Abstract
Hypothyroidism is the common clinical condition of thyroid hormone deficiency and, if left untreated, can lead to serious adverse health effects on multiple organ systems, with the cardiovascular system as the most robustly studied target. Overt primary hypothyroidism is defined as elevated thyroid-stimulating hormone (TSH) concentration in combination with free thyroxine (fT4) concentration below the reference range. Subclinical hypothyroidism, commonly considered an early sign of thyroid failure, is defined by elevated TSH concentrations but fT4 concentrations within the reference range. Hypothyroidism is classified as primary, central or peripheral based on pathology in the thyroid, the pituitary or hypothalamus, or peripheral tissue, respectively. Acquired primary hypothyroidism is the most prevalent form and can be caused by severe iodine deficiency but is more frequently caused by chronic autoimmune thyroiditis in iodine-replete areas. The onset of hypothyroidism is insidious in most cases and symptoms may present relatively late in the disease process. There is a large variation in clinical presentation and the presence of hypothyroid symptoms, especially in pregnancy and in children. Levothyroxine (LT4) is the mainstay of treatment and is one of the most commonly prescribed drugs worldwide. After normalization of TSH and fT4 concentrations, a considerable proportion of patients treated with LT4 continue to have persistent complaints, compromising quality of life. Further research is needed regarding the appropriateness of currently applied reference ranges and treatment thresholds, particularly in pregnancy, and the potential benefit of LT4/liothyronine combination therapy for thyroid-related symptom relief, patient satisfaction and long-term adverse effects.
Similar content being viewed by others
Introduction
Thyroid hormone is essential for the normal development of many human tissues and regulates the metabolism of virtually all cells and organs of the human body throughout life. Hypothyroidism, the clinical condition of thyroid hormone deficiency, is a common disorder in the general population. Overt hypothyroidism is defined by thyroid-stimulating hormone (TSH) levels above the upper limit of the reference range while levels of free thyroxine (fT4) are below the lower limit of the reference range. The reference range is typically statistically defined by the 2.5th and 97.5th percentiles of the measured circulating thyroid hormone values in populations defined as healthy. In subclinical hypothyroidism, TSH levels are elevated but fT4 levels are still within the reference range. Untreated hypothyroidism, especially overt hypothyroidism, can lead to serious adverse effects on multiple organ systems, both in the short and long term. Most adult patients with hypothyroidism have acquired hypothyroidism, which originates either in the thyroid (primary hypothyroidism) or in the pituitary or hypothalamus (central hypothyroidism). Hypothyroidism can also result from severe iodine deficiency because the synthesis of thyroid hormone requires the trace element iodine. Chronic autoimmune thyroid disease, Hashimoto thyroiditis, is the most common cause of primary hypothyroidism in iodine-replete areas. Because thyroid hormone is also essential for multiple aspects of normal development in childhood, most developed countries have established neonatal screening programmes to detect congenital hypothyroidism (prevalence of 1 in 500–3,000 newborns, depending on ethnicity)1,2 as well as programmes to prevent severe iodine deficiency (for example, universal salt iodization).
The onset of hypothyroidism is insidious in most cases and symptoms may present late in the disease process. Symptoms are generally non-specific and the most common include fatigue, cold intolerance and constipation. As a consequence, there is a large variation in clinical presentation and the presence of symptoms has a low sensitivity and positive predictive value (that is, symptoms are not specific to hypothyroidism) for diagnosis. Therefore, detection of high TSH and low fT4 levels is the hallmark of diagnosis3 (Fig. 1).
Most symptoms attributed to hypothyroidism are common in the general population and are non-specific. Less common symptoms of hypothyroidism (not shown) include dry skin (when severe, a non-pitting oedema termed myxoedema), hoarseness, anaemia (usually normochromic and normocytic but occasionally macrocytic), increased thrombosis risk (due to impaired coagulation and fibrinolysis) and various neurological (carpal tunnel syndrome and encephalopathy), musculoskeletal (myalgia and increased serum creatine kinase levels) and metabolic (hyponatraemia and increase in serum creatine kinase levels) symptoms.
Levothyroxine (LT4), a synthetic form of T4 that is the mainstay of treatment for hypothyroidism, is the third most commonly prescribed drug in the United States, with over 100 million prescriptions4,5. Data from commercially insured patients revealed that an estimated 7% of the population have an active LT4 prescription, with 30% of patients displaying normal thyroid function values at initiation of therapy6. After normalization of TSH and fT4 concentrations, up to 15% of patients treated with LT4 continue to have persistent complaints, which may require evaluation for alternative diagnoses7. In this Primer, we discuss the pathophysiology, diagnosis, optimal treatment and quality of life of patients with hypothyroidism as well as current knowledge gaps and research priorities.
Epidemiology
Primary hypothyroidism
Primary hypothyroidism is a common disorder worldwide (Fig. 2a; Supplementary Table 1), with iodine deficiency and Hashimoto thyroiditis as the principal causes. Other less common causes of primary hypothyroidism include congenital, drug-related, iatrogenic and infiltrative diseases (Table 1). Prevalence estimates from the general population typically do not distinguish between causes of primary hypothyroidism and depend on several factors, including the population studied and the definition used (for example, whether subclinical hypothyroidism is included). In the USA, National Health and Nutrition Examination Survey data showed a prevalence of hypothyroidism (both overt and subclinical) of 4.6%8. A US screening study showed a prevalence of 0.4% and 9% for overt and subclinical hypothyroidism, respectively, with the latter increasing to over 20% among women 75 years of age or older9. In a meta-analysis from Europe, the prevalence of overt and subclinical hypothyroidism was 0.37% and 3.8%, respectively, including both diagnosed and undiagnosed cases, and the estimated incidence of hypothyroidism was 226 cases per 100,000 individuals per year10.
a | Worldwide prevalence of overt hypothyroidism based on epidemiological studies (Supplementary Table 1). The median value was calculated for countries for which data are available from multiple studies. b | Global iodine nutrition status in 2021 (ref.186) based on iodine intake in the general population as assessed by median urinary iodine concentration (mUIC) in school-aged children from studies conducted between 2005 and 2020.
Primary hypothyroidism prevalence is highest in populations with high iodine intake or severe iodine deficiency as compared with populations with a sufficient iodine status11 (Fig. 2b). The prevalence decreases with the declining severity of iodine deficiency and increases as iodine intake shifts from mild deficiency to optimal or excessive intake. Furthermore, improvement in iodine status also increases thyroid antibody positivity12 and therefore the risk of Hashimoto thyroiditis.
Hypothyroidism occurrence is dependent on genetic, inherent (for example, sex) and environmental factors. A meta-analysis of genome-wide association studies (GWAS), which included more than 70,000 participants from 22 cohorts, identified 42 loci for circulating TSH levels within the reference range13. Only 7 of these 42 loci were linked to hypothyroidism, including thyroid peroxidase (TPO), which encodes an enzyme crucial for the synthesis of thyroid hormone13. Individuals with a TSH-based genetic risk score in the highest quartile had a 2.5-fold increased odds of hypothyroidism compared with individuals with a genetic risk score in the lowest quartile13. No differences were found between men and women in genetic variants for TSH and fT4 in sex-stratified GWAS meta-analyses13. Nevertheless, the risk of developing primary hypothyroidism is up to tenfold higher in women than in men, suggesting an important contribution of non-genetic factors14.
TPO antibody concentrations are lower in smokers than in non-smokers15,16. Furthermore, TSH levels are lower in current smokers than in former smokers, and lower in former smokers than in never smokers15,16. Smoking initiation results in a significant decrease in serum TSH levels after 1 year in men. Obesity is associated with higher serum TSH levels in adults and children, although the directionality of the relationship is under discussion and may even be bidirectional17,18. Children born small for gestational age have higher serum TSH levels than children born appropriate for gestational age and are consequently more often diagnosed with subclinical hypothyroidism19. Environmental factors that have been implicated in hypothyroidism and Hashimoto thyroiditis include vitamin D and selenium deficiency, and moderate alcohol intake16.
Central and peripheral hypothyroidism
Central hypothyroidism is a rare disorder that may be due to secondary hypothyroidism (pathology of the pituitary) or due to tertiary hypothyroidism (pathology of the hypothalamus) and can be congenital or acquired (Table 1). Incidence estimates for congenital central hypothyroidism range from 1:21,000 to 1:160,000 (refs20,21), with variability attributed at least in part to differences in neonatal diagnostic strategies. The most common causes of central hypothyroidism in adults include pituitary adenoma, infiltrative disease and radiotherapy. Increased use of immune-checkpoint inhibitors in the setting of cancer treatment over the past decade has resulted in a surge in hypophysitis-related central hypothyroidism, although the exact mechanisms are still poorly understood22. Peripheral hypothyroidism (that is, extra-thyroidal) denotes a diverse group of disorders with defects that reduce the effectiveness of thyroid hormone through altered cell membrane transport and metabolism. These rare disorders may be caused by (congenital) genetic alterations causing decreased sensitivity to the biological activity of chemically intact hormones and typically tissue-specific hypothyroidism. Another example of peripheral hypothyroidism is consumptive hypothyroidism, which is due to an increased expression of type 3 iodothyronine deiodinase (DIO3, an enzyme that inactivates thyroid hormone) in tumours (for example, gastrointestinal stromal tumours)23.
Mechanisms/pathophysiology
Physiological aspects
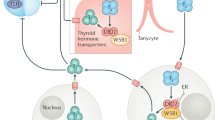
Thyroid hormone production and release are regulated by a very sensitive feedback loop, the hypothalamus–pituitary–thyroid axis (Fig. 3). Thyrotropin-releasing hormone (TRH) produced in the hypothalamus controls production of TSH by the anterior pituitary gland. TSH in turn regulates the production and secretion of the two forms of thyroid hormone by the thyroid gland: T4 and the more bioactive hormone triiodothyronine (T3)24. Serum TSH levels follow a circadian rhythm: levels are highest between 9 pm and 5 am and lowest between 4 pm and 7 pm (ref.25). The thyroid gland secretes predominantly T4 and, to a lesser extent, T3, which accounts for up to only ~20% of circulating T3. The remaining T3 is produced by peripheral tissues, such as liver and skeletal muscle, by the activating enzymes type 1 and type 2 iodothyronine deiodinase (DIO1 and DIO2, respectively), which cleave an iodine atom from T4 (ref.26). Most circulating T4 and T3 is bound to transport proteins such as thyroxine-binding globulin (TBG), transthyretin and albumin. Only ~0.02% of T4 and ~0.2% of T3 are present in an unbound form and this fT4 and free T3 (fT3) can be measured for diagnostic purposes27.
Thyrotropin-releasing hormone (TRH) neurons receive various central modulators and other inputs. Thyroid hormone receptor-β2 (TRβ2) expressed in TRH neurons mediates feedback regulation by thyroid hormones. Type 2 iodothyronine deiodinase (DIO2) is required for the production of the active thyroid hormone triiodothyronine (T3) from thyroxine (T4). Production and release of thyroid-stimulating hormone (TSH) from the anterior pituitary is modulated by both thyroid hormones and TRH. In the periphery, only the free fraction of thyroid hormones can be transported into target cells. The effects of thyroid hormones are mediated via interaction of the active hormone T3 with the nuclear T3 receptor, which together bind to thyroid response elements (TREs) and modulate the expression of thyroid hormone-responsive genes. TRs, nuclear T3 receptors. Adapted from refs187,188, Springer Nature Limited.
Most biological activities of thyroid hormone are mediated by binding of T3 to the nuclear T3 receptors (TRs), which bind to thyroid response elements (TREs) in thyroid hormone-responsive genes and modulate their expression. The two thyroid receptor genes THRA and THRB encode thyroid hormone receptor-α (TRα; also known as THRα) and TRβ (also known as THRβ), respectively. Alternative splicing and different promoter usage results in the production of three THRα and three THRβ isoforms, of which TRα1, TRβ1 and TRβ2 bind to T3. TRα1 and TRβ1 are ubiquitously expressed, TRα1 preferentially in brain, heart, and bone and TRβ1 preferentially in liver, kidney and thyroid. TRβ2 has a more restricted expression pattern but is the predominant isoform expressed in the pituitary gland and is thereby essential for the negative regulation of TSH28. Intracellular T3 concentrations strongly determine the biological activity of thyroid hormone. Different processes are involved in the regulation of intracellular thyroid hormone concentrations, such as the fT4 and fT3 concentrations in serum, the activity of the intracellular DIO1, DIO2 and DIO3 enzymes that can activate or inactivate thyroid hormone, and transporter proteins at the cell membrane that facilitate uptake and efflux of T4 and T3. Only fT4 and fT3 are available for transport into thyroid hormone-target cells29,30,31.
Primary hypothyroidism
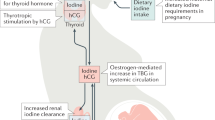
Primary hypothyroidism has various causes, mostly affecting thyrocyte function, and a wide range of underlying pathophysiological mechanisms (Table 1). Chronic autoimmune thyroiditis is the most common cause of primary hypothyroidism and most typically manifests as Hashimoto thyroiditis. Many factors potentially contribute to and might interact in the development of chronic autoimmune thyroiditis, including genetic and environmental factors, micronutrients (mainly iodine and selenium), drugs, infiltration and/or infection, immune system defects (for example, polyglandular syndromes), and molecular mimicry between microbial and host antigens32. High concentrations of anti-thyroid antibodies (predominantly anti-TPO (TPOAb) and antithyroglobulin antibodies) are present in most patients with autoimmune thyroiditis but also occur in ~10% of the euthyroid general population8. In pregnancy, TPOAb positivity is seen in 2–17% of women and may accompany higher serum TSH levels during the first trimester14. In more than 40% of pregnant women with thyroid autoimmunity, serum fT4 concentration falls in the hypothyroid range during late pregnancy, which may complicate diagnosing overt hypothyroidism during the third trimester33. This is due to inadequate maternal thyroid capacity in response to increased demands in thyroid hormone production imposed by stimulation of the thyroid by human chorionic gonadotropin, increases in TBG, and changes in placental deiodination and renal clearance of iodine during pregnancy34. The rates of miscarriages and preterm delivery are increased in pregnant women with thyroid autoimmunity35. A negative association of TPOAb positivity during pregnancy with neurodevelopment of offspring has been suggested but needs to be further investigated36.
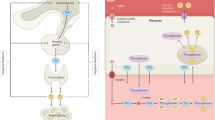
Chronic autoimmune thyroiditis has been attributed to failure of T cell-mediated inflammatory responses through complex mechanisms involving antigen-presenting T cells and B cells, amongst others (Fig. 4). Infiltration of thyroid tissue by lymphocytes, mainly T helper 1 (TH1) cells, can directly alter thyroid follicular cell function through the actions of IL-1, TNF and IFNγ37,38. Chemokines (small chemoattractant molecules that are structurally similar to cytokines) have also been implicated in thyroid infiltration, which may be induced by IFNγ39.
Thyroid autoimmunity is the result of the interplay of genetic and environmental factors that cause damage to thyroid cells, leading to autoantigen release and presentation. Entry of autoreactive immune cells into the thyroid leads to activation of cellular and humoral immune responses, cytokine production, and cytotoxicity and apoptosis. TH1, T helper 1; TSHR, thyroid-stimulating hormone receptor.
Iodine is an essential micronutrient and is crucial for the biosynthesis of thyroid hormone. Both iodine deficiency and iodine excess may cause hypothyroidism but overt hypothyroidism mainly occurs in the context of severe iodine deficiency and is commonly accompanied by goitre40. A high dietary intake of iodine is well tolerated in most individuals, but following exposure to high iodine levels in high-risk individuals (such as those who are prone to Hashimoto thyroiditis), the synthesis of thyroid hormone can be inhibited by the so-called Wolff–Chaikoff effect without resumption of iodine organification after a few days41. Hypothyroidism with or without goitre may therefore be observed following chronic administration of large doses of iodine from iodinated contrast material, the heavily iodinated antiarrhythmic amiodarone, the topical antiseptic povidone-iodine or iodine-containing thyroid supplements that are available without prescription42. Optimal functioning of the thyroid gland is also dependent on the essential trace element selenium, which directly affects thyroid hormone metabolism and redox processes43; insufficient selenium intake is associated with elevated risk of thyroid disease44.
Both internal and external irradiation of the thyroid gland may cause hypothyroidism. Radioactive iodine (131I) therapy may be administered for treatment of hyperthyroidism or thyroid cancer. Ablative doses of radioiodine recommended to treat Graves disease (autoimmune hyperthyroidism) cause permanent hypothyroidism in most patients45. Radioiodine treatment of toxic nodular goitre or non-toxic nodular goitre (enlarged thyroid with or without hyperthyroidism, respectively) results in hypothyroidism in ~25% of patients46,47. External radiation with doses ≥25 Gy (2,500 rad) to the head and neck region for malignant tumours may cause permanent hypothyroidism in >50% of patients48. Total thyroidectomy or near-total thyroidectomy in the context of thyroid cancer, Graves disease or multinodular goitre treatment are important causes of iatrogenic overt hypothyroidism. Subtotal thyroidectomy results in hypothyroidism in ~50% of patients49.
Other causes of hypothyroidism include transient thyroiditis or forms of destructive thyroiditis. Postpartum thyroiditis with subsequent hypothyroidism is common, resulting in an estimated prevalence of postpartum thyroid dysfunction of 2–10%50. Overt and subclinical hypothyroidism are seen in 14–27% of patients with primary thyroid lymphoma and 30–40% of patients with Reidel thyroiditis51,52,53. Hypothyroidism due to thyroid infection with Pneumocystis jirovecii, tuberculosis and brucellosis have been reported54,55. COVID-19-related thyroid dysfunction has been reported, with hypothyroidism mainly described as a consequence of subacute thyroiditis, although the exact mechanism is not yet clear56. Hypothyroidism is also more common in patients with other autoimmune diseases, particularly type 1 diabetes mellitus, autoimmune gastric atrophy and coeliac disease (for example, occurring as part of autoimmune poly-endocrinopathies).
Hypothyroidism may occur following administration of a range of medications through various processes that interfere with endogenous thyroid function57. For example, 20% of patients treated with lithium will develop hypothyroidism. Lithium increases intrathyroidal iodine content, diminishes coupling of iodotyrosine residues to T4 and T3, and inhibits thyroid hormone release58. Hypothyroidism occurs in 5–15% of individuals receiving amiodarone59. Clinically relevant thyroid dysfunction occurs in 58% and 32% of patients treated with IFNα and IL-2, respectively, in part due to activation of autoimmune processes60. Hypothyroidism occurs in 18–52% of patients receiving tyrosine kinase inhibitor therapy61. Immune-checkpoint inhibitors are associated with immune-related adverse events, including thyroid dysfunction and hypophysitis, which can lead to central hypothyroidism. More than 20% of patients treated with anti-CTLA4 or anti-PD1 monoclonal antibodies, but mainly with both in combination, develop either thyroiditis, with potentially subsequent hypothyroidism, or primary hypothyroidism62, and up to 15% develop hypophysitis63,64.
Cigarette smoking causes a decrease in serum TSH and TPOAb levels and a decreased risk of hypothyroidism in patients with underlying chronic autoimmune thyroiditis15. Numerous naturally occurring environmental chemicals, herbicides, pesticides and industrial chemicals have been reported to cause thyroid hypofunction65.
Congenital primary hypothyroidism may be caused by an absent, underdeveloped or ectopic thyroid gland (dysgenesis) or by defective thyroid hormone biosynthesis (dyshormonogenesis). Mutations in TSHR, FOXE1, NKX2-1, PAX8 and NKX2-5 have been implicated in thyroid dysgenesis and those in SLC5A5, TPO, DUOX2, DUOXA2, SLC6A4 and DHEAL1 have been implicated in dyshormonogenesis. Most cases of congenital hypothyroidism are due to thyroid ectopy, with fewer than 5% of cases attributable to a mutation in a gene involved in differentiation, migration or growth of the thyroid66,67.
Central and peripheral hypothyroidism
Central hypothyroidism is characterized by insufficient stimulation of the normal thyroid gland by TSH, resulting in a defect in thyroid hormone production. Congenital central hypothyroidism is very rare, whereas acquired disease is more common, occurs mainly in adults, has a variable pathogenesis and is most frequently caused by pituitary adenoma (Table 1). In most cases, the thyrotroph defect is combined with multiple other hormone deficiencies68. The concentration of serum TSH is often within the reference range in patients with central hypothyroidism but the secreted TSH isoform, although immunoreactive, has severely impaired biological activity. Therefore, the combination of inappropriately normal serum TSH and low circulating fT4 levels is common in patients with central hypothyroidism69. In addition, transient or reversible forms of the disease may occur in patients with prolonged thyrotoxicosis, newborns of hyperthyroid mothers, and individuals treated with somatostatin, glucocorticoids, antineoplastic agents or dopaminergic compounds68,69,70.
Peripheral hypothyroidism denotes a diverse group of disorders with defects that reduce the effectiveness of thyroid hormone through altered cell membrane transport and metabolism and are rare causes of hypothyroidism71. These disorders include consumptive hypothyroidism (for example, upregulation of DIO3 in tumour cells) as well as tissue-specific hypothyroidism due to decreased sensitivity to thyroid hormone in patients with mutations in, for example, MCT8, THRA or THRB, each of which contribute to a specific set of clinical signs and symptoms.
Diagnosis, screening and prevention
Common symptoms and clinical presentation
Hypothyroidism has clinical implications for nearly all organs and can therefore be associated with a multitude of symptoms of varying severity, depending on the degree of thyroid hormone deficiency, irrespective of the cause72.
Almost all of the manifestations associated with hypothyroidism are either due to a generalized reduction of metabolic processes (for example, symptoms such as fatigue, cold intolerance, bradycardia and weight gain) or to accumulation of matrix glycosaminoglycans in tissue interstitial spaces (leading to coarse hair and hoarseness of voice). The symptoms of hypothyroidism can range from mild, with few or almost no symptoms (especially in subclinical hypothyroidism) to very severe (including presentation with life-threatening myxoedema coma). The onset of hypothyroidism in most cases is insidious and, therefore, symptoms and signs can be vague, wide-ranging and present late in the disease process, thus making it difficult to distinguish from other conditions. One of the most common symptoms is fatigue or tiredness. Other common symptoms include dry skin, weight gain and constipation (Fig. 1). Reduced gastrointestinal tract and gallbladder motility73 have been suggested as underlying mechanisms for constipation, gallbladder hypotonia and bile duct stone formation in hypothyroidism. Mild hepatocellular dysfunction may occur and hypothyroidism is considered a risk factor for non-alcoholic fatty liver disease and occasional steatohepatitis74. Impaired glomerular function, changes in renal tubular function, impaired free water clearance and hyponatraemia have all been described in hypothyroidism75. Overt hypothyroidism in adults can contribute to entrapment neuropathies (such as carpal tunnel syndrome) and metabolic polyneuropathies, impaired memory, poor concentration, musculoskeletal symptoms, sleep apnoea, depression, and other psychiatric disturbances76. Severe and sustained hypothyroidism results in increased vascular resistance, decreased cardiac output, and decreased left ventricular function77. Other cardiovascular effects include myocardial injury, pericardial effusion and elements of metabolic syndrome, including hypertension, increased waist circumference and dyslipidaemia78,79.
However, most symptoms and signs associated with hypothyroidism are non-specific and do not by their presence confirm the diagnosis. Furthermore, a number of the common symptoms attributed to hypothyroidism have a high prevalence in adults80. For example, in a study of individuals attending a health fair, 12% of individuals with overt hypothyroidism, 7.4% of those with mild (or subclinical) hypothyroidism and 7.7% of those who were euthyroid reported hypothyroid symptoms9. Thus, the presence of symptoms of hypothyroidism alone has a low sensitivity and positive predictive value. Furthermore, it is uncertain how many of the symptoms attributed to hypothyroidism (such as fatigue or weight gain) might be related to ageing, particularly as serum TSH levels have been suggested to increase with age81. In fact, an increased severity of symptoms might predict hypothyroidism as a study reported a likelihood ratio of 8.7 for the presence of hypothyroidism when patients reported changes in seven or more symptoms in the previous year3. Nevertheless, utilizing symptoms alone to diagnose hypothyroidism would lead to an unacceptably high proportion of euthyroid individuals being falsely diagnosed with hypothyroidism82,83. Patients with hypothyroidism might present with one or more symptoms of hypothyroidism or when abnormal thyroid test results are noted as part of routine screening tests in the setting of other medical conditions such as dyslipidaemia, atrial fibrillation, cognitive decline, unexplained weight gain or subfertility. As thyroid function testing is frequently ordered, it is not surprising that many individuals are diagnosed with incidental, usually subclinical, hypothyroidism84. Diagnosing hypothyroidism is particularly challenging in pregnant women and in children. In pregnancy, patients with hypothyroidism may present with one or more symptoms that are typically associated with hypothyroidism (for example, tiredness or weight gain) but may be misattributed to the pregnancy itself. In practice, especially in regions without screening strategies, most women are diagnosed late in the course of pregnancy, either incidentally or due to screening for thyroid dysfunction in those presenting with associated complications such as gestational hypertension or pre-eclampsia85,86. Because of the importance of thyroid hormone for normal brain development in early life, gestational hypothyroidism caused by iodine deficiency, untreated or undiagnosed overt hypothyroidism during pregnancy, and untreated congenital hypothyroidism can cause severe neurocognitive and psychomotor dysfunction in offspring. Cretinism, a condition caused by a severe gestational iodine deficiency, is characterized by severely stunted physical and mental growth in early childhood87. In children, prolonged and severe overt hypothyroidism might present not only with the typical symptoms observed in adults (such as fatigue, unexplained weight gain or cold intolerance) but also with goitre, delayed growth or delayed puberty88.
Myxoedema coma
Myxoedema coma is the most extreme form of hypothyroidism and can progress to death unless diagnosed and treated promptly. Myxoedema coma has a mortality rate of 50–60%; thus, early recognition is vital89. Myxoedema coma might present de novo or, more likely, might be precipitated in a patient with hypothyroidism by a number of drugs, systemic illnesses (such as pneumonia) or other causes89. Myxoedema coma is more commonly seen in older women in winter and might present with the typical signs of severe hypothyroidism as well as hypothermia, hyponatraemia, hypercarbia and hypoxaemia. It is critical that treatment with thyroid hormone therapy is initiated promptly, ideally in an intensive care unit setting89. However, the type of thyroid hormone to administer (thyroxine, triiodothyronine or both) is unclear. In addition to thyroid hormone therapy, adjunctive measures, such as ventilation, warming, fluids, antibiotics, vasopressor agents and corticosteroids, are equally essential for survival89.
Diagnostic workflow
Prior to the advent of biochemical testing of thyroid function, symptoms and signs of hypothyroidism were the mainstays of hypothyroidism diagnosis. The presence of signs such as delayed ankle reflexes, low basal metabolic rate and bradycardia helped to confirm the diagnosis but both mild and severe forms of hypothyroidism were likely under-recognized.
The advent of assays for estimating thyroid function (initially the levels of circulating thyroid hormones in the 1950s and then TSH in the 1960s) was a game-changer. The ability to measure serum TSH and thyroid hormone levels (initially by radio-immunoassays and later by immunoassays) meant that milder forms could be detected and also that patients could be treated with much lower doses of thyroid hormone90.
Biochemical testing of thyroid function, usually with third-generation immunoassays, currently remains the cornerstone of accurate diagnosis of thyroid dysfunction. Measurement of serum TSH levels is the most reliable marker for assessing thyroid status in most patients, provided that pituitary disease is excluded and patients are not on medications that alter TSH secretion. There is a log-linear relationship between TSH and thyroxine: a twofold decrease in fT4 levels is associated with a 100-fold increase in circulating TSH91. However, the TSH–fT4 relationship might be non-linear in some individuals and influenced by age, sex, smoking and TPOAb status16. An abnormal circulating TSH level is the earliest indicator of thyroid dysfunction as the hypothalamus and pituitary register that fT4 has changed from its genetically determined set point for a particular individual92. There are various patterns of thyroid function tests to help diagnose thyroid dysfunction (Fig. 5).
The first step in assessing a patient with suspected primary hypothyroidism is to measure serum thyroid-stimulating hormone (TSH) levels. If serum TSH levels are persistently elevated, peripheral thyroid hormone levels should be measured to differentiate between subclinical and overt hypothyroidism. Levothyroxine (LT4) therapy should be initiated for all patients with overt hypothyroidism. LT4 treatment might be initiated for persistently subclinical hypothyroidism in patients who are ≤70 years of age, who have symptoms potentially caused by hypothyroidism, with cardiovascular risk factors, goitre, positive thyroid peroxidase antibody (TPOAb), are planning pregnancy, and/or have a serum TSH level persistently >10 mIU/l. Most patients with subclinical hypothyroidism and over 70 years of age can be monitored without therapy. Patients with subclinical hypothyroidism not started on LT4 therapy should have their thyroid function monitored periodically. A low serum TSH level together with low peripheral thyroid hormone levels raises suspicion of central hypothyroidism and pituitary function should be assessed. Elevated serum TSH with elevated peripheral thyroid hormone levels can be due to assay interference, a TSH-secreting pituitary adenoma (TSHoma), resistance to thyroid hormone (RTH) or familial dysalbuminaemic hyperthyroxinaemia (FDH). CVD, cardiovascular disease; fT3, free triiodothyronine; fT4, free thyroxine. aCentral hypothyroidism can present with a normal TSH level and a low fT4 level. In individuals with a high risk of central hypothyroidism (for example, pituitary adenoma), simultaneous measurement of TSH and fT4 is recommended.
Measuring circulating markers of thyroid autoimmunity (TPOAb or anti-thyroglobulin antibodies) or detecting a diffusely hypoechogenic, heterogeneous pattern by ultrasonography are not required to diagnose hypothyroidism but may be useful to confirm autoimmunity as the underlying cause.
Differential diagnosis
As the symptoms of hypothyroidism are non-specific and variable, many other conditions with similar presentations should be considered (Box 1). True subclinical hypothyroidism must be distinguished from the recovery phase of non-thyroidal illness when serum TSH levels are often transiently elevated (having been low or normal during the acute phase) and serum fT4 levels are usually normal93. For patients with apparent subclinical hypothyroidism, it is therefore recommended that thyroid function should be retested after 8–12 weeks to determine whether the TSH elevation is persistent. Observational studies showed that 30–50% of individuals who initially had high serum TSH levels have normal levels on retesting94. Interference with the laboratory analytes, usually by human anti-animal antibodies, can also lead to a diagnostic conundrum. Overestimation of TSH levels due to interference with the TSH assay or the presence of macro-TSH can lead to misdiagnosed hypothyroidism95. Rarely, individuals with resistance to thyroid hormone due to THRB mutation (high TSH and high fT4 levels), or TSH or TRH resistance (normal fT4 levels), can occasionally be misdiagnosed with hypothyroidism. A lack of typical hypothyroidism symptoms or an unusual pattern of thyroid function test results should lead to the diagnosis being questioned.
Screening for hypothyroidism
Screening for hypothyroidism entails assessing thyroid function in asymptomatic individuals who are not known to have thyroid dysfunction but are at risk of having thyroid disease. Despite the high prevalence of hypothyroidism in the general population, easy diagnosis (by a simple serum TSH test) and availability of cheap treatment, there is no evidence that early detection and treatment improves clinical outcomes. Some organizations, such as the American Thyroid Association, American Association of Clinical Endocrinologists and the Latin American Thyroid Society, recommend screening at different intervals among individuals above a particular age, ranging from every 5 years for individuals >35 years of age to an unspecified period for individuals ≥60 years of age, particularly among women96,97. However, in 1996, the Royal College of Physicians in the UK concluded that screening of the general population is unjustified given the low number of overt hypothyroidism cases detected with screening98. Similarly, in 2015, the US Preventive Services Task Force concluded that the available evidence was inadequate to determine the balance of benefits and harms of screening99. A potential explanation for the variation in screening strategies among different organizations could be differences in emphasis. Screening could potentially identify many individuals with mildly increased serum TSH levels, particularly among older individuals or those with obesity, but robust evidence that treatment is beneficial is lacking. However, individuals at high risk of thyroid dysfunction might benefit from screening, including those with risk factors for hypothyroidism (for example, goitre, previous treatment for hyperthyroidism such as radioactive iodine therapy or partial thyroidectomy, a history of neck irradiation, on medications affecting thyroid function, or the presence of other autoimmune diseases). Screening for hypothyroidism should be considered in patients with dyslipidaemia, hyponatraemia, unexplained high levels of muscle enzymes, macrocytic anaemia, or pericardial or pleural effusions without any other cause97. Furthermore, individuals at high risk of developing thyroid disease, such as those with Down syndrome, Turner syndrome or pituitary disease, should also be assessed regularly for the development of hypothyroidism.
In women of childbearing age, targeted case-finding for thyroid dysfunction should be considered in pregnant women from areas of moderate to severe iodine deficiency, women with symptoms potentially attributable to thyroid dysfunction, those with a personal and/or family history of thyroid disease, or in women with recurrent miscarriage or unexplained infertility97. In pregnancy, screening for milder forms of hypothyroidism is controversial and remains a matter of debate owing to the possibility of overtreatment and the lack of evidence that treatment of mild thyroid dysfunction with thyroid hormones improves neurocognitive outcomes in offspring100,101.
The benefit of screening for congenital hypothyroidism in newborns using dried blood spot tests for thyroid function is beyond doubt; this has been one of the major success stories of newborn screening programmes. Congenital hypothyroidism is one of the most common preventable causes of intellectual impairment. As most infants with this condition have no obvious clinical manifestations and no family history, it is not possible to target a high-risk group. Universal newborn screening programmes are available in many countries and have led to normal or near-normal neurocognitive outcomes in the majority of infants with congenital hypothyroidism102.
Dietary modifications to prevent hypothyroidism in individuals at risk
Universal salt iodization has been successful in reducing hypothyroidism in areas where severe iodine deficiency was previously prevalent11. Even so, iodine deficiency remains an important public health concern despite global efforts to combat it with iodization programmes. Adequate intake of iodine is important for all individuals but might be especially important in those with underlying autoimmune thyroid disease as iodine deficiency may trigger or worsen hypothyroidism11. The recommended daily dose of iodine is 90 μg for pre-school children, 120 μg for school children, 150 μg in adults and 250 μg in pregnancy103. Chronic excessive iodine intake can also cause alterations in thyroid function — usually increased serum TSH levels — in susceptible individuals. Maintaining an optimal iodine intake is crucial, particularly in pregnancy104.
Selenium supplementation may reduce TPOAb levels in patients with autoimmune thyroid disease in the short to medium term105. However, the clinical relevance of TPOAb reduction is unclear and the long-term safety of selenium supplementation is yet to be established. There is insufficient evidence that such therapy normalizes increased serum TSH levels in individuals with chronic autoimmune thyroid disease106.
Management
Thyroid hormone replacement with LT4
LT4 therapy is the mainstay of treatment for hypothyroidism. Treatment can reduce tissue manifestations and improve quality of life and might also benefit neurodevelopment in infants and young children. Typical full replacement doses in adults are 1.6 µg/kg/day (ref.7). Lower starting doses might be used in older individuals, those with mild hypothyroidism or those with untreated cardiovascular disease. Because weight-based dosing might overestimate the requirements of individuals with obesity, BMI-adjusted dosing algorithms have been developed107. In patients with primary hypothyroidism, treatment is targeted to the normalization of serum TSH levels. In patients with central hypothyroidism, serum TSH levels are largely uninformative and treatment should instead target a serum fT4 level in the upper half of the reference range108. Treatment leads to resolution of hypothyroidism symptoms in most patients, although the non-specific nature of symptoms means that they could also be due to other causes. Serum TSH levels should be monitored 6 weeks after initiation of treatment or any change in dose and then every 6–12 months thereafter. Thyroid hormone under-replacement and over-replacement (outside the setting of thyroid cancer, when TSH-suppressive dosing might be desired to reduce the risk for tumour recurrence) should be avoided owing to the potential for cardiac and bone toxicity and the increased mortality risk108,109,110. LT4 should be taken consistently, ideally 60 min before breakfast but taking LT4 30 min before breakfast or at bedtime on an empty stomach is also acceptable111. TSH levels should be monitored after starting or stopping medications that might interfere with LT4 absorption, binding or metabolism57 (Table 2). Malabsorption of LT4 might also occur following bariatric surgery or owing to gastrointestinal disorders. In patients with malabsorption, treatment with liquid rather than tablet LT4 formulations might help to stabilize TSH levels112.
Treatment of subclinical hypothyroidism in adults
The risk for progression from subclinical to overt hypothyroidism is ~2–4% annually and is more likely when patients have postitive TPOAb113. Elevated serum TSH levels, particularly >10 mIU/l, are associated with increased cardiovascular and mortality risks114,115. However, no consensus currently exists as to whether subclinical hypothyroidism requires treatment. Trials of LT4 in individuals ≥65 years of age have found no clear symptomatic benefit116,117. Similarly, a trial of LT4 in patients with acute myocardial infarction and subclinical hypothyroidism found no improvement in left ventricular function118. A meta-analysis suggested that LT4 might decrease mortality in patients with subclinical hypothyroidism aged <65–70 years old but not in older individuals119. Although one guideline recommends against treatment when TSH levels are <20 mIU/l (ref.120), most authors suggest individualized consideration of low-dose LT4 in patients, especially those ≤70 years of age, who have symptoms potentially referable to hypothyroidism, cardiovascular risk factors, goitre, positive TPOAb, are planning pregnancy, and/or have a serum TSH level persistently >10 mIU/l (refs97,121,122,123) (Fig. 5).
Treatment in pregnant women
The developing fetus relies entirely on maternal thyroid hormones during critical phases of early brain development (usually before gestation weeks 16–20). Untreated overt hypothyroidism in pregnancy is associated with increased risks for miscarriage, preterm delivery, gestational hypertension, pre-eclampsia, low birthweight, fetal death and impaired child intellectual development97,124. Overt hypothyroidism in pregnancy requires prompt LT4 initiation34,125. Whether milder forms of maternal thyroid hypofunction require treatment in pregnancy remains controversial. Maternal subclinical hypothyroidism is associated with increased risks for pregnancy loss, placental abruption, premature rupture of membranes, preterm delivery, and neonatal death126,127. Maternal hypothyroxinaemia (low fT4 in the setting of normal serum TSH levels) has also been associated with adverse obstetric and child neurodevelopmental outcomes126,128. However, clinical trials to date have not clearly shown a benefit of LT4 treatment for subclinical hypothyroidism or hypothyroxinaemia in pregnancy129,130,131. Current recommendations in clinical practice guidelines are variable (Table 3). Most pregnant women on LT4 therapy will require an increase in LT4 dosing (25–30% as soon as pregnancy is diagnosed) to maintain euthyroidism during gestation, when serum TBG levels are markedly increased and thyroid hormone is rapidly metabolized by placental DIO3. Serum TSH levels should be closely monitored, approximately every 4 weeks during the first half of gestation34. In pregnancy and the pre-conception period, LT4 dosing should target a serum TSH level of <2.5 mIU/l (refs34,125).
Treatment of women with subfertility
Small randomized trials have demonstrated that LT4 treatment started before conception improves assisted reproductive technology outcomes when the baseline TSH level is >4.0 mIU/l, particularly in women who are positive for TPOAb132. The recommended TSH target level in treated women is <2.5 mIU/l (ref.133). Although overt hypothyroidism should always be treated, it is not known whether pre-conception treatment of subclinical hypothyroidism improves fertility or pregnancy outcomes in women who conceive without assisted reproduction134.
Treatment in infants and children
Because thyroid hormone is important for normal growth and neurodevelopment in early life, infants with congenital hypothyroidism are unable to produce an adequate amount of thyroid hormones to maintain physiological tissue levels after birth and require rapid initiation of LT4 therapy within the first 2 weeks after delivery. Starting LT4 doses in infants should be 10–15 µg/kg daily7. Follow-up laboratory and clinical evaluations should occur every 1–2 weeks until the serum TSH level normalizes, every 1–3 months until 12 months of age, and then every few months thereafter until growth is completed. Therapy should be targeted to keep the serum TSH within the age-specific reference range in children with primary hypothyroidism and fT4 in the upper half of the reference range in children with central hypothyroidism. In children without a clear underlying cause of permanent congenital hypothyroidism, re-evaluation of the pituitary–thyroid axis should be performed at about 3 years of age to determine whether ongoing LT4 treatment is needed.
Younger children require higher doses of LT4 per kilogram of body weight than older children: 4–6 µg/kg is recommended from 1–3 years of age, 3–5 µg/kg from 3–10 years of age, and 2–4 µg/kg for 10–16 years of age7. Most children with subclinical hypothyroidism do not progress to overt hypothyroidism and most are asymptomatic. Treatment of subclinical hypothyroidism in children over 3 years of age is generally considered only when serum TSH levels are >10 mIU/l, particularly in the setting of TPOAb positivity, hyperlipidaemia135 or concerns about growth velocity136. Children with milder TSH elevation can be monitored without therapy137.
Treatment in older patients
Individuals >65–70 years of age are at increased risk of adverse effects from excessive LT4 dosing, including cardiac arrhythmia, progressive heart failure, increased bone turnover leading to osteoporosis, catabolic muscle loss, impaired quality of life and increased mortality138. Therefore, it is often recommended to start with low LT4 doses (25–50 µg daily) and to titrate doses gradually in individuals >65 years of age, particularly in those with known cardiovascular disease. There may be a physiological increase in serum TSH levels with normal ageing139, which argues against treatment of modest TSH elevations in older patients. If therapy for subclinical hypothyroidism is elected, it is particularly important to confirm the persistence of TSH elevations above age-appropriate levels over time prior to treatment initiation as thyroid function might normalize spontaneously in almost 50% of individuals ≥65 years of age140. It is also important to avoid overtreatment and targeting a serum TSH levels of 4–6 mIU/l has been advocated in patients >70 years of age7.
Challenges in treatment
A minority of patients feel unwell on LT4 despite optimal TSH levels141. Normalization of serum TSH level typically might not fully normalize serum T3 levels, and it is plausible that persistent symptoms on LT4 monotherapy might result from low systemic or tissue-specific T3 levels, particularly in individuals with polymorphisms in DIO2, who might not efficiently convert T4 into the active hormone T3 (ref.142). Although multiple trials have examined whether therapy with a combination of LT4 and liothyronine (LT3; a synthetic form of T3) improves quality of life, results are inconclusive141. Combination T3 and T4 therapy can be tried in individual patients who feel unwell on LT4 alone as long as they are closely monitored and other causes for symptoms have been excluded143. However, there is agreement that T3-containing therapies should not be used in pregnancy34,97,125,143 or in young children144. During gestation, maternal T3 does not reach the fetal brain and thus treatment of pregnant women with T3-containing therapies incurs a risk for fetal hypothyroidism and impaired brain development, even when maternal TSH level is normalized. The effects of T3 administration on brain development in paediatric patients have not been studied. Hypothyroidism following postpartum thyroiditis is common and may be transient. Therefore, withdrawal of LT4 therapy after 6–12 months might be appropriate in these cases.
Quality of life
Overt hypothyroidism is a chronic disease that might be associated with a decrease in health-related quality of life (HRQOL)145,146. Although several instruments are available to evaluate HRQOL in patients with thyroid disease, the 84-item instrument Thyroid Patient Related Outcome (ThyPRO) is the most commonly used and has been identified as the most appropriate tool for patients with hypothyroidism147. ThyPRO has good responsiveness for the detection of relevant treatment effects for use in clinical trials148,149. A 39-item shortened version, ThyPRO-39, is currently available and includes a composite measure score to evaluate HRQOL150.
ThyPRO scores in patients with untreated overt and subclinical hypothyroidism show that HRQOL is severely impacted in these patients compared with the general population145. After 6 months of LT4 treatment, improvement in HRQOL is observed but full recovery is not achieved. These results are in agreement with observational studies that report persistence of hypothyroid symptoms after treatment with LT4 (refs151,152,153,154,155,156). Persistence of residual symptoms in patients with hypothyroidism after treatment has been described in up to 15% of patients145,155,157 and has been attributed to the inability of LT4 monotherapy to fully restore tissue-specific euthyroidism. Factors unrelated to thyroid dysfunction, such as the stigma and the labelling effect of receiving the diagnosis of a chronic disease158, the need to take medications every day, or even a specific effect of autoimmunity on HRQOL145,157, have also been hypothesized to contribute to reduced HRQOL. However, it is important to highlight that these persistent symptoms might also be related to the presence of other chronic diseases or even to the medication used to treat these comorbidities.
Three meta-analyses of randomized, placebo-controlled trials in patients with hypothyroidism reported no difference in HRQOL using only LT4 compared with a combination of LT4 and LT3 (refs159,160,161). There was also no improvement in HRQOL using different doses of LT4 (refs162,163,164) or desiccated thyroid extract165. Another meta-analysis showed no difference in clinical outcomes, including quality of life, between adults with hypothyroidism treated with combination LT4 and LT3 therapy and those treated with LT4 monotherapy (although a higher proportion of patients preferred combination therapy); however, the evidence was of low-to-moderate quality159. Of note, most randomized controlled trials were limited by small sample sizes162,163,164,165,166,167,168,169,170,171,172. Furthermore, men have been under-represented in many studies. Only a few trials have used HRQOL167,169,172 or thyroid-specific HRQOL instruments162,163,165,166,171,172 as the primary outcome. Furthermore, trials have included participants with variable underlying causes of hypothyroidism163,165,166,169,171,172.
The TRUST trial is the largest trial to compare LT4 versus placebo for the treatment of subclinical hypothyroidism using ThyPRO as the main outcome in individuals ≥65 years of age. This trial demonstrated no clear benefit of LT4 treatment on HRQOL during 1 year of follow-up117. A meta-analysis of 21 randomized controlled trials conducted between 1984 and 2017, including the TRUST Trial, reported similar findings173. A more recent analysis that combined data from the TRUST study and the IEMO 80-plus study did not show any benefits of treatment of subclinical hypothyroidism in individuals ≥80 years of age116. However, in both TRUST and IEMO, the HRQOL score at baseline of included individuals with subclinical hypothyroidism was better than in the general population, which might have compromised the ability to detect an improvement in HRQOL after treatment174.
The benefit of thyroid hormone treatment for subclinical hypothyroidism in younger adults remains unclear as previous findings suggest a differential effect of treatment on HRQOL dependent on age175, whereas the majority of high-quality randomized controlled trials were performed in older people116,117. Although studies have consistently underlined the issue of residual symptoms in treated patients with hypothyroidism155, it is unclear what the underlying mechanisms are and how to tackle this issue in a personalized manner.
Outlook
Although the causes and consequences of hypothyroidism were initially described over a century ago90, essential information regarding prevalence, genetic causes, environmental factors, and thresholds for diagnosis, treatment and management optimization for hypothyroidism are still limited.
Prevalence and incidence data for primary hypothyroidism are lacking for many regions around the world. Furthermore, despite the importance of newborn screening programmes for the early detection and treatment of congenital hypothyroidism, over 70% of the world population is not screened at birth, hampering estimates of congenital hypothyroidism occurrence176 and, more importantly, hampering timely treatment. With ever-changing risk factors for thyroid disease (for example, iodine nutrition status and ageing populations), researchers, clinicians and policymakers require available and up-to-date statistics.
The heritability of TSH levels has been estimated at 65% while, in the largest GWAS to date all 42 significant associations together accounted for 33% of the genetic variance in TSH levels, displaying clear polygenicity13. While increasing the sample size in future GWAS will contribute to the search for the missing heritability, improved techniques (such as whole-genome sequencing) are expected to have a substantial impact as well. In addition to genetic and inherent factors (for example, sex), environmental risk factors, including smoking and BMI, are known to influence thyroid function. However, the variability explained by age, sex, smoking, BMI, TPOAb levels and alcohol use combined only accounts for ~7% of TSH and 5% fT4 variation16. Therefore, study of other risk factors, including endocrine-disrupting chemicals, is needed to determine their contribution to thyroid dysfunction, perhaps starting at pre-conception or conception.
Optimal iodine intake is required to avoid hypothyroidism. Although salt iodization has been implemented in more than 120 countries worldwide, mild-to-moderate iodine deficiency is still a public health problem in many regions. Additionally, salt is increasingly consumed from commercially processed foods that typically do not use fortified salt, leading to shifts in iodine status at a national and regional level. Salt iodization programmes require careful monitoring to avoid either population iodine deficiency or excess177. The groups most vulnerable to iodine deficiency are young infants, especially up to 6 months of age, and pregnant and lactating women as iodine deficiency in utero and in early life may lead to cognitive impairment in offspring. Low iodine intake by lactating mothers leads to low breast milk iodine content, which is important as this might be the only source of iodine for neonates up to 6 months of age178. Several national surveys suggest that many pregnant women currently have an insufficient iodine intake179. In a study in pregnant women from the UK, the median urinary iodine concentration was 85.3 μg/l, classifying this group as iodine deficient179. WHO recommends that optimal iodine nutrition in pregnant women is achieved when the median urinary iodine concentration in women is 150–249 μg/l (outside of pregnancy, this value is 100–199 µg/l). Achieving the recommended iodine intake in pregnancy remains a challenge.
Hypothyroidism in pregnancy poses particular challenges with regard to diagnosis and, subsequently, treatment thresholds. Maternal subclinical hypothyroidism has been reported in ~4% of all pregnancies180 and has been suggested to be adversely related to pregnancy outcomes and child development181. However, to date, consensus is lacking regarding serum TSH thresholds for treatment initiation and discussion concerning the definition of thyroid dysfunction in pregnancy is ongoing.
Diagnosis of hypothyroidism relies on laboratory measurements of serum TSH and fT4 levels. Reference ranges of TSH and fT4, defined by the 2.5th and 97.5th percentiles, have been criticized owing to their arbitrary nature and that they do not consider the potential long-term risk of serious diseases99. Both subclinical and overt hypothyroidism as well as low-to-normal thyroid function, have been associated with an increased risk of coronary heart disease and cardiovascular risk factors such as non-alcoholic fatty liver disease. These associations have been replicated in large collaborations involving individual participant-level meta-analyses of longitudinal cohort studies115 but also in multiple Mendelian randomization studies, underlining the causal relationship17,182. To date, it has proved difficult to incorporate adverse clinical outcomes in the definition of subclinical hypothyroidism. However, identifying relevant cut-offs of serum TSH and fT4 levels is imperative for a clinically meaningful definition of hypothyroidism and establishing potential treatment thresholds.
Furthermore, age-specific cut-offs for serum TSH levels have been suggested to avoid overtreatment of older individuals, particularly in the context of subclinical hypothyroidism140. This notion is based on the assumption that TSH levels increase with ageing. To date, three cohorts have investigated serum TSH levels longitudinally, with conflicting results16,81,183. Two studies, one from Australia (n = 1,100)184 and the other from the USA (n = 834)81,183, found that serum TSH levels increase with age, while a study from the Netherlands (n = 1,225) reported no change in TSH level with increasing age16. Furthermore, the Australian study showed that the increase in serum TSH levels over time was smallest in people with the highest TSH level at baseline184. These discrepant findings warrant further research in larger populations of community-dwelling adults to identify relevant age-specific cut-offs.
Thyroid hormones undergo tissue-specific metabolism, including deiodination, sulfation, glucuronidation, deamination and decarboxylation, thereby producing a wide variety and quantity of thyroid hormone metabolites184. In animal studies and a few human studies with a small sample size, different thyroid hormone metabolites have distinct effects on the cardiovascular system and cardiovascular risk factors185. However, to date, information is lacking on the importance of these thyroid hormone metabolites in predicting outcomes in patients with cardiovascular disease and their potential usefulness as determinants of cardiovascular disease incidence in the general population, including in individuals with hypothyroidism.
Residual symptoms and other hypothyroidism manifestations in patients treated for hypothyroidism have been attributed to the inability of LT4 monotherapy to restore truly normal thyroid physiology, especially a normal serum T4 to T3 ratio. Therefore, several trials have investigated the effectiveness of LT3 and LT4 combination therapy, with mixed results. This inconsistency has been attributed to generally small sample sizes and the potential variability in the proportion of participants carrying the DIO2T92A single-nucleotide polymorphism (SNP). Unresponsiveness to monotherapy in a subset of patients with hypothyroidism is hypothesized to be related to the presence of this SNP, which reduces the activity of DIO2 and therefore decreases the conversion of T4 into T3. However, in a meta-analysis assessing the effect of combination therapy, none of the included studies reported the proportion of patients with this SNP159. The T3-4-Hypo trial (NL74281.078.21), a national randomized, placebo-controlled, double-blind, multicentre trial of LT4 and LT3 combination therapy in the Netherlands in patients with autoimmune hypothyroidism, with an anticipated target size of 600 participants, might provide more information about the potential benefit of combination therapy in the future. Whether LT4 therapy in combination with slow-release LT3 would be beneficial is not clear, especially as the safety of long-term therapy has yet to be investigated.
Change history
10 June 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41572-022-00373-7
References
Mehran, L. et al. Evaluation of the congenital hypothyroidism screening programme in Iran: a 3-year retrospective cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 104, F176–F181 (2019).
Deladoey, J., Ruel, J., Giguere, Y. & Van Vliet, G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Quebec. J. Clin. Endocrinol. Metab. 96, 2422–2429 (2011).
Canaris, G. J., Steiner, J. F. & Ridgway, E. C. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J. Gen. Intern. Med. 12, 544–550 (1997).
Kantor, E. D., Rehm, C. D., Haas, J. S., Chan, A. T. & Giovannucci, E. L. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 314, 1818–1831 (2015).
ClinCalc DrugStats Database. Levothyroxine: Drug Usage Statistics, U. S., 2013–2019. ClinCalc.com https://clincalc.com/DrugStats/Drugs/Levothyroxine (2021).
Brito, J. P. et al. Levothyroxine use in the United States, 2008-2018. JAMA Intern. Med. 181, 1402–1405 (2021).
Jonklaas, J. et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 24, 1670–1751 (2014).
Hollowell, J. G. et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 87, 489–499 (2002).
Canaris, G. J., Manowitz, N. R., Mayor, G. & Ridgway, E. C. The Colorado Thyroid Disease Prevalence study. Arch. Intern. Med. 160, 526–534 (2000).
Garmendia Madariaga, A., Santos Palacios, S., Guillen-Grima, F. & Galofre, J. C. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J. Clin. Endocrinol. Metab. 99, 923–931 (2014).
Zimmermann, M. B. & Boelaert, K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 3, 286–295 (2015).
Pedersen, I. B. et al. A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin. Endocrinol. 75, 120–126 (2011).
Teumer, A. et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat. Commun. 9, 4455 (2018).
Vanderpump, M. P. The epidemiology of thyroid disease. Br. Med. Bull. 99, 39–51 (2011).
Belin, R. M., Astor, B. C., Powe, N. R. & Ladenson, P. W. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 89, 6077–6086 (2004).
Chaker, L. et al. Thyroid function characteristics and determinants: the rotterdam study. Thyroid 26, 1195–1204 (2016).
Kus, A. et al. Variation in normal range thyroid function affects serum cholesterol levels, blood pressure, and type 2 diabetes risk: a mendelian randomization study. Thyroid 31, 721–731 (2021).
Wang, X. et al. Causal association between serum thyrotropin and obesity: a bidirectional, mendelian randomization study. J. Clin. Endocrinol. Metab. 106, e4251–e4259 (2021).
Liu, C. et al. Small for gestational age is a risk factor for thyroid dysfunction in preterm newborns. BMC Pediatr. 20, 179 (2020).
Asakura, Y., Tachibana, K., Adachi, M., Suwa, S. & Yamagami, Y. Hypothalamo-pituitary hypothyroidism detected by neonatal screening for congenital hypothyroidism using measurement of thyroid-stimulating hormone and thyroxine. Acta Paediatr. 91, 172–177 (2002).
Kempers, M. J. et al. Neonatal screening for congenital hypothyroidism based on thyroxine, thyrotropin, and thyroxine-binding globulin measurement: potentials and pitfalls. J. Clin. Endocrinol. Metab. 91, 3370–3376 (2006).
Wright, J. J., Powers, A. C. & Johnson, D. B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol. 17, 389–399 (2021).
Maynard, M. A. et al. Thyroid hormone inactivation in gastrointestinal stromal tumors. N. Engl. J. Med. 370, 1327–1334 (2014).
Fekete, C. & Lechan, R. M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 35, 159–194 (2014).
Brabant, G. et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. J. Clin. Endocrinol. Metab. 70, 403–409 (1990).
Kopp, P. in Werner & Ingbar’s the Thyroid: A Fundamental and Clinical Text Ch. 6, 97–126 (Wolters Kluwer, 2021).
Schweizer, U., Johannes, J., Bayer, D. & Braun, D. Structure and function of thyroid hormone plasma membrane transporters. Eur. Thyroid J. 3, 143–153 (2014).
Ortiga-Carvalho, T. M., Sidhaye, A. R. & Wondisford, F. E. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 10, 582–591 (2014).
Peeters, R. P., van der Deure, W. M. & Visser, T. J. Genetic variation in thyroid hormone pathway genes; polymorphisms in the TSH receptor and the iodothyronine deiodinases. Eur. J. Endocrinol. 155, 655–662 (2006).
Drigo, R. A. & Bianco, A. C. Type 2 deiodinase at the crossroads of thyroid hormone action. Int. J. Biochem. Cell Biol. 43, 1432–1441 (2011).
Schweizer, U. & Steegborn, C. New insights into the structure and mechanism of iodothyronine deiodinases. J. Mol. Endocrinol. 55, R37–R52 (2015).
Merrill, S. J. & Minucci, S. B. Thyroid autoimmunity: an interplay of factors. Vitam. Hormones 106, 129–145 (2018).
Glinoer, D., Riahi, M., Grün, J.-P. & Kinthaert, J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J. Clin. Endocrinol. Metab. 79, 197–204 (1994).
Alexander, E. K. et al. 2017 Guidelines of the american thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27, 315–389 (2017).
Nazarpour, S., Tehrani, F. R., Amiri, M., Yarandi, R. B. & Azizi, F. Levothyroxine treatment and pregnancy outcomes in women with subclinical hypothyroidism: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 300, 805–819 (2019).
Derakhshan, A. et al. The association of maternal thyroid autoimmunity during pregnancy with child IQ. J. Clin. Endocrinol. Metab. 103, 3729–3736 (2018).
Theofilopoulos, A. N., Kono, D. H. & Baccala, R. The multiple pathways to autoimmunity. Nat. Immunol. 18, 716–724 (2017).
Rapoport, B. & McLachlan, S. M. Reflections on thyroid autoimmunity: a personal overview from the past into the future. Horm. Metab. Res. 50, 840–852 (2018).
Wu, F. et al. Decreased β-catenin expression contributes to IFNγ-induced chemokine secretion and lymphocyte infiltration in Hashimoto’s thyroiditis. Endocr. Connect. 11, e210451 (2022).
Azizi, F. et al. Impairment of neuromotor and cognitive development in iodine-deficient schoolchildren with normal physical growth. Eur. J. Endocrinol. 129, 501–504 (1993).
Wolff, J. et al. The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology 45, 504–513 (1949).
Leung, A. M. & Braverman, L. E. Iodine-induced thyroid dysfunction. Curr. Opin. Endocrinol. Diabetes Obes. 19, 414 (2012).
Rayman, M. P. Selenium and human health. Lancet 379, 1256–1268 (2012).
Wu, Q. et al. Low population selenium status is associated with increased prevalence of thyroid disease. J. Clin. Endocrinol. Metab. 100, 4037–4047 (2015).
Aung, E. T. et al. Predicting outcomes and complications following radioiodine therapy in Graves’ thyrotoxicosis. Clin. Endocrinol. 90, 192–199 (2019).
Kahraman, D. et al. Development of hypothyroidism during long-term follow-up of patients with toxic nodular goitre after radioiodine therapy. Clin. Endocrinol. 76, 297–303 (2012).
Bonnema, S. J. et al. The feasibility of high dose iodine 131 treatment as an alternative to surgery in patients with a very large goiter: effect on thyroid function and size and pulmonary function. J. Clin. Endocrinol. Metab. 84, 3636–3641 (1999).
Alba, J., Basterra, J., Ferrer, J., Santonja, F. & Zapater, E. Hypothyroidism in patients treated with radiotherapy for head and neck carcinoma: standardised long-term follow-up study. J. Laryngol. Otol. 130, 478–481 (2016).
Lin, Y.-S., Lin, J.-D., Hsu, C.-C. & Yu, M.-C. The long-term outcomes of thyroid function after subtotal thyroidectomy for Graves’ hyperthyroidism. J. Surg. Res. 220, 112–118 (2017).
Azizi, F. The occurrence of permanent thyroid failure in patients with subclinical postpartum thyroiditis. Eur. J. Endocrinol. 153, 367–371 (2005).
Hennessey, J. V. Riedel’s thyroiditis: a clinical review. J. Clin. Endocrinol. Metab. 96, 3031–3041 (2011).
Ozdemir, D., Dagdelen, S. & Erbas, T. Endocrine involvement in systemic amyloidosis. Endocr. Pract. 16, 1056–1063 (2010).
Antonelli, A. et al. Clinical and subclinical autoimmune thyroid disorders in systemic sclerosis. Eur. J. Endocrinol. 156, 431–437 (2007).
Guttler, R. & Singer, P. A. Pneumocystis carinii thyroiditis: report of three cases and review of the literature. Arch. Intern. Med. 153, 393–396 (1993).
Azizi, F. & Katchoui, A. Brucella infection of the thyroid gland. Thyroid 6, 461–463 (1996).
Murugan, A. K. & Alzahrani, A. S. SARS-CoV-2: emerging role in the pathogenesis of various thyroid diseases. J. Inflamm. Res. 14, 6191–6221 (2021).
Burch, H. B. Drug effects on the thyroid. N. Engl. J. Med. 381, 749–761 (2019).
Lazarus, J. H. Lithium and thyroid. Best. Pract. Res. Clin. Endocrinol. Metab. 23, 723–733 (2009).
Eskes, S. A. & Wiersinga, W. M. Amiodarone and thyroid. Best. Pract. Res. Clin. Endocrinol. Metab. 23, 735–751 (2009).
Reid, I. et al. Thyroid dysfunction can predict response to immunotherapy with interleukin-2 and interferon-2α. Br. J. Cancer 64, 915–918 (1991).
Wong, E. et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid 17, 351–355 (2007).
Ryder, M., Callahan, M., Postow, M. A., Wolchok, J. & Fagin, J. A. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr. Relat. Cancer 21, 371–381 (2014).
Min, L. et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin. Cancer Res. 21, 749–755 (2015).
Chang, L.-S. et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr. Rev. 40, 17–65 (2019).
Pearce, E. N. & Braverman, L. E. Environmental pollutants and the thyroid. Best. Pract. Res. Clin. Endocrinol. Metab. 23, 801–813 (2009).
Peters, C., Van Trotsenburg, A. & Schoenmakers, N. Diagnosis of endocrine disease: congenital hypothyroidism: update and perspectives. Eur. J. Endocrinol. 179, R297–R317 (2018).
Kostopoulou, E., Miliordos, K. & Spiliotis, B. Genetics of primary congenital hypothyroidism — a review. Hormones 20, 225–236 (2021).
Persani, L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J. Clin. Endocrinol. Metab. 97, 3068–3078 (2012).
Persani, L., Ferretti, E., Borgato, S., Faglia, G. & Beck-Peccoz, P. Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J. Clin. Endocrinol. Metab. 85, 3631–3635 (2000).
Beck-Peccoz, P., Rodari, G., Giavoli, C. & Lania, A. Central hypothyroidism — a neglected thyroid disorder. Nat. Rev. Endocrinol. 13, 588–598 (2017).
Refetoff, S. et al. Classification and proposed nomenclature for inherited defects of thyroid hormone action, cell transport, and metabolism. Thyroid 24, 407–409 (2014).
Chaker, L., Bianco, A. C., Jonklaas, J. & Peeters, R. P. Hypothyroidism. Lancet 390, 3550–3562 (2017).
Ai, J., Leonhardt, J. M. & Heymann, W. R. Autoimmune thyroid diseases: etiology, pathogenesis, and dermatologic manifestations. J. Am. Acad. Dermatol. 48, 641–662 (2003).
Mantovani, A. et al. Association between primary hypothyroidism and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Thyroid 28, 1270–1284 (2018).
Rhee, C. M. The interaction between thyroid and kidney disease: an overview of the evidence. Curr. Opin. Endocrinol. Diabetes Obes. 23, 407 (2016).
Thvilum, M. et al. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid 24, 802–808 (2014).
Razvi, S. et al. Thyroid hormones and cardiovascular function and diseases. J. Am. Coll. Cardiol. 71, 1781–1796 (2018).
Chaker, L., Bianco, A. C., Jonklaas, J. & Peeters, R. P. Hypothyroidism. Lancet 390, 1550–1562 (2017).
Tiller, D. et al. Association of serum thyrotropin with anthropometric markers of obesity in the general population. Thyroid 26, 1205–1214 (2016).
Carle, A. et al. Hypothyroid symptoms and the likelihood of overt thyroid failure: a population-based case-control study. Eur. J. Endocrinol. 171, 593–602 (2014).
Waring, A. C. et al. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J. Clin. Endocrinol. Metab. 97, 3944–3950 (2012).
Seshadri, M. S., Samuel, B. U., Kanagasabapathy, A. S. & Cherian, A. M. Clinical scoring system for hypothyroidism: is it useful? J. Gen. Intern. Med. 4, 490–492 (1989).
Billewicz, W. Z. et al. Statistical methods applied to the diagnosis of hypothyroidism. Q. J. Med. 38, 255–266 (1969).
Allahabadia, A., Razvi, S., Abraham, P. & Franklyn, J. Diagnosis and treatment of primary hypothyroidism. BMJ 338, b725 (2009).
Leung, A. S., Millar, L. K., Koonings, P. P., Montoro, M. & Mestman, J. H. Perinatal outcome in hypothyroid pregnancies. Obstet. Gynecol. 81, 349–353 (1993).
Davis, L. E., Leveno, K. J. & Cunningham, F. G. Hypothyroidism complicating pregnancy. Obstet. Gynecol. 72, 108–112 (1988).
Batistuzzo, A. & Ribeiro, M. O. Clinical and subclinical maternal hypothyroidism and their effects on neurodevelopment, behavior and cognition. Arch. Endocrinol. Metab. 64, 89–95 (2020).
de Vries, L., Bulvik, S. & Phillip, M. Chronic autoimmune thyroiditis in children and adolescents: at presentation and during long-term follow-up. Arch. Dis. Child. 94, 33–37 (2009).
Wartofsky, L. Myxedema coma. Endocrinol. Metab. Clin. North. Am. 35, 687–698 (2006).
McAninch, E. A. & Bianco, A. C. The history and future of treatment of hypothyroidism. Ann. Intern. Med. 164, 50–56 (2016).
Andersen, S., Bruun, N. H., Pedersen, K. M. & Laurberg, P. Biologic variation is important for interpretation of thyroid function tests. Thyroid 13, 1069–1078 (2003).
Hansen, P. S., Brix, T. H., Sorensen, T. I., Kyvik, K. O. & Hegedus, L. Major genetic influence on the regulation of the pituitary-thyroid axis: a study of healthy Danish twins. J. Clin. Endocrinol. Metab. 89, 1181–1187 (2004).
Fliers, E., Bianco, A. C., Langouche, L. & Boelen, A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 3, 816–825 (2015).
Jonklaas, J. & Razvi, S. Reference intervals in the diagnosis of thyroid dysfunction: treating patients not numbers. Lancet Diabetes Endocrinol. 7, 473–483 (2019).
Favresse, J., Burlacu, M. C., Maiter, D. & Gruson, D. Interferences with thyroid function immunoassays: clinical implications and detection algorithm. Endocr. Rev. 39, 830–850 (2018).
Brenta, G. et al. Clinical practice guidelines for the management of hypothyroidism. Arq. Bras. Endocrinol. Metab. 57, 265–291 (2013).
Garber, J. R. et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 18, 988–1028 (2012).
Vanderpump, M. P., Ahlquist, J. A., Franklyn, J. A. & Clayton, R. N. Consensus statement for good practice and audit measures in the management of hypothyroidism and hyperthyroidism. The Research Unit of the Royal College of Physicians of London, the Endocrinology and Diabetes Committee of the Royal College of Physicians of London, and the Society for Endocrinology. BMJ 313, 539–544 (1996).
LeFevre, M. L. & U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 162, 641–650 (2015).
Taylor, P. N. & Lazarus, J. H. Hypothyroidism in pregnancy. Endocrinol. Metab. Clin. North. Am. 48, 547–556 (2019).
Lee, S. Y. & Pearce, E. N. Testing, monitoring, and treatment of thyroid dysfunction in pregnancy. J. Clin. Endocrinol. Metab. 106, 883–892 (2021).
LaFranchi, S. H. Newborn screening strategies for congenital hypothyroidism: an update. J. Inherit. Metab. Dis. 33, S225–S233 (2010).
Leung, A. M. & Braverman, L. E. Consequences of excess iodine. Nat. Rev. Endocrinol. 10, 136–142 (2014).
Pearce, E. N., Lazarus, J. H., Moreno-Reyes, R. & Zimmermann, M. B. Consequences of iodine deficiency and excess in pregnant women: an overview of current knowns and unknowns. Am. J. Clin. Nutr. 104 (Suppl. 3), 918S–923S (2016).
Wichman, J., Winther, K. H., Bonnema, S. J. & Hegedus, L. Selenium supplementation significantly reduces thyroid autoantibody levels in patients with chronic autoimmune thyroiditis: a systematic review and meta-analysis. Thyroid 26, 1681–1692 (2016).
Qiu, Y. et al. Insufficient evidence to support the clinical efficacy of selenium supplementation for patients with chronic autoimmune thyroiditis. Endocrine 73, 384–397 (2021).
Elfenbein, D. M. et al. Prospective intervention of a novel levothyroxine dosing protocol based on body mass index after thyroidectomy. J. Am. Coll. Surg. 222, 83–88 (2016).
Persani, L. et al. 2018 European Thyroid Association (ETA) guidelines on the diagnosis and management of central hypothyroidism. Eur. Thyroid J. 7, 225–237 (2018).
Lillevang-Johansen, M., Abrahamsen, B., Jorgensen, H. L., Brix, T. H. & Hegedus, L. Duration of over- and under-treatment of hypothyroidism is associated with increased cardiovascular risk. Eur. J. Endocrinol. 180, 407–416 (2019).
Lillevang-Johansen, M., Abrahamsen, B., Jorgensen, H. L., Brix, T. H. & Hegedus, L. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid 28, 566–574 (2018).
Jonklaas, J. Optimal thyroid hormone replacement. Endocr. Rev. 43, 366–404 (2022).
Laurent, I. et al. Liquid L-thyroxine versus tablet L-thyroxine in patients on L- thyroxine replacement or suppressive therapy: a meta-analysis. Endocrine 61, 28–35 (2018).
Vanderpump, M. P. et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin. Endocrinol. 43, 55–68 (1995).
Moon, S., Kim, M. J., Yu, J. M., Yoo, H. J. & Park, Y. J. Subclinical hypothyroidism and the risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Thyroid 28, 1101–1110 (2018).
Rodondi, N. et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304, 1365–1374 (2010).
Mooijaart, S. P. et al. Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. JAMA 322, 1977–1986 (2019).
Stott, D. J. et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N. Engl. J. Med. 376, 2534–2544 (2017).
Jabbar, A. et al. Effect of levothyroxine on left ventricular ejection fraction in patients with subclinical hypothyroidism and acute myocardial infarction: a randomized clinical trial. JAMA 324, 249–258 (2020).
Peng, C. C. et al. Association of thyroid hormone therapy with mortality in subclinical hypothyroidism: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 106, 292–303 (2021).
Bekkering, G. E. et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ 365, l2006 (2019).
Peeters, R. P. Subclinical hypothyroidism. N. Engl. J. Med. 376, 2556–2565 (2017).
National Institute for Health and Care Excellence. Thyroid disease: assessment and management. NICE https://www.nice.org.uk/guidance/ng145 (2019).
Biondi, B., Cappola, A. R. & Cooper, D. S. Subclinical hypothyroidism: a review. JAMA 322, 153–160 (2019).
Haddow, J. E. et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 341, 549–555 (1999).
Thyroid Disease in Pregnancy.ACOG Practice Bulletin, Number 223. Obstet. Gynecol. 135, e261–e274 (2020).
The Consortium on Thyroid and Pregnancy — Study Group on Preterm Birth. Association of Thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA 322, 632–641 (2019).
Maraka, S. et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 26, 580–590 (2016).
Levie, D. et al. Thyroid function in early pregnancy, child IQ, and autistic traits: a meta-analysis of individual participant data. J. Clin. Endocrinol. Metab. 103, 2967–2979 (2018).
Nazarpour, S., Ramezani Tehrani, F., Rahmati, M., Amiri, M. & Azizi, F. Effects of isolated maternal hypothyroxinemia on adverse pregnancy outcomes. Arch. Gynecol. Obstet. 305, 903–911 (2022).
Casey, B. M. et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N. Engl. J. Med. 376, 815–825 (2017).
Lazarus, J. H. et al. Antenatal thyroid screening and childhood cognitive function. N. Engl. J. Med. 366, 493–501 (2012).
Velkeniers, B. et al. Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: systematic review and meta-analysis of RCTs. Hum. Reprod. Update 19, 251–258 (2013).
Poppe, K. et al. 2021 European thyroid association guideline on thyroid disorders prior to and during assisted reproduction. Eur. Thyroid J. 9, 281–295 (2021).
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil. Steril. 104, 545–553 (2015).
Unal, E. et al. Association of subclinical hypothyroidism with dyslipidemia and increased carotid intima-media thickness in children. J. Clin. Res. Pediatr. Endocrinol. 9, 144–149 (2017).
Cerbone, M. et al. Linear growth and intellectual outcome in children with long-term idiopathic subclinical hypothyroidism. Eur. J. Endocrinol. 164, 591–597 (2011).
Lazarus, J. et al. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur. Thyroid J. 3, 76–94 (2014).
Effraimidis, G., Watt, T. & Feldt-Rasmussen, U. Levothyroxine therapy in elderly patients with hypothyroidism. Front. Endocrinol. 12, 641560 (2021).
Surks, M. I. & Hollowell, J. G. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 92, 4575–4582 (2007).
Somwaru, L. L., Rariy, C. M., Arnold, A. M. & Cappola, A. R. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J. Clin. Endocrinol. Metab. 97, 1962–1969 (2012).
Jonklaas, J. et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Eur. Thyroid J. 10, 10–38 (2021).
Ettleson, M. D. & Bianco, A. C. Individualized therapy for hypothyroidism: is T4 enough for everyone? J. Clin. Endocrinol. Metab. 105, e3090–e3104 (2020).
Okosieme, O. et al. Management of primary hypothyroidism: statement by the british thyroid association executive committee. Clin. Endocrinol. 84, 799–808 (2016).
van Trotsenburg, P. et al. Congenital hypothyroidism: a 2020-2021 Consensus Guidelines Update — an ENDO-European reference network initiative endorsed by the european society for pediatric endocrinology and the european society for endocrinology. Thyroid 31, 387–419 (2021).
Winther, K. H. et al. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS One 11, e0156925 (2016).
Bianchi, G. P. et al. Health-related quality of life in patients with thyroid disorders. Qual. Life Res. 13, 45–54 (2004).
Wong, C. K., Lang, B. H. & Lam, C. L. A systematic review of quality of thyroid-specific health-related quality-of-life instruments recommends ThyPRO for patients with benign thyroid diseases. J. Clin. Epidemiol. 78, 63–72 (2016).
Watt, T. et al. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. J. Clin. Endocrinol. Metab. 99, 3708–3717 (2014).
Watt, T. et al. Establishing construct validity for the thyroid-specific patient reported outcome measure (ThyPRO): an initial examination. Qual. Life Res. 18, 483–496 (2009).
Watt, T. et al. Development of a short version of the thyroid-related patient-reported outcome ThyPRO. Thyroid 25, 1069–1079 (2015).
Moron-Diaz, M. et al. Correlation between TSH levels and quality of life among subjects with well-controlled primary hypothyroidism. Endocrine 72, 190–197 (2021).
Kelderman-Bolk, N., Visser, T. J., Tijssen, J. P. & Berghout, A. Quality of life in patients with primary hypothyroidism related to BMI. Eur. J. Endocrinol. 173, 507–515 (2015).
Quinque, E. M., Villringer, A., Kratzsch, J. & Karger, S. Patient-reported outcomes in adequately treated hypothyroidism - insights from the German versions of ThyDQoL, ThySRQ and ThyTSQ. Health Qual. Life Outcomes 11, 68 (2013).
Wekking, E. M. et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur. J. Endocrinol. 153, 747–753 (2005).
Saravanan, P. et al. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin. Endocrinol. 57, 577–585 (2002).
Jaeschke, R., Guyatt, G., Cook, D., Harper, S. & Gerstein, H. C. Spectrum of quality of life impairment in hypothyroidism. Qual. Life Res. 3, 323–327 (1994).
Groenewegen, K. L., Mooij, C. F. & van Trotsenburg, A. S. P. Persisting symptoms in patients with Hashimoto’s disease despite normal thyroid hormone levels: Does thyroid autoimmunity play a role? A systematic review. J. Transl. Autoimmun. 4, 100101 (2021).
Jorgensen, P., Langhammer, A., Krokstad, S. & Forsmo, S. Diagnostic labelling influences self-rated health. A prospective cohort study: the HUNT Study, Norway. Fam. Pract. 32, 492–499 (2015).
Millan-Alanis, J. M. et al. Benefits and harms of levothyroxine/l-triiodothyronine versus levothyroxine monotherapy for adult patients with hypothyroidism: systematic review and meta-analysis. Thyroid 31, 1613–1625 (2021).
Ma, C. et al. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism. Nucl. Med. Commun. 30, 586–593 (2009).
Grozinsky-Glasberg, S., Fraser, A., Nahshoni, E., Weizman, A. & Leibovici, L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 91, 2592–2599 (2006).
Samuels, M. H., Kolobova, I., Niederhausen, M., Janowsky, J. S. & Schuff, K. G. Effects of altering levothyroxine (L-T4) doses on quality of life, mood, and cognition in L-T4 treated subjects. J. Clin. Endocrinol. Metab. 103, 1997–2008 (2018).
Walsh, J. P. et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J. Clin. Endocrinol. Metab. 91, 2624–2630 (2006).
Roos, A., Linn-Rasker, S. P., van Domburg, R. T., Tijssen, J. P. & Berghout, A. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch. Intern. Med. 165, 1714–1720 (2005).
Hoang, T. D., Olsen, C. H., Mai, V. Q., Clyde, P. W. & Shakir, M. K. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J. Clin. Endocrinol. Metab. 98, 1982–1990 (2013).
Kaminski, J., Miasaki, F. Y., Paz-Filho, G., Graf, H. & Carvalho, G. A. Treatment of hypothyroidism with levothyroxine plus liothyronine: a randomized, double-blind, crossover study. Arch. Endocrinol. Metab. 60, 562–572 (2016).
Nygaard, B., Jensen, E. W., Kvetny, J., Jarlov, A. & Faber, J. Effect of combination therapy with thyroxine (T4) and 3,5,3’-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur. J. Endocrinol. 161, 895–902 (2009).
Appelhof, B. C. et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J. Clin. Endocrinol. Metab. 90, 2666–2674 (2005).
Escobar-Morreale, H. F. et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann. Intern. Med. 142, 412–424 (2005).
Sawka, A. M., Gerstein, H. C., Marriott, M. J., MacQueen, G. M. & Joffe, R. T. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J. Clin. Endocrinol. Metab. 88, 4551–4555 (2003).
Walsh, J. P. et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J. Clin. Endocrinol. Metab. 88, 4543–4550 (2003).
Clyde, P. W., Harari, A. E., Getka, E. J. & Shakir, K. M. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 290, 2952–2958 (2003).
Feller, M. et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA 320, 1349–1359 (2018).
Korevaar, T. I. M., Chaker, L. & Peeters, R. P. Improving the clinical impact of randomised trials in thyroidology. Lancet Diabetes Endocrinol. 6, 523–525 (2018).
Recker, S. et al. Thyroid related quality of life in elderly with subclinical hypothyroidism and improvement on levothyroxine is distinct from that in young patients (TSAGE). Horm. Metab. Res. 51, 568–574 (2019).
Ford, G. & LaFranchi, S. H. Screening for congenital hypothyroidism: a worldwide view of strategies. Best. Pract. Res. Clin. Endocrinol. Metab. 28, 175–187 (2014).
Gorstein, J. L., Bagriansky, J., Pearce, E. N., Kupka, R. & Zimmermann, M. B. Estimating the health and economic benefits of universal salt iodization programs to correct iodine deficiency disorders. Thyroid 30, 1802–1809 (2020).
World Health Organization. Guideline: fortification of food-grade salt with iodine for the prevention and control of iodine deficiency disorders. WHO https://www.who.int/publications/i/item/9789241507929 (2014).
Bath, S. C., Walter, A., Taylor, A., Wright, J. & Rayman, M. P. Iodine deficiency in pregnant women living in the South East of the UK: the influence of diet and nutritional supplements on iodine status. Br. J. Nutr. 111, 1622–1631 (2014).
Dong, A. C. & Stagnaro-Green, A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta-analysis. Thyroid 29, 278–289 (2019).
Korevaar, T. I. M., Medici, M., Visser, T. J. & Peeters, R. P. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat. Rev. Endocrinol. 13, 610–622 (2017).
van Vliet, N. A. et al. Higher thyrotropin leads to unfavorable lipid profile and somewhat higher cardiovascular disease risk: evidence from multi-cohort Mendelian randomization and metabolomic profiling. BMC Med. 19, 266 (2021).
Bremner, A. P. et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J. Clin. Endocrinol. Metab. 97, 1554–1562 (2012).
Kohrle, J. Thyroid hormones and derivatives: endogenous thyroid hormones and their targets. Methods Mol. Biol. 1801, 85–104 (2018).
Rutigliano, G. & Zucchi, R. Cardiac actions of thyroid hormone metabolites. Mol. Cell Endocrinol. 458, 76–81 (2017).
The Iodine Global Network. Global Scorecard of Iodine Nutrition in 2020 in the General Population Based on School-age Children (SAC) (IGN, 2021).
Ikegami, K., Refetoff, S., Van Cauter, E. & Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 15, 590–600 (2019).
Le Tissier, P. et al. An updated view of hypothalamic–vascular–pituitary unit function and plasticity. Nat. Rev. Endocrinol. 13, 257–267 (2017).
Author information
Authors and Affiliations
Contributions
Introduction (L.C., S.R., I.M.B., F.A., E.N.P. and R.P.P.); Epidemiology (L.C., S.R., I.M.B., F.A., E.N.P. and R.P.P.); Mechanisms/pathophysiology (L.C., S.R., I.M.B., F.A., E.N.P. and R.P.P.); Diagnosis, screening and prevention (L.C., S.R., I.M.B., F.A., E.N.P. and R.P.P.); Management (L.C., S.R., I.M.B., F.A., E.N.P. and R.P.P.); Quality of life (L.C., S.R., I.M.B., F.A., E.N.P. and R.P.P.); Outlook (S.R., I.M.B., F.A., E.N.P. and R.P.P.); Overview of Primer (R.P.P.).
Corresponding author
Ethics declarations
Competing interests
R.P.P. has received speaker fees from IBSA Pharmaceuticals. S.R. has received speaker fees from Merck, IBSA and Abbott Pharmaceuticals (manufacturers of thyroid hormones). L.C., I.M.B., F.A. and E.N.P. declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks P. Fallahi, W. Teng, L. Persani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Wolff–Chaikoff effect
-
The phenomenon that consists of the acute inhibition of iodine organification in thyroid follicles and thyroid hormone release due to iodine excess.
- Iodine organification
-
The incorporation of iodine into thyroglobulin.
- Total thyroidectomy
-
Removal of the entire thyroid gland.
- Near-total thyroidectomy
-
Removal of most of the thyroid gland except for <1 g of thyroid tissue to minimize risk of damaging the recurrent laryngeal nerve.
- Subtotal thyroidectomy
-
Removal of most of the thyroid gland except for ~4–8 g of tissue to maintain euthyroidism.
- Thyrotroph
-
Anterior pituitary cells that produce thyroid-stimulating hormone in response to thyrotropin-releasing hormone from hypothalamic thyrotropin-releasing hormone-producing neurons.
- Myxoedema coma
-
Severe hypothyroidism resulting in a loss of homeostasis, often presenting as altered mental state, hypothermia, hypotension, hyponatraemia and other symptoms, which can lead to coma.
Rights and permissions
About this article
Cite this article
Chaker, L., Razvi, S., Bensenor, I.M. et al. Hypothyroidism. Nat Rev Dis Primers 8, 30 (2022). https://doi.org/10.1038/s41572-022-00357-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-022-00357-7
This article is cited by
-
Association of major depression, schizophrenia and bipolar disorder with thyroid cancer: a bidirectional two-sample mendelian randomized study
BMC Psychiatry (2024)
-
Causal relationship between hypothyroidism and temporomandibular disorders: evidence from complementary genetic methods
BMC Oral Health (2024)
-
Clinical and laboratory characteristics of early-onset and delayed-onset lupus nephritis patients: A single-center retrospective study
Rheumatology International (2024)
-
Obesity and Obesity-Related Thyroid Dysfunction: Any Potential Role for the Very Low-Calorie Ketogenic Diet (VLCKD)?
Current Nutrition Reports (2024)
-
Severity of hypothyroidism is inversely associated with impaired quality of life in patients referred to an endocrine clinic
Thyroid Research (2023)