Abstract
Osteosarcoma is the most common primary malignant tumour of the bone. Osteosarcoma incidence is bimodal, peaking at 18 and 60 years of age, and is slightly more common in males. The key pathophysiological mechanism involves several possible genetic drivers of disease linked to bone formation, causing malignant progression and metastasis. While there have been significant improvements in the outcome of patients with localized disease, with event-free survival outcomes exceeding 60%, in patients with metastatic disease, event-free survival outcomes remain poor at less than 30%. The suspicion of osteosarcoma based on radiographs still requires pathological evaluation of a bone biopsy specimen for definitive diagnosis and CT imaging of the chest should be performed to identify lung nodules. So far, population-based screening and surveillance strategies have not been implemented due to the rarity of osteosarcoma and the lack of reliable markers. Current screening focuses only on groups at high risk such as patients with genetic cancer predisposition syndromes. Management of osteosarcoma requires a multidisciplinary team of paediatric and medical oncologists, orthopaedic and general surgeons, pathologists, radiologists and specialist nurses. Survivors of osteosarcoma require specialized medical follow-up, as curative treatment consisting of chemotherapy and surgery has long-term adverse effects, which also affect the quality of life of patients. The development of osteosarcoma model systems and related research as well as the evaluation of new treatment approaches are ongoing to improve disease outcomes, especially for patients with metastases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Change history
30 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41572-022-00416-z
References
Mirabello, L., Troisi, R. J. & Savage, S. A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer 115, 1531–1543 (2009).
Bielack, S. S. et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 20, 776–790 (2002). A study that affirms tumour site and size, primary metastases, response to chemotherapy, and surgical remission as independent prognostic factors in patients with osteosarcoma.
Klein, M. J. & Siegal, G. P. Osteosarcoma: anatomic and histologic variants. Am. J. Clin. Pathol. 125, 555–581 (2006).
Piperdi, S. et al. β-Catenin does not confer tumorigenicity when introduced into partially transformed human mesenchymal stem cells. Sarcoma 2012, 164803 (2012).
Bertoni, F. & Bacchini, P. Classification of bone tumors. Eur. J. Radiol. 27 (Suppl. 1), S74–S76 (1998).
Kager, L. et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J. Clin. Oncol. 21, 2011–2018 (2003).
Isakoff, M. S., Bielack, S. S., Meltzer, P. & Gorlick, R. Osteosarcoma: current treatment and a collaborative pathway to success. J. Clin. Oncol. 33, 3029–3035 (2015).
Smith, M. A. et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J. Clin. Oncol. 28, 2625–2634 (2010).
Gill, J. & Gorlick, R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 18, 609–624 (2021).
Mirabello, L., Troisi, R. J. & Savage, S. A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 125, 229–234 (2009).
Parkin, D. M., Stiller, C. A., Draper, G. J. & Bieber, C. The international incidence of childhood cancer. Int. J. Cancer 42, 511–520 (1988).
Mirabello, L. et al. Frequency of pathogenic germline variants in cancer-susceptibility genes in patients with osteosarcoma. JAMA Oncol. 6, 724–734 (2020). This study demonstrates a high frequency of potentially pathogenic germline mutations in patients with osteosarcoma, supporting the role of germline genetic testing.
Glass, A. G. & Fraumeni, J. F. Jr. Epidemiology of bone cancer in children. J. Natl Cancer Inst. 44, 187–199 (1970).
Czerniak, B. Dorfman and Czerniak’s Bone Tumors E-Book (Elsevier Health Sciences, 2015).
Brown, H. K., Schiavone, K., Gouin, F., Heymann, M.-F. & Heymann, D. Biology of bone sarcomas and new therapeutic developments. Calcif. Tissue Int. 102, 174–195 (2018).
Cole, S., Gianferante, D. M., Zhu, B. & Mirabello, L. Osteosarcoma: a surveillance, epidemiology, and end results program‐based analysis from 1975 to 2017. Cancer 128, 2107–2118 (2022).
Ilcisin, L. A. S. et al. Poverty, race, ethnicity, and survival among U.S. children with non-metastatic osteosarcoma treated on EURAMOS-1: a report from the Children’s Oncology Group. J. Clin. Oncol. 40, 10004 (2022).
Zhang, J. et al. Germline mutations in predisposition genes in pediatric cancer. N. Engl. J. Med. 373, 2336–2346 (2015).
Vlachos, A., Rosenberg, P. S., Atsidaftos, E., Alter, B. P. & Lipton, J. M. Incidence of neoplasia in Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood 119, 3815–3819 (2012).
Wang, L. L. et al. Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome. J. Natl Cancer Inst. 95, 669–674 (2003).
Lu, L., Jin, W. & Wang, L. L. RECQ DNA helicases and osteosarcoma. Adv. Exp. Med. Biol. 1258, 37–54 (2020).
Hameed, M. & Mandelker, D. Tumor syndromes predisposing to osteosarcoma. Adv. Anat. Pathol. 25, 217–222 (2018).
Calvert, G. T. et al. At-risk populations for osteosarcoma: the syndromes and beyond. Sarcoma 2012, 152382 (2012).
Mirabello, L. et al. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control 22, 899–908 (2011).
Tucker, M. A. et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N. Engl. J. Med. 317, 588–593 (1987).
Cundy, T. Paget’s disease of bone. Metabolism 80, 5–14 (2018).
Ruggieri, P., Sim, F. H., Bond, J. R. & Krishnan Unni, K. Malignancies in fibrous dysplasia. Cancer 73, 1411–1424 (1994).
Picci, P. et al. Late sarcoma development after curettage and bone grafting of benign bone tumors. Eur. J. Radiol. 77, 19–25 (2011).
Jones, K. B. Osteosarcomagenesis: modeling cancer initiation in the mouse. Sarcoma 2011, 694136 (2011).
Mutsaers, A. J. & Walkley, C. R. Cells of origin in osteosarcoma: mesenchymal stem cells or osteoblast committed cells? Bone 62, 56–63 (2014).
Lin, Y. H. et al. Osteosarcoma: molecular pathogenesis and iPSC modeling. Trends Mol. Med. 23, 737–755 (2017).
Cortés-Ciriano, I. et al. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat. Genet. 52, 331–341 (2020).
Chen, X. et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 7, 104–112 (2014). The majority of TP53 loss in osteosarcoma occurs through intron 1 rearrangements or deletions rather than through point mutations.
Perry, J. A. et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc. Natl Acad. Sci. USA 111, E5564–E5573 (2014).
Sayles, L. C. et al. Genome-informed targeted therapy for osteosarcoma. Cancer Discov. 9, 46–63 (2019). A study that defines potentially actionable molecular subtypes of osteosarcoma.
Wu, C. C. et al. Immuno-genomic landscape of osteosarcoma. Nat. Commun. 11, 1008 (2020). Molecular profiling of samples from patients with osteosarcoma characterizes immune subsets, including immune enrichment among older patients.
Rajan, S. et al. Remarkably stable copy-number profiles in osteosarcoma revealed using single-cell DNA sequencing. Preprint at bioRxiv https://doi.org/10.1101/2021.08.30.458268 (2021).
Behjati, S. et al. Recurrent mutation of IGF signalling genes and distinct patterns of genomic rearrangement in osteosarcoma. Nat. Commun. 8, 15936 (2017).
Overholtzer, M. et al. The presence of p53 mutations in human osteosarcomas correlates with high levels of genomic instability. Proc. Natl Acad. Sci. USA 100, 11547–11552 (2003). One of the first studies to show that p53 mutations correlate with high levels of genomic instability in osteosarcomas.
Eischen, C. M. Genome stability requires p53. Cold Spring Harb. Perspect. Med. 6, a026096 (2016).
Hanel, W. & Moll, U. M. Links between mutant p53 and genomic instability. J. Cell. Biochem. 113, 433–439 (2012).
Gerstung, M. et al. The evolutionary history of 2,658 cancers. Nature 578, 122–128 (2020).
Lawlor, R. T. et al. Alternative lengthening of telomeres (ALT) influences survival in soft tissue sarcomas: a systematic review with meta-analysis. BMC Cancer 19, 232 (2019).
Kovac, M. et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat. Commun. 6, 8940 (2015).
Tellez-Gabriel, M. et al. Analysis of gap junctional intercellular communications using a dielectrophoresis-based microchip. Eur. J. Cell Biol. 96, 110–118 (2017).
Bénédicte Brounais, L.-R. & Frédéric, L. In Bone Cancer 221–239, Ch. 18 (Academic Press, 2022).
Lan, M. et al. Extracellular vesicles-mediated signaling in the osteosarcoma microenvironment: roles and potential therapeutic targets. J. Bone Oncol. 12, 101–104 (2018).
Cackowski, F. C. et al. Osteoclasts are important for bone angiogenesis. Blood 115, 140–149 (2010).
Endo-Munoz, L., Evdokiou, A. & Saunders, N. A. The role of osteoclasts and tumour-associated macrophages in osteosarcoma metastasis. Biochim. Biophys. Acta 1826, 434–442 (2012).
Khanna, C. et al. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin. Cancer Res. 20, 4200–4209 (2014).
Heymann, M.-F., Lézot, F. & Heymann, D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell. Immunol. https://doi.org/10.1016/j.cellimm.2017.10.011 (2017).
Brown, H. K., Tellez-Gabriel, M. & Heymann, D. Cancer stem cells in osteosarcoma. Cancer Lett. 386, 189–195 (2017).
Grunewald, T. G. et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 12, e11131 (2020).
Zhou, Y. et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 11, 6322 (2020).
Guo, J. et al. Single-cell profiling of tumor microenvironment heterogeneity in osteosarcoma identifies a highly invasive subcluster for predicting prognosis. Front. Oncol. 12, 732862 (2022).
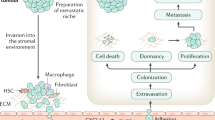
Mazumdar, A. et al. Exploring the role of osteosarcoma-derived extracellular vesicles in pre-metastatic niche formation and metastasis in the 143-B xenograft mouse osteosarcoma model. Cancers https://doi.org/10.3390/cancers12113457 (2020).
Stamatopoulos, A. et al. Mesenchymal stromal cells for bone sarcoma treatment: roadmap to clinical practice. J. Bone Oncol. 16, 100231 (2019).
Perrot, P. et al. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS ONE 5, e10999 (2010).
Cortini, M., Avnet, S. & Baldini, N. Mesenchymal stroma: role in osteosarcoma progression. Cancer Lett. 405, 90–99 (2017).
Baglio, S. R. et al. Blocking tumor-educated MSC paracrine activity halts osteosarcoma progression. Clin. Cancer Res. 23, 3721–3733 (2017).
Tu, B., Du, L., Fan, Q. M., Tang, Z. & Tang, T. T. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 325, 80–88 (2012).
Gross, A. C. et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight https://doi.org/10.1172/jci.insight.99791 (2018).
Mazumdar, A. et al. Osteosarcoma-derived extracellular vesicles induce lung fibroblast reprogramming. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21155451 (2020).
Zhang, W. et al. Adaptive fibrogenic reprogramming of osteosarcoma stem cells promotes metastatic growth. Cell Rep. 24, 1266–1277.e5 (2018).
Yui, Y., Kumai, J., Watanabe, K., Wakamatsu, T. & Sasagawa, S. Lung fibrosis is a novel therapeutic target to suppress lung metastasis of osteosarcoma. Int. J. Cancer https://doi.org/10.1002/ijc.34008 (2022).
Kurzman, I. D. et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin. Cancer Res. 1, 1595–1601 (1995).
Meyers, P. A. et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival–a report from the Children’s Oncology Group. J. Clin. Oncol. 26, 633–638 (2008).
Kleinerman, E. S. et al. Phase II study of liposomal muramyl tripeptide in osteosarcoma: the cytokine cascade and monocyte activation following administration. J. Clin. Oncol. 10, 1310–1316 (1992).
Mason, N. J. et al. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin. Cancer Res. 22, 4380–4390 (2016).
Chen, K. et al. Wnt10b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr. Blood Cancer 51, 349–355 (2008).
Goldstein, S. D., Trucco, M., Bautista Guzman, W., Hayashi, M. & Loeb, D. M. A monoclonal antibody against the Wnt signaling inhibitor dickkopf-1 inhibits osteosarcoma metastasis in a preclinical model. Oncotarget 7, 21114–21123 (2016).
Khanna, C. et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat. Med. 10, 182–186 (2004).
Bulut, G. et al. Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene 31, 269–281 (2012).
Ren, L. et al. Dysregulation of Ezrin phosphorylation prevents metastasis and alters cellular metabolism in osteosarcoma. Cancer Res. 72, 1001–1012 (2012).
Morrow, J. J. et al. Positively selected enhancer elements endow osteosarcoma cells with metastatic competence. Nat. Med. 24, 176–185 (2018).
Ichikawa, J. et al. Thrombin induces osteosarcoma growth, a function inhibited by low molecular weight heparin in vitro and in vivo. Cancer 118, 2494–2506 (2012).
Charan, M. et al. Tumor secreted ANGPTL2 facilitates recruitment of neutrophils to the lung to promote lung pre-metastatic niche formation and targeting ANGPTL2 signaling affects metastatic disease. Oncotarget 11, 510–522 (2020).
Navet, B. et al. The intrinsic and extrinsic implications of RANKL/RANK signaling in osteosarcoma: from tumor initiation to lung metastases. Cancers https://doi.org/10.3390/cancers10110398 (2018).
Church, A. J. et al. Clinical impact of molecular tumor profiling in pediatric, adolescent, and young adult patients with extra-cranial solid malignancies: an interim report from the GAIN/iCat2 study. J. Clin. Oncol. 39, 10005 (2021).
Suehara, Y. et al. Clinical genomic sequencing of pediatric and adult osteosarcoma reveals distinct molecular subsets with potentially targetable alterations. Clin. Cancer Res. 25, 6346–6356 (2019).
Meyers, P. A. et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival — a report from the Children’s Oncology Group. J. Clin. Oncol. 26, 633–638 (2008).
Bielack, S. S. et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon Alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 Good Response Randomized Controlled Trial. J. Clin. Oncol. 33, 2279–2287 (2015).
Gröbner, S. N. et al. The landscape of genomic alterations across childhood cancers. Nature 555, 321–327 (2018).
Tawbi, H. A. et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 18, 1493–1501 (2017).
Le Cesne, A. et al. Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: results from the PEMBROSARC study. Eur. J. Cancer 119, 151–157 (2019).
Hingorani, P. et al. ABBV-085, antibody–drug conjugate targeting LRRC15, is effective in osteosarcoma: a report by the pediatric preclinical testing consortium. Mol. Cancer Ther. 20, 535–540 (2021).
Hingorani, P. et al. Trastuzumab deruxtecan, antibody-drug conjugate targeting HER2, is effective in pediatric malignancies: a report by the pediatric preclinical testing consortium. Mol. Cancer Ther. 21, 1318–1325 (2022).
Lange, S. et al. A chimeric GM-CSF/IL18 receptor to sustain CAR T-cell function. Cancer Discov. 11, 1661–1671 (2021).
Tullius, B. P., Setty, B. A. & Lee, D. A. In Current Advances in Osteosarcoma: Clinical Perspectives: Past, Present and Future (eds Kleinerman, E. S. & Gorlick, R.) 141–154 (Springer International Publishing, 2020).
Kendsersky, N. M. et al. The B7-H3–targeting antibody–drug conjugate m276-SL-PBD is potently effective against pediatric cancer preclinical solid tumor models. Clin. Cancer Res. 27, 2938–2946 (2021).
Hingorani, P. et al. Abstract LB-217: Preclinical evaluation of trastuzumab deruxtecan (T-DXd; DS-8201a), a HER2 antibody-drug conjugate, in pediatric solid tumors by the Pediatric Preclinical Testing Consortium (PPTC).Cancer Res. 80 (Suppl. 16), LB-217 (2020).
Bayles, I. et al. Ex vivo screen identifies CDK12 as a metastatic vulnerability in osteosarcoma. J. Clin. Invest. 129, 4377–4392 (2019).
Chang, L.-S. et al. Targeting protein translation by rocaglamide and didesmethylrocaglamide to treat MPNST and other sarcomas. Mol. Cancer Ther. 19, 731–741 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04040205 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03242382 (2022).
Teven, C. M., Farina, E. M., Rivas, J. & Reid, R. R. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis. 1, 199–213 (2014).
Ikebuchi, Y. Coupling of bone resorption and formation by RANKL reverse signalling. Nature https://doi.org/10.1038/s41586-018-0482-7 (2018).
Li, Y. S., Liu, Q., He, H. B. & Luo, W. The possible role of insulin-like growth factor-1 in osteosarcoma. Curr. Probl. Cancer 43, 228–235 (2019).
Regan, D. P. et al. Losartan blocks osteosarcoma-elicited monocyte recruitment, and combined with the kinase inhibitor toceranib, exerts significant clinical benefit in canine metastatic osteosarcoma. Clin. Cancer Res. 28, 662–676 (2022).
Nomura, M. et al. Tegavivint and the β-catenin/ALDH axis in chemotherapy-resistant and metastatic osteosarcoma. J. Natl Cancer Inst. 111, 1216–1227 (2019).
Meltzer, P. S. & Helman, L. J. New horizons in the treatment of osteosarcoma. N. Engl. J. Med. 385, 2066–2076 (2021).
Zhou, Y. et al. The effect of pathological fractures on the prognosis of patients with osteosarcoma: a meta-analysis of 14 studies. Oncotarget 8, 73037–73049 (2017).
Papagelopoulos, P. J. et al. Current concepts in the evaluation and treatment of osteosarcoma. Orthopedics 23, 858–867 (2000).
Strauss, S. J. et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 32, 1520–1536 (2021).
Casali, P. G. et al. Bone sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv79–iv95 (2018).
WHO Classification of Tumours Editorial Board. WHO Classification of Tumours: Soft Tissue and Bone Tumours (International Agency for Research on Cancer, 2020).
Meyer, J. S. et al. Imaging guidelines for children with Ewing sarcoma and osteosarcoma: a report from the Children’s Oncology Group Bone Tumor Committee. Pediatr. Blood Cancer 51, 163–170 (2008).
Wolf, R. E. & Enneking, W. F. The staging and surgery of musculoskeletal neoplasms. Orthop. Clin. North. Am. 27, 473–481 (1996).
Sheth, D. S. et al. Conventional and dedifferentiated parosteal osteosarcoma. Diagnosis, treatment, and outcome. Cancer 78, 2136–2145 (1996).
Grimer, R. J. et al. Periosteal osteosarcoma — a European review of outcome. Eur. J. Cancer 41, 2806–2811 (2005).
Roberts, R. D. et al. Provocative questions in osteosarcoma basic and translational biology: a report from the Children’s Oncology Group. Cancer 125, 3514–3525 (2019).
Gorlick, R., Janeway, K., Lessnick, S., Randall, R. L. & Marina, N. Children’s Oncology Group’s 2013 blueprint for research: bone tumors. Pediatr. Blood Cancer 60, 1009–1015 (2013).
Aljubran, A. H., Griffin, A., Pintilie, M. & Blackstein, M. Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Ann. Oncol. 20, 1136–1141 (2009).
Marina, N. M. et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 17, 1396–1408 (2016). An international randomized clinical trial fails to show benefit of addition of I/E to MAP chemotherapy in the primary treatment of osteosarcoma.
Rosen, G., Murphy, M. L., Huvos, A. G., Gutierrez, M. & Marcove, R. C. Chemotherapy, en bloc resection, and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer 37, 1–11 (1976).
Rosen, G. et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer 49, 1221–1230 (1982).
Bishop, M. W. et al. Assessing the prognostic significance of histologic response in osteosarcoma: a comparison of outcomes on CCG-782 and INT0133 — a report from the Children’s Oncology Group Bone Tumor Committee. Pediatr. Blood Cancer 63, 1737–1743 (2016). This study validates the prognostic significance of pathological treatment response in osteosarcoma following neoadjuvant chemotherapy.
Bacci, G. et al. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Cancer 97, 3068–3075 (2003).
Meyers, P. A. et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J. Clin. Oncol. 16, 2452–2458 (1998).
Villani, A. et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol. 17, 1295–1305 (2016).
Diessner, B. J. et al. Nearly half of TP53 germline variants predicted to be pathogenic in patients with osteosarcoma are de novo: a report from the Children’s Oncology Group. JCO Precis. Oncol. 4, 1187–1195 (2020).
Kratz, C. P. et al. Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin. Cancer Res. 23, e38–e45 (2017).
Marees, T. et al. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J. Natl Cancer Inst. 100, 1771–1779 (2008).
Hendrickson, P. G. et al. Radiation therapy and secondary malignancy in Li-Fraumeni syndrome: a hereditary cancer registry study. Cancer Med. 9, 7954–7963 (2020).
Smeland, S. et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 109, 36–50 (2019).
Kempf-Bielack, B. et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J. Clin. Oncol. 23, 559–568 (2005).
Jaffe, N., Puri, A. & Gelderblom, H. Osteosarcoma: evolution of treatment paradigms. Sarcoma 2013, 203531 (2013).
Anninga, J. K. et al. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur. J. Cancer 47, 2431–2445 (2011).
Bielack, S. S. et al. Osteosarcoma: the same old drugs or more. J. Clin. Oncol. 26, 3102–3103 (2008).
Chou, A. J. et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children’s Oncology Group. Cancer 115, 5339–5348 (2009).
Brard, C. et al. Sarcome-13/OS2016 trial protocol: a multicentre, randomised, open-label, phase II trial of mifamurtide combined with postoperative chemotherapy for patients with newly diagnosed high-risk osteosarcoma. BMJ Open 9, e025877 (2019).
Grimer, R. J. et al. Osteosarcoma over the age of forty. Eur. J. Cancer 39, 157–163 (2003).
Ferrari, S. et al. EURO-B.O.S.S.: a European study on chemotherapy in bone-sarcoma patients aged over 40: Outcome in primary high-grade osteosarcoma. Tumori 104, 30–36 (2018).
Picci, P. et al. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J. Clin. Oncol. 12, 2699–2705 (1994).
Ruggieri, P. et al. Outcome of expandable prostheses in children. J. Pediatr. Orthop. 33, 244–253 (2013).
Tate, R., Gerrand, C. & Hale, J. Tibial turn-up procedure as an alternative to rotationplasty in a 4-year-old with osteosarcoma of the distal femur. J. Pediatr. Orthop. B 24, 50–55 (2015).
Hebert, J. S., Rehani, M. & Stiegelmar, R. Osseointegration for lower-limb amputation: a systematic review of clinical outcomes. JBJS Rev. 5, e10 (2017).
Ciernik, I. F. et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer 117, 4522–4530 (2011).
Matsunobu, A. et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer 118, 4555–4563 (2012).
Seidensaal, K. et al. The role of combined ion-beam radiotherapy (CIBRT) with protons and carbon ions in a multimodal treatment strategy of inoperable osteosarcoma. Radiother. Oncol. 159, 8–16 (2021).
NCCN. Treatment by cancer type. NCCN https://www.nccn.org/guidelines/category_1 (2022).
Gentet, J. C. et al. Ifosfamide and etoposide in childhood osteosarcoma. A phase II study of the French Society of Paediatric Oncology. Eur. J. Cancer 33, 232–237 (1997).
Lee, J. A. et al. Higher gemcitabine dose was associated with better outcome of osteosarcoma patients receiving gemcitabine-docetaxel chemotherapy. Pediatr. Blood Cancer 63, 1552–1556 (2016).
Miser, J. S. et al. Ifosfamide with mesna uroprotection and etoposide: an effective regimen in the treatment of recurrent sarcomas and other tumors of children and young adults. J. Clin. Oncol. 5, 1191–1198 (1987).
Rodríguez-Galindo, C. et al. Treatment of refractory osteosarcoma with fractionated cyclophosphamide and etoposide. J. Pediatr. Hematol. Oncol. 24, 250–255 (2002).
Davis, L. E. et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J. Clin. Oncol. 37, 1424–1431 (2019).
Grignani, G. et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann. Oncol. 23, 508–516 (2012).
Italiano, A. et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 21, 446–455 (2020).
Xie, L. et al. Apatinib for advanced osteosarcoma after failure of standard multimodal therapy: an open label phase II clinical trial. Oncologist 24, e542–e550 (2019).
Gaspar, N. et al. Lenvatinib with etoposide plus ifosfamide in patients with refractory or relapsed osteosarcoma (ITCC-050): a multicentre, open-label, multicohort, phase 1/2 study. Lancet Oncol. 22, 1312–1321 (2021).
Duffaud, F. et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 20, 120–133 (2019).
Lagmay, J. P. et al. Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: learning from the past to move forward. J. Clin. Oncol. 34, 3031–3038 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04154189 (2022).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Hecker-Nolting, S., Langer, T., Blattmann, C., Kager, L. & Bielack, S. S. Current insights into the management of late chemotherapy toxicities in pediatric osteosarcoma patients. Cancer Manag. Res. 13, 8989–8998 (2021).
Mason, G. E. et al. Quality of life following amputation or limb preservation in patients with lower extremity bone sarcoma. Front. Oncol. 3, 210 (2013).
Kratz, C. P. et al. Predisposition to cancer in children and adolescents. Lancet Child Adolesc. Health 5, 142–154 (2021).
Leone, G., Pagano, L., Ben-Yehuda, D. & Voso, M. T. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica 92, 1389–1398 (2007).
Boddu, P. et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv. 1, 1312–1323 (2017).
Armstrong, G. T. et al. Late mortality among 5-year survivors of childhood cancer: a summary from the childhood cancer survivor study. J. Clin. Oncol. 27, 2328–2338 (2009).
Mancilla, T. R., Iskra, B. & Aune, G. J. Doxorubicin-induced cardiomyopathy in children. Compr. Physiol. 9, 905–931 (2019).
Bhagat, A. & Kleinerman, E. S. Anthracycline-induced cardiotoxicity: causes, mechanisms, and prevention. Adv. Exp. Med. Biol. 1257, 181–192 (2020).
Rawat, P. S., Jaiswal, A., Khurana, A., Bhatti, J. S. & Navik, U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 139, 111708 (2021).
Armenian, S. H. et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 16, e123–e136 (2015).
Bock, M. J. et al. Cancer recurrence and mortality after pediatric heart transplantation for anthracycline cardiomyopathy: a report from the Pediatric Heart Transplant Study (PHTS) group. Pediatr. Transpl. https://doi.org/10.1111/petr.12923 (2017).
Shugh, S. B. & Ryan, T. D. Heart transplantation in survivors of childhood cancer. Transl Pediatr. 8, 314–321 (2019).
Curigliano, G. et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 31, 171–190 (2020).
Moke, D. J. et al. Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: a multi-institutional North American cohort study. Lancet Child Adolesc. Health 5, 274–283 (2021).
Romano, A. et al. Assessment and management of platinum-related ototoxicity in children treated for cancer. Cancers https://doi.org/10.3390/cancers12051266 (2020).
Clemens, E. et al. Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium. Lancet Oncol. 20, e29–e41 (2019).
Skinner, R. Late renal toxicity of treatment for childhood malignancy: risk factors, long-term outcomes, and surveillance. Pediatr. Nephrol. 33, 215–225 (2018).
Laws, H. J. et al. Impfen bei Immundefizienz : Anwendungshinweise zu den von der Ständigen Impfkommission empfohlenen Impfungen. (III) Impfen bei hämatologischen und onkologischen Erkrankungen (antineoplastische Therapie, Stammzelltransplantation), Organtransplantation und Asplenie [German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 63, 588–644 (2020).
Pittet, L. F. & Posfay-Barbe, K. M. Vaccination of immune compromised children-an overview for physicians. Eur. J. Pediatr. 180, 2035–2047 (2021).
Bader, M. S. Herpes zoster: diagnostic, therapeutic, and preventive approaches. Postgrad. Med. 125, 78–91 (2013).
van Santen, H. M. et al. Reproductive complications in childhood cancer survivors. Pediatr. Clin. North Am. 67, 1187–1202 (2020).
Oktay, K. et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 36, 1994–2001 (2018).
Stokke, J., Sung, L., Gupta, A., Lindberg, A. & Rosenberg, A. R. Systematic review and meta-analysis of objective and subjective quality of life among pediatric, adolescent, and young adult bone tumor survivors. Pediatr. Blood Cancer 62, 1616–1629 (2015).
Edelmann, M. N. et al. Neurocognitive and patient-reported outcomes in adult survivors of childhood osteosarcoma. JAMA Oncol. 2, 201–208 (2016).
Bekkering, W. P. et al. Quality of life after bone sarcoma surgery around the knee: a long-term follow-up study. Eur. J. Cancer Care https://doi.org/10.1111/ecc.12603 (2017).
Koirala, P. et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 6, 30093 (2016).
Landuzzi, L., Manara, M. C., Lollini, P. L. & Scotlandi, K. Patient derived xenografts for genome-driven therapy of osteosarcoma. Cells https://doi.org/10.3390/cells10020416 (2021).
Higuchi, T. et al. Osteosarcoma patient-derived orthotopic xenograft (PDOX) models used to identify novel and effective therapeutics: a review. Anticancer. Res. 41, 5865–5871 (2021).
Loh, A. H. P. et al. Combinatorial screening using orthotopic patient derived xenograft-expanded early phase cultures of osteosarcoma identify novel therapeutic drug combinations. Cancer Lett. 442, 262–270 (2019).
Lilienthal, I. & Herold, N. Targeting molecular mechanisms underlying treatment efficacy and resistance in osteosarcoma: a review of current and future strategies. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21186885 (2020).
DeRenzo, C. & Gottschalk, S. Genetically modified T-cell therapy for osteosarcoma: into the roaring 2020s. Adv. Exp. Med. Biol. 1257, 109–131 (2020).
Wang, Y. et al. Comprehensive surfaceome profiling to identify and validate novel cell-surface targets in osteosarcoma. Mol. Cancer Ther. https://doi.org/10.1158/1535-7163.Mct-21-0836 (2022).
Whittle, S. B. et al. Charting a path for prioritization of novel agents for clinical trials in osteosarcoma: a report from the Children’s Oncology Group New Agents for Osteosarcoma Task Force. Pediatr. Blood Cancer 68, e29188 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05135975 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04055220 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04833582 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04417062 (2021).
FDA. Considerations for the inclusion of adolescent patients in adult oncology clinical trials: guidance for industry. FDA https://bit.ly/2IMiAdT (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03635632 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04539366 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03721068 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00902044 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03618381 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04483778 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04897321 (2022).
Gianferante, D. M., Mirabello, L. & Savage, S. A. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 13, 480–491 (2017).
Meyers, P. A. & Gorlick, R. Osteosarcoma. Pediatr. Clin. North. Am. 44, 973–989 (1997).
Kansara, M., Teng, M. W., Smyth, M. J. & Thomas, D. M. Translational biology of osteosarcoma. Nat. Rev. Cancer 14, 722–735 (2014).
Jubelin, C. et al. Biological evidence of cancer stem-like cells and recurrent disease in osteosarcoma. Cancer Drug Resist. 5, 184–198 (2022).
Ségaliny, A. I., Tellez-Gabriel, M., Heymann, M. F. & Heymann, D. Receptor tyrosine kinases: characterisation, mechanism of action and therapeutic interests for bone cancers. J. Bone Oncol. 4, 1–12 (2015).
Bray, S. J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 (2006).
Ségaliny, A. I. et al. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int. J. Cancer 137, 73–85 (2015).
Ory, B. et al. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma‐bearing mice. Cancer 104, 2522–2529 (2005).
Dharia, N. V. et al. A first-generation pediatric cancer dependency map. Nat. Genet. 53, 529–538 (2021).
Jia, S.-F., Worth, L. L. & Kleinerman, E. S. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin. Exp. Metastasis 17, 501–506 (1999).
Khanna, C. et al. An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin. Exp. Metastasis 18, 261–271 (2000).
Boyle, D. B. & Coupar, B. E. H. Identification and cloning of the Fowlpox virus thymidine kinase gene using Vaccinia virus. J. Gen. Virol. 67, 1591–1600 (1986).
Fan, T. M., Roberts, R. D. & Lizardo, M. M. Understanding and modeling metastasis biology to improve therapeutic strategies for combating osteosarcoma progression. Front. Oncol. https://doi.org/10.3389/fonc.2020.00013 (2020).
Zhao, S. et al. NKD2, a negative regulator of Wnt signaling, suppresses tumor growth and metastasis in osteosarcoma. Oncogene 34, 5069–5079 (2015).
Mendoza, A. et al. Modeling metastasis biology and therapy in real time in the mouse lung. J. Clin. Invest. 120, 2979–2988 (2010).
Lizardo, M. M. & Sorensen, P. H. Practical considerations in studying metastatic lung colonization in osteosarcoma using the pulmonary metastasis assay. J. Vis. Exp. https://doi.org/10.3791/56332 (2018).
Tsai, Y. C. et al. The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat. Med. 13, 1504–1509 (2007).
Lizardo, M. M. et al. Upregulation of glucose-regulated protein 78 in metastatic cancer cells is necessary for lung metastasis progression. Neoplasia 18, 699–710 (2016).
Morrow, J. J. et al. mTOR inhibition mitigates enhanced mRNA translation associated with the metastatic phenotype of osteosarcoma cells in vivo. Clin. Cancer Res. 22, 6129–6141 (2016).
Yu, P. Y. et al. Target specificity, in vivo pharmacokinetics, and efficacy of the putative STAT3 inhibitor LY5 in osteosarcoma, Ewing’s sarcoma, and rhabdomyosarcoma. PLoS ONE 12, e0181885 (2017).
Gillet, J.-P. et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc. Natl Acad. Sci. USA 108, 18708–18713 (2011).
Wilding, J. L. & Bodmer, W. F. Cancer cell lines for drug discovery and development. Cancer Res. 74, 2377–2384 (2014).
Phan, N. et al. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol. https://doi.org/10.1038/s42003-019-0305-x (2019).
Stewart, E. et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature 549, 96–100 (2017).
Houghton, P. J. et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr. Blood Cancer 49, 928–940 (2007).
Morton, C. L. & Houghton, P. J. Establishment of human tumor xenografts in immunodeficient mice. Nat. Protoc. 2, 247–250 (2007).
Mundi, P. S. et al. Pre-clinical validation of an RNA-based precision oncology platform for patient-therapy alignment in a diverse set of human malignancies resistant to standard treatments. Preprint at bioRxiv https://doi.org/10.1101/2021.10.03.462951 (2021).
Ben-David, U. et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 49, 1567–1575 (2017).
Woo, X. Y. et al. Conservation of copy number profiles during engraftment and passaging of patient-derived cancer xenografts. Nat. Genet. 53, 86–99 (2021).
Jacques, C. et al. Murine models of bone sarcomas. Methods Mol. Biol. 1914, 331–342 (2019).
Wang, Z. Q., Liang, J., Schellander, K., Wagner, E. F. & Grigoriadis, A. E. c-fos-induced osteosarcoma formation in transgenic mice: cooperativity with c-jun and the role of endogenous c-fos. Cancer Res. 55, 6244–6251 (1995).
Fenger, J. M., London, C. A. & Kisseberth, W. C. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J. 55, 69–85 (2014).
Gardner, H. L. et al. Canine osteosarcoma genome sequencing identifies recurrent mutations in DMD and the histone methyltransferase gene SETD2. Commun. Biol. 2, 266 (2019).
LeBlanc, A. K. et al. Perspectives from man’s best friend: National Academy of Medicine’s Workshop on Comparative Oncology. Sci. Transl Med. 8, 324ps325 (2016).
Paoloni, M. et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics 10, 625 (2009).
Dow, S. A role for dogs in advancing cancer immunotherapy research. Front. Immunol. 10, 2935 (2020).
Acknowledgements
R.G. is supported as the H. Grant Taylor, M.D., W.W. Sutow, M.D. and Margaret P. Sullivan, M.D. Distinguished Chair in Paediatrics. J.G. and R.G. both acknowledge the support of the Foster Foundation, Swim Across America Inc., the Osteosarcoma Institute, the QuadW Foundation and the Barbara Epstein Foundation. A.M.F. is supported by the Tom Prince Cancer Trust, the Bone Cancer Research Trust, Sarcoma UK, the Cancer Research UK University College London Experimental Cancer Medicine Centre, the RNOH Research and Development Department, and the National Institute for Health Research, University College London Hospitals Biomedical Research Centre. H.C.B. acknowledges support by Triumph Over Kid Cancer Foundation (to Valerae Lewis), A Shelter for Cancer Families, formerly Amshwand Sarcoma Cancer Foundation (to the Sarcoma Medical Oncology Department at MD Anderson Cancer Center), QuadW Foundation (to the Sarcoma Oncology Group), and Cancer Prevention Research Institute of Texas. S.S. is funded in part by the National Institute for Health Research, University College London Hospitals Biomedical Research Centre. J.A.L. acknowledges the support of the Osteosarcoma Institute, the Rally Foundation, and the Make It Better (MIB) Agents. R.D.R. is supported by the National Institutes of Health/National Cancer Institute, the Osteosarcoma Institute, the Hyundai Hope on Wheels Foundation, the CancerFREE Kids Foundation, Steps for Sarcoma, and the St. Baldrick’s Foundation. D.H. acknowledges support from ICO Cancer Center, France (ref# “DorSarc-2018-ICO-DH”), Ouest Valorisation SATT (France) and the Bone Cancer Research Trust (UK). K.J. acknowledges support from Pan Mass Challenge and philanthropic funds supporting osteosarcoma research at Dana-Farber Cancer Institute. The work of S.B. is charitably supported by Förderkreis krebskranke Kinder Stuttgart e.V. We are very thankful to W.-L. Wang and A. Lazar of the Division of Pathology at MD Anderson Cancer Center for providing the histology figures as well as their descriptions.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all sections of the Primer. Overview of Primer (R.G.).
Corresponding author
Ethics declarations
Competing interests
Since 2019, S.B. has been on Advisory Boards for Eli Lilly, Ipsen, Hoffmann La Roche, Bayer Healthcare, Boehringer Ingelheim, EISAI and MAP Biopharma. All other authors declare no competing interests.
Peer review
Peer Review information
Nature Reviews Disease Primers thanks G. Stein, K. Ogura, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beird, H.C., Bielack, S.S., Flanagan, A.M. et al. Osteosarcoma. Nat Rev Dis Primers 8, 77 (2022). https://doi.org/10.1038/s41572-022-00409-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-022-00409-y
This article is cited by
-
Prognostic and predictive value of super-enhancer-derived signatures for survival and lung metastasis in osteosarcoma
Journal of Translational Medicine (2024)
-
Harnessing ferroptosis for enhanced sarcoma treatment: mechanisms, progress and prospects
Experimental Hematology & Oncology (2024)
-
Soy isoflavones induces mitophagy to inhibit the progression of osteosarcoma by blocking the AKT/mTOR signaling pathway
Molecular Medicine (2024)
-
High-performance pyrite nano-catalyst driven photothermal/chemodynamic synergistic therapy for Osteosarcoma
Journal of Nanobiotechnology (2024)
-
In vivo therapy of osteosarcoma using anion transporters-based supramolecular drugs
Journal of Nanobiotechnology (2024)