 279–288 (2018).
Al-Chami, E., Tormo, A., Khodayarian, F. & Rafei, M. Therapeutic utility of the newly discovered properties of interleukin-21. Cytokine 82, 33–37 (2016).
Kannappan, V. et al. Interleukin 21 inhibits cancer-mediated FOXP3 induction in naive human CD4 T cells. Cancer Immunol. Immunother. 66, 637–645 (2017).
Spolski, R. & Leonard, W. J. IL-21 and T follicular helper cells. Int. Immunol. 22, 7–12 (2010).
Lewis, K. E. et al. Interleukin-21 combined with PD-1 or CTLA-4 blockade enhances antitumor immunity in mouse tumor models. Oncoimmunology 7, e1377873 (2017).
Chapuis, A. G. et al. Combined IL-21-primed polyclonal CTL plus CTLA4 blockade controls refractory metastatic melanoma in a patient. J. Exp. Med. 213, 1133–1139 (2016).
Kaiser, J. ‘Liquid biopsy’ for cancer promises early detection. Science 359, 259 (2018).
Tavare, R. et al. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 76, 73–82 (2016).
Ali, H. R., Chlon, L., Pharoah, P. D., Markowetz, F. & Caldas, C. Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLOS Med. 13, e1002194 (2016).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457 (2015).
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G. & Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220 (2017).
Li, T. et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 77, e108–e110 (2017).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830 (2018).
Zappia, L., Phipson, B. & Oshlack, A. Exploring the single-cell RNA-seq analysis landscape with the scRNA-tools database. PLOS Comput. Biol. 14, e1006245 (2018).
Chen, X., Sun, Y. C., Church, G. M., Lee, J. H. & Zador, A. M. Efficient in situ barcode sequencing using padlock probe-based BaristaSeq. Nucleic Acids Res. 46, e22 (2018).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 24, 207–212 (2012).
Ayers, M. et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Davoli, T., Uno, H., Wooten, E. C. & Elledge, S. J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 355, eaaf8399 (2017).
Papaccio, F. et al. Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med. 6, 2115–2125 (2017).
Jolly, M. K., Ware, K. E., Gilja, S., Somarelli, J. A. & Levine, H. EMT and MET: necessary or permissive for metastasis? Mol. Oncol. 11, 755–769 (2017).
Moustakas, A. & de Herreros, A. G. Epithelial-mesenchymal transition in cancer. Mol. Oncol. 11, 715–717 (2017).
Terry, S. et al. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 11, 824–846 (2017).
Acknowledgements
The authors thank the following institutions for their financial support: the National Cancer Institute of France (INCa), the Plan Cancer, the Canceropole Ile de France, INSERM, Cancer Research for Personalized Medicine (CARPEM), the Paris Alliance of Cancer Research Institutes (PACRI), H2020 PHC-32-2014 APERIM grant number EEAA15006DDA, MedImmune (grant number RVE15004DSA) and LabEx Immuno-oncology.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
Immunoscore is a registered trademark from INSERM. J.G. is co-founder and chairman of the scientific advisory board of HalioDx. J.G. has patents associated with an ‘in vitro method for the prognosis of progression of a cancer’ (PCT/IB2006/003168 and PCT/EP2013/062405). J.G. established Collaborative Research Agreement (grants) with Perkin-Elmer, IO Biotech, MedImmune, Astra Zeneca, Janssen, Imcheck Therapeutics. J.G. participated to Scientific Advisory Boards of BMS, MedImmune, Astra Zeneca, Novartis, Definiens, Merck Serono, IO Biotech, ImmunID, Nanostring, Illumina, Northwest Biotherapeutics, Actelion, Amgen, Kite Pharma and Merck MSD. J.G. was a consultant for BMS, Roche, GSK, Compugen, Mologen and Sanofi.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- TNM system
-
The tumour-node-metastasis (TNM) staging system is a globally recognized classification of tumours based on their anatomical extent. T refers to the size and extent of the primary tumour, N refers to the involvement of regional lymph nodes and M describes the presence of distant metastases.
- Pathologic T (pT) stage
-
The staging assigned post-surgery to guide treatment stratification, patient selection for clinical trials and prognosis prediction (as opposed to clinical staging that relies on physical exams and imaging tests).
- Immunoproteasome
-
Proteasome isoform constitutively expressed in haematopoietic cells and induced in non-immune cells following exposure to interferon-γ (IFNγ) and other pro-inflammatory cytokines (type I interferons and tumour necrosis factor (TNF)). It is involved in antigen processing and in the expansion, maintenance and differentiation of T cell populations during an immune response.
- T cell receptor (TCR) repertoire
-
The variety of the TCR diversity, as generated by the somatic recombination of the germ line V, D and J gene segments and the deletion and insertion of nucleotides at the V(D)J junctions. Such variety is required to recognize a wide spectrum of antigens.
- TCR Vß subfamilies
-
Human TCR ß locus is on chromosome 7, comprising nine multimember V subfamilies plus additional elements on chromosome 9. The presence of multiple subfamilies is due to evolutionary duplication events.
- Tumour-specific antigen
-
(TSA). Antigen not encoded in the normal genome, expressed exclusively by tumour cells.
- Antigenicity
-
Presence of tumour-associated antigens (TAAs) capable of engaging with T cell receptors or antibodies (B cell receptors), thereby driving adaptive immunity.
- Abscopal effect
-
Phenomenon characterized by the regression of metastases outside the field of radiation after irradiation of one tumour site. Although rarely detected, it is well documented in patients with more immunogenic tumours.
- Adjuvanticity
-
Presence of damage-associated molecular patterns (DAMPs) and stress signals driving the innate immunity.
- Genotoxic chemotherapies
-
Chemical agents that cause DNA damage, such as single-strand and double-strand breaks, loss of excision repair, crosslinking, alkali-labile sites, point mutations and structural and numerical chromosomal aberrations.
- Damage-associated molecular patterns
-
(DAMPs). Intracellular molecules that are hidden from immune recognition under physiological conditions. These molecules are secreted, exposed or released upon cellular stress or tissue injury and recognized by pattern-recognition receptors expressed on innate immune cells.
- Tumour-associated antigens
-
(TAAs). Antigens that are preferentially expressed by tumour cells but they can also be found in normal tissues (except for the TSAs, which are exclusively expressed by tumour cells). They can be broadly categorized into aberrantly expressed self-antigens, mutated self-antigens and TSAs.
- (LC3B+) puncta
-
LC3 is a protein involved in the formation of autophagosomes and autolysosomes. Punctate (as opposed to diffused) LC3 staining indicates autophagy, as determined by fluorescence microscopy.
- Differentiation antigens
-
Antigens derived from proteins that are expressed in a given type of tumour and the corresponding healthy tissue, often in lower amounts.
- Neoantigen fitness
-
The likelihood of a peptide to be immunogenic, as measured by its binding affinity to major histocompatibility complex (MHC) and subsequent recognition by T cells.
Rights and permissions
About this article
Cite this article
Galon, J., Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 18, 197–218 (2019). https://doi.org/10.1038/s41573-018-0007-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-018-0007-y
This article is cited by
-
AIM2 promotes irradiation resistance, migration ability and PD-L1 expression through STAT1/NF-κB activation in oral squamous cell carcinoma
Journal of Translational Medicine (2024)
-
Modulating ferroptosis sensitivity: environmental and cellular targets within the tumor microenvironment
Journal of Experimental & Clinical Cancer Research (2024)
-
T-cell infiltration and its regulatory mechanisms in cancers: insights at single-cell resolution
Journal of Experimental & Clinical Cancer Research (2024)
-
Magnetic resonance imaging-based approaches for detecting the efficacy of combining therapy following VEGFR-2 and PD-1 blockade in a colon cancer model
Journal of Translational Medicine (2024)
-
Regulation of tumor metastasis and CD8+ T cells infiltration by circRNF216/miR-576-5p/ZC3H12C axis in colorectal cancer
Cellular & Molecular Biology Letters (2024)