Abstract
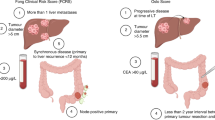
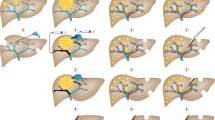
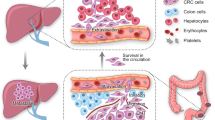
Liver metastases are commonly detected in a range of malignancies including colorectal cancer (CRC), pancreatic cancer, melanoma, lung cancer and breast cancer, although CRC is the most common primary cancer that metastasizes to the liver. Interactions between tumour cells and the tumour microenvironment play an important part in the engraftment, survival and progression of the metastases. Various cells including liver sinusoidal endothelial cells, Kupffer cells, hepatic stellate cells, parenchymal hepatocytes, dendritic cells, resident natural killer cells as well as other immune cells such as monocytes, macrophages and neutrophils are implicated in promoting and sustaining metastases in the liver. Four key phases (microvascular, pre-angiogenic, angiogenic and growth phases) have been identified in the process of liver metastasis. Imaging modalities such as ultrasonography, CT, MRI and PET scans are typically used for the diagnosis of liver metastases. Surgical resection remains the main potentially curative treatment among patients with resectable liver metastases. The role of liver transplantation in the management of liver metastasis remains controversial. Systemic therapies, newer biologic agents (for example, bevacizumab and cetuximab) and immunotherapeutic agents have revolutionized the treatment options for liver metastases. Moving forward, incorporation of genetic tests can provide more accurate information to guide clinical decision-making and predict prognosis among patients with liver metastases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hess, K. R. et al. Metastatic patterns in adenocarcinoma. Cancer 106, 1624–1633 (2006). Registry analysis of the metastatic patterns for the main sites of primary adenocarcinoma.
Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8, 98–101 (1989).
Brodt, P. Role of the microenvironment in liver metastasis: from pre- to prometastatic niches. Clin. Cancer Res. 22, 5971–5982 (2016).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Wagner, J. S., Adson, M. A., Van Heerden, J. A., Adson, M. H. & Ilstrup, D. M. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann. Surg. 199, 502–508 (1984).
Anaya, D. A., Becker, N. S. & Abraham, N. S. Global graying, colorectal cancer and liver metastasis: new implications for surgical management. Crit. Rev. Oncol. Hematol. 77, 100–108 (2011).
Edwards, B. K. et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 120, 1290–1314 (2014). Analysis over a period of 35 years on how cancer incidence changed for colorectal, breast, lung and prostate cancer.
Horn, S. R. et al. Epidemiology of liver metastases. Cancer Epidemiol. 67, 101760 (2020). Recent publication with specific epidemiological information on liver metastasis and metastatic patterns of their primary tumours.
de Ridder, J. et al. Incidence and origin of histologically confirmed liver metastases: an explorative case-study of 23,154 patients. Oncotarget 7, 55368–55376 (2016).
Golubnitschaja, O. & Sridhar, K. C. Liver metastatic disease: new concepts and biomarker panels to improve individual outcomes. Clin. Exp. Metastasis 33, 743–755 (2016).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Hackl, C. et al. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 14, 810 (2014).
Manfredi, S. et al. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 244, 254–259 (2006).
Slesser, A. A. et al. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surgical Oncol. 22, 36–47 (2013).
Siegel, R. L., Jakubowski, C. D., Fedewa, S. A., Davis, A. & Azad, N. S. Colorectal cancer in the young: epidemiology, prevention, management. Am. Soc. Clin. Oncol. Educ. Book. 40, 1–14 (2020).
Siegel, R. L. et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70, 145–164 (2020).
Nordlinger, B. et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 14, 1208–1215 (2013).
Engstrand, J., Nilsson, H., Stromberg, C., Jonas, E. & Freedman, J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer 18, 78 (2018).
Kobayashi, S. et al. Survival outcomes of resected BRAF V600E mutant colorectal liver metastases: a multicenter retrospective cohort study in Japan. Ann. Surg. Oncol. 27, 3307–3315 (2020).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Rawla, P., Sunkara, T. & Gaduputi, V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J. Oncol. 10, 10–27 (2019).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Ouyang, H. et al. Multimodality treatment of pancreatic cancer with liver metastases using chemotherapy, radiation therapy, and/or Chinese herbal medicine. Pancreas 40, 120–125 (2011).
Tas, F. Metastatic behavior in melanoma: timing, pattern, survival, and influencing factors. J. Oncol. 2012, 647684–647684 (2012).
Damsky, W. E., Rosenbaum, L. E. & Bosenberg, M. Decoding melanoma metastasis. Cancers 3, 126–163 (2010).
Damsky, W. E., Theodosakis, N. & Bosenberg, M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene 33, 2413–2422 (2014).
Lee, Y. T. Malignant melanoma: pattern of metastasis. CA Cancer J. Clin. 30, 137–142 (1980).
Rose, D. M. et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch. Surg. 136, 950–955 (2001).
Bustamante, P., Piquet, L., Landreville, S. & Burnier, J. V. Uveal melanoma pathobiology: metastasis to the liver. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.05.003 (2020).
Aronow, M. E., Topham, A. K. & Singh, A. D. Uveal melanoma: 5-year update on incidence, treatment, and survival (SEER 1973–2013). Ocul. Oncol. Pathol. 4, 145–151 (2018).
Sisley, K. et al. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosomes Cancer 19, 22–28 (1997).
Piperno-Neumann, S. et al. Prospective study of surveillance testing for metastasis in 100 high-risk uveal melanoma patients. J. Fr. Ophtalmol. 38, 526–534 (2015).
Riihimäki, M. et al. Metastatic sites and survival in lung cancer. Lung Cancer 86, 78–84 (2014).
Nakazawa, K. et al. Specific organ metastases and survival in small cell lung cancer. Oncol. Lett. 4, 617–620 (2012).
Tamura, T. et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol. Clin. Oncol. 3, 217–221 (2015).
Funazo, T., Nomizo, T. & Kim, Y. H. Liver metastasis is associated with poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. J. Thorac. Oncol. 12, e140–e141 (2017).
Elliott, J. A., Osterlind, K., Hirsch, F. R. & Hansen, H. H. Metastatic patterns in small-cell lung cancer: correlation of autopsy findings with clinical parameters in 537 patients. J. Clin. Oncol. 5, 246–254 (1987).
Adam, R. et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann. Surg. 244, 897–907; discussion 907–898 (2006).
Yao, J. C. et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 26, 3063–3072 (2008).
Modlin, I. M., Lye, K. D. & Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97, 934–959 (2003).
Fairweather, M. et al. Management of neuroendocrine tumor liver metastases: long-term outcomes and prognostic factors from a large prospective database. Ann. Surg. Oncol. 24, 2319–2325 (2017).
Soreide, K. et al. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 40, 39–46 (2016).
Corless, C. L., Fletcher, J. A. & Heinrich, M. C. Biology of gastrointestinal stromal tumors. J. Clin. Oncol. 22, 3813–3825 (2004).
Seesing, M. F. et al. Resection of liver metastases in patients with gastrointestinal stromal tumors in the imatinib era: a nationwide retrospective study. Eur. J. Surg. Oncol. 42, 1407–1413 (2016).
Bauer, S. et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib – analysis of prognostic factors (EORTC-STBSG collaborative study). Eur. J. Surg. Oncol. 40, 412–419 (2014).
Van den Eynden, G. G. et al. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 73, 2031–2043 (2013).
Vidal-Vanaclocha, F. in Liver Metastasis: Biology and Clinical Management (ed. Brodt, P.) 1 Online Resource (Springer Science+Business Media B.V. 2011).
Wisse, E., De Zanger, R. B., Charels, K., Van Der Smissen, P. & McCuskey, R. S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology 5, 683–692 (1985).
Wisse, E. & Knook, D. L. Kupffer cells and other liver sinusoidal cells: proceedings of the International Kupffer Cell Symposium held in Noordwijkerhout. 4-7 (Elsevier/North-Holland Biomedical Press 1977).
Stanger, B. Z. Cellular homeostasis and repair in the mammalian liver. Annu. Rev. Physiol. 77, 179–200 (2015).
Malato, Y. et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J. Clin. Invest. 121, 4850–4860 (2011).
Kolios, G., Valatas, V. & Kouroumalis, E. Role of Kupffer cells in the pathogenesis of liver disease. World J. Gastroententerol. 12, 7413–7420 (2006).
Keirsse, J. et al. The role of hepatic macrophages in liver metastasis. Cell Immunol. 330, 202–215 (2018).
Kubes, P. & Jenne, C. Immune responses in the liver. Annu. Rev. Immunol. 36, 247–277 (2018).
Crispe, I. N. The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 (2009).
Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. A thymic precursor to the NK T cell lineage. Science 296, 553–555 (2002).
Dashtsoodol, N. et al. Alternative pathway for the development of Vα14+ NKT cells directly from CD4−CD8− thymocytes that bypasses the CD4+CD8+ stage. Nat. Immunol. 18, 274–282 (2017).
Doherty, D. G. & O’Farrelly, C. Innate and adaptive lymphoid cells in the human liver. Immunol. Rev. 174, 5–20 (2000).
Friedman, S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172 (2008).
Muhanna, N. et al. Activation of hepatic stellate cells after phagocytosis of lymphocytes: a novel pathway of fibrogenesis. Hepatology 48, 963–977 (2008).
Crispe, I. N. Liver antigen-presenting cells. J. Hepatol. 54, 357–365 (2011).
Doherty, D. G. Immunity, tolerance and autoimmunity in the liver: a comprehensive review. J. Autoimmun. 66, 60–75 (2016).
Thery, C. & Amigorena, S. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 13, 45–51 (2001).
Xia, S. et al. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood 112, 3175–3185 (2008).
Kim, J. & Bae, J. S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016, 6058147 (2016).
Krenkel, O. & Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 17, 306–321 (2017).
Grossman, J. G. et al. Recruitment of CCR2+ tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology 7, e1470729 (2018).
Zhao, L. et al. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 57, 829–839 (2013).
Aalto, K. et al. Siglec-9 is a novel leukocyte ligand for vascular adhesion protein-1 and can be used in PET imaging of inflammation and cancer. Blood 118, 3725–3733 (2011).
Kivi, E. et al. Human siglec-10 can bind to vascular adhesion protein-1 and serves as its substrate. Blood 114, 5385–5392 (2009).
Paiva, A. E. et al. Pericytes in the premetastatic niche. Cancer Res. 78, 2779–2786 (2018).
Kruger, A. Premetastatic niche formation in the liver: emerging mechanisms and mouse models. J. Mol. Med. 93, 1193–1201 (2015).
Matsumura, H. et al. Kupffer cells decrease metastasis of colon cancer cells to the liver in the early stage. Int. J. Oncol. 45, 2303–2310 (2014).
Kimura, Y. et al. The innate immune receptor Dectin-2 mediates the phagocytosis of cancer cells by Kupffer cells for the suppression of liver metastasis. Proc. Natl Acad. Sci. USA 113, 14097–14102 (2016).
Braet, F. et al. The hepatic sinusoidal endothelial lining and colorectal liver metastases. World J. Gastroenterol. 13, 821–825 (2007).
Ramadori, G., Moriconi, F., Malik, I. & Dudas, J. Physiology and pathophysiology of liver inflammation, damage and repair. J. Physiol. Pharmacol. 59 (Suppl. 1), 107–117 (2008).
Spicer, J., Ferri, L. & Brodt. P. in Liver Metastasis: Biology and Clinical Management Cancer Metastasis - Biology and Treatment (ed Brodt. P.) Ch. 6, 155-185 (Springer 2011).
Ou, J. et al. Endothelial cell-derived fibronectin extra domain A promotes colorectal cancer metastasis via inducing epithelial-mesenchymal transition. Carcinogenesis 35, 1661–1670 (2014).
Barbazan, J. et al. Liver metastasis is facilitated by the adherence of circulating tumor cells to vascular fibronectin deposits. Cancer Res. 77, 3431–3441 (2017).
Taylor, D. P., Clark, A., Wheeler, S. & Wells, A. Hepatic nonparenchymal cells drive metastatic breast cancer outgrowth and partial epithelial to mesenchymal transition. Breast Cancer Res. Treat. 144, 551–560 (2014).
Lucotti, S. et al. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A2. J. Clin. Invest. 129, 1845–1862 (2019).
Wen, S. W., Ager, E. I. & Christophi, C. Bimodal role of Kupffer cells during colorectal cancer liver metastasis. Cancer Biol. Ther. 14, 606–613 (2013).
Ciner, A. T., Jones, K., Muschel, R. J. & Brodt, P. The unique immune microenvironment of liver metastases: challenges and opportunities. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.06.003 (2020). Recent and comprehensive review of the liver immune microenvironment and its targeting.
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Yu, X. et al. Immune modulation of liver sinusoidal endothelial cells by melittin nanoparticles suppresses liver metastasis. Nat. Commun. 10, 574 (2019).
Shaul, M. E. & Fridlender, Z. G. Cancer-related circulating and tumor-associated neutrophils - subtypes, sources and function. FEBS J. 285, 4316–4342 (2018).
Mizuno, R. et al. The role of tumor-associated neutrophils in colorectal cancer. Int. J. Mol. Sci. 20, 529 (2019).
Spicer, J., Brodt, P. & Ferri, L. E. in Liver Metastasis: Biology and Clinical Management (ed Brodt, P.) 155-185 (Springer 2011).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123, 3446–3458 (2013).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018).
Giese, M. A., Hind, L. E. & Huttenlocher, A. Neutrophil plasticity in the tumor microenvironment. Blood 133, 2159–2167 (2019).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009).
Rayes, R. F. et al. Loss of neutrophil polarization in colon carcinoma liver metastases of mice with an inducible, liver-specific IGF-I deficiency. Oncotarget 9, 15691–15704 (2018).
Tabaries, S. et al. Granulocytic immune infiltrates are essential for the efficient formation of breast cancer liver metastases. Breast Cancer Res. 17, 45 (2015).
Gordon-Weeks, A. N. et al. Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2-dependent angiogenesis in mice. Hepatology 65, 1920–1935 (2017).
Coffelt, S. B., Wellenstein, M. D. & de Visser, K. E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446 (2016).
Qian, B. Z. Inflammation fires up cancer metastasis. Semin. Cancer Biol. 47, 170–176 (2017).
Li, X. et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66, 157–167 (2017).
Mills, C. D. Anatomy of a discovery: m1 and m2 macrophages. Front. Immunol. 6, 212 (2015).
Dey, A., Allen, J. & Hankey-Giblin, P. A. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front. Immunol. 5, 683 (2014).
Ehling, J. et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut 63, 1960–1971 (2014).
Karlmark, K. R. et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274 (2009).
Milette, S., Sicklick, J. K., Lowy, A. M. & Brodt, P. Molecular pathways: targeting the microenvironment of liver metastases. Clin. Cancer Res. 23, 6390–6399 (2017).
Condamine, T. & Gabrilovich, D. I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32, 19–25 (2011).
Zhao, W. et al. Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Lab. Invest. 94, 182–191 (2014).
Gabrilovich, D. I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 5, 3–8 (2017).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Sade-Feldman, M. et al. Tumor necrosis factor-alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity 38, 541–554 (2013).
Svoronos, N. et al. Tumor cell-independent estrogen signaling drives disease progression through mobilization of myeloid-derived suppressor cells. Cancer Discov. 7, 72–85 (2017).
Lin, Q. et al. The mechanism of the premetastatic niche facilitating colorectal cancer liver metastasis generated from myeloid-derived suppressor cells induced by the S1PR1-STAT3 signaling pathway. Cell Death Dis. 10, 693 (2019).
Milette, S. et al. Sexual dimorphism and the role of estrogen in the immune microenvironment of liver metastases. Nat. Commun. 10, 5745 (2019).
Nielsen, S. R. et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat. Cell Biol. 18, 549–560 (2016).
Taura, K. et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology 135, 1729–1738 (2008).
Vinas, O. et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology 38, 919–929 (2003).
Charles, R. et al. Human hepatic stellate cells inhibit T-cell response through B7-H1 pathway. Transplantation 96, 17–24 (2013).
Jiang, G. et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation 86, 1492–1502 (2008).
Hochst, B. et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J. Hepatol. 59, 528–535 (2013).
Shimizu, S. et al. Ultrastructure of early phase hepatic metastasis of human colon carcinoma cells with special reference to desmosomal junctions with hepatocytes. Pathol. Int. 50, 953–959 (2000).
Mook, O. R. F. et al. Interactions between colon cancer cells and hepatocytes in rats in relation to metastasis. J. Cell. Mol. Med. 12, 2052–2061 (2008).
Huang, J., Pan, C., Hu, H., Zheng, S. & Ding, L. Osteopontin-enhanced hepatic metastasis of colorectal cancer cells. PLoS ONE 7, e47901 (2012).
Tabaries, S. et al. Claudin-2 promotes breast cancer liver metastasis by facilitating tumor cell interactions with hepatocytes. Mol. Cell Biol. 32, 2979–2991 (2012).
Georges, R. et al. Sequential biphasic changes in claudin1 and claudin4 expression are correlated to colorectal cancer progression and liver metastasis. J. Cell. Mol. Med. 16, 260–272 (2012).
Zvibel, I. et al. Transcriptional profiling identifies genes induced by hepatocyte-derived extracellular matrix in metastatic human colorectal cancer cell lines. Clin. Exp. Metastasis 30, 189–200 (2013).
Li, H. et al. Human and mouse colon cancer utilizes CD95 signaling for local growth and metastatic spread to liver. Gastroenterology 137, 934–944.e934 (2009).
Yoshioka, T. et al. Significance of integrin αvβ5 and erbB3 in enhanced cell migration and liver metastasis of colon carcinomas stimulated by hepatocyte-derived heregulin. Cancer Sci. 101, 2011–2018 (2010).
Dome, B., Hendrix, M. J., Paku, S., Tovari, J. & Timar, J. Alternative vascularization mechanisms in cancer: pathology and therapeutic implications. Am. J. Pathol. 170, 1–15 (2007).
Tiegs, G. & Lohse, A. W. Immune tolerance: what is unique about the liver. J. Autoimmun. 34, 1–6 (2010).
Heymann, F. & Tacke, F. Immunology in the liver–from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13, 88–110 (2016).
Bilen, M. A. et al. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer 19, 857 (2019).
Tumeh, P. C. et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol. Res. 5, 417–424 (2017).
Yu, J. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 27, 152–164 (2021).
Burnier, J. V. et al. Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene 30, 3766–3783 (2011).
Qi, S., Perrino, S., Miao, X., Lamarche-Vane, N. & Brodt, P. The chemokine CCL7 regulates invadopodia maturation and MMP-9 mediated collagen degradation in liver-metastatic carcinoma cells. Cancer Lett. 483, 98–113 (2020).
Vaniotis, G. et al. Collagen IV-conveyed signals can regulate chemokine production and promote liver metastasis. Oncogene 37, 3790–3805 (2018).
Goddard, E. T. et al. The rodent liver undergoes weaning-induced involution and supports breast cancer metastasis. Cancer Discov. 7, 177–187 (2017).
Salarian, M. et al. Precision detection of liver metastasis by collagen-targeted protein MRI contrast agent. Biomaterials 224, 119478 (2019).
Nystrom, H., Tavelin, B., Bjorklund, M., Naredi, P. & Sund, M. Improved tumour marker sensitivity in detecting colorectal liver metastases by combined type IV collagen and CEA measurement. Tumour Biol. 36, 9839–9847 (2015).
Steele, C. W. et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29, 832–845 (2016).
Barnhill, R. et al. Replacement and desmoplastic histopathological growth patterns in cutaneous melanoma liver metastases: frequency, characteristics, and robust prognostic value. J. Pathol. Clin. Res. 6, 195–206 (2020).
Van den Eynden, G. G. et al. Tumor stromal phenotypes define VEGF sensitivity–letter. Clin. Cancer Res. 20, 5140 (2014).
Stessels, F. et al. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br. J. Cancer 90, 1429–1436 (2004).
Watanabe, K. et al. The “histological replacement growth pattern” represents aggressive invasive behavior in liver metastasis from pancreatic cancer. Cancer Med. 9, 3130–3141 (2020).
Frentzas, S. et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 22, 1294–1302 (2016).
Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Invest. 126, 1208–1215 (2016).
Wortzel, I., Dror, S., Kenific, C. M. & Lyden, D. Exosome-mediated metastasis: communication from a distance. Dev. Cell 49, 347–360 (2019).
Zhang, H. et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 8, 15016 (2017).
Desmond, B. J., Dennett, E. R. & Danielson, K. M. Circulating extracellular vesicle microRNA as diagnostic biomarkers in early colorectal cancer-a review. Cancers 12, 52 (2019).
Shao, Y. et al. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis 39, 1368–1379 (2018).
Rahbari, N. N. et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl. Med. 8, 360ra135 (2016).
Yang, Y. et al. Discontinuation of anti-VEGF cancer therapy promotes metastasis through a liver revascularization mechanism. Nat. Commun. 7, 12680 (2016).
Im, J. H. et al. G-CSF rescues tumor growth and neo-angiogenesis during liver metastasis under host angiopoietin-2 deficiency. Int. J. Cancer 132, 315–326 (2013).
Sahani, D. V., Bajwa, M. A., Andrabi, Y., Bajpai, S. & Cusack, J. C. Current status of imaging and emerging techniques to evaluate liver metastases from colorectal carcinoma. Ann. Surg. 259, 861–872 (2014). Comprehensive review on the different imaging techniques to evaluate liver metastases from colorectal cancer.
Minami, Y. & Kudo, M. Hepatic malignancies: correlation between sonographic findings and pathological features. World J. Radiol. 2, 249–256 (2010).
Estrella, J. S. et al. Intrabiliary growth of liver metastases: clinicopathologic features, prevalence, and outcome. Am. J. Surg. Pathol. 37, 1571–1579 (2013).
Niekel, M. C., Bipat, S. & Stoker, J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 257, 674–684 (2010).
Floriani, I. et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J. Magn. Reson. Imaging 31, 19–31 (2010).
Ito, T. et al. The diagnostic advantage of EOB-MR imaging over CT in the detection of liver metastasis in patients with potentially resectable pancreatic cancer. Pancreatology 17, 451–456 (2017).
Bahri, H. et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J. Nucl. Med. 55, 1786–1790 (2014).
Weber, W. A. Use of PET for monitoring cancer therapy and for predicting outcome. J. Nucl. Med. 46, 983–995 (2005).
De Bruyne, S. et al. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br. J. Cancer 106, 1926–1933 (2012).
Tan, M. C., Linehan, D. C., Hawkins, W. G., Siegel, B. A. & Strasberg, S. M. Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J. Gastrointest. Surg. 11, 1112–1119 (2007).
Has Simsek, D. et al. Can complementary 68Ga-DOTATATE and 18F-FDG PET/CT establish the missing link between histopathology and therapeutic approach in gastroenteropancreatic neuroendocrine tumors? J. Nucl. Med. 55, 1811–1817 (2014).
Hope, T. A. et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J. Nucl. Med. 59, 66–74 (2018).
Balogova, S. et al. 18F-fluorodihydroxyphenylalanine vs other radiopharmaceuticals for imaging neuroendocrine tumours according to their type. Eur. J. Nucl. Med. Mol. Imaging 40, 943–966 (2013).
Koopmans, K. P. et al. Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J. Clin. Oncol. 26, 1489–1495 (2008).
Fiebrich, H. B. et al. Total 18F-Dopa PET tumour uptake reflects metabolic endocrine tumour activity in patients with a carcinoid tumour. Eur. J. Nucl. Med. Mol. Imaging 38, 1854–1861 (2011).
Heinemann, V. et al. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur. J. Cancer 51, 1927–1936 (2015).
Chun, Y. S. et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 302, 2338–2344 (2009).
Frankel, T. L., Gian, R. K. & Jarnagin, W. R. Preoperative imaging for hepatic resection of colorectal cancer metastasis. J. Gastrointest. Oncol. 3, 11–18 (2012).
Peloso, A. et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. HPB 15, 928–934 (2013).
Tot, T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur. J. Cancer 38, 758–763 (2002).
Shimonishi, T., Miyazaki, K. & Nakanuma, Y. Cytokeratin profile relates to histological subtypes and intrahepatic location of intrahepatic cholangiocarcinoma and primary sites of metastatic adenocarcinoma of liver. Histopathology 37, 55–63 (2000).
Liu, H., Shi, J., Wilkerson, M. L. & Lin, F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am. J. Clin. Pathol. 138, 57–64 (2012).
Chu, P. G., Schwarz, R. E., Lau, S. K., Yen, Y. & Weiss, L. M. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am. J. Surg. Pathol. 29, 359–367 (2005).
Mukhopadhyay, S. & Katzenstein, A. L. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am. J. Surg. Pathol. 35, 15–25 (2011).
Gould, V. E. et al. Synaptophysin expression in neuroendocrine neoplasms as determined by immunocytochemistry. Am. J. Pathol. 126, 243–257 (1987).
Wiedenmann, B., Franke, W. W., Kuhn, C., Moll, R. & Gould, V. E. Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc. Natl Acad. Sci. USA 83, 3500–3504 (1986).
Wilson, B. S. & Lloyd, R. V. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am. J. Pathol. 115, 458–468 (1984).
Gown, A. M., Fulton, R. S. & Kandalaft, P. L. Markers of metastatic carcinoma of breast origin. Histopathology 68, 86–95 (2016).
Truong, L. D. & Shen, S. S. Immunohistochemical diagnosis of renal neoplasms. Arch. Pathol. Lab. Med. 135, 92–109 (2011).
Laury, A. R. et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am. J. Surg. Pathol. 35, 816–826 (2011).
Terada, T., Ohta, T., Sasaki, M., Nakanuma, Y. & Kim, Y. S. Expression of MUC apomucins in normal pancreas and pancreatic tumours. J. Pathol. 180, 160–165 (1996).
Brimo, F. & Epstein, J. I. Immunohistochemical pitfalls in prostate pathology. Hum. Pathol. 43, 313–324 (2012).
Sepulveda, A. R. et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 35, 1453–1486 (2017).
Tsilimigras, D. I. et al. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: a systematic review of the current evidence. Surg. Oncol. 27, 280–288 (2018). Comprehensive systematic review on the significance of genetic mutations in resectable and unresectable colorectal liver metastases.
Schwarz, R. E. et al. Systemic cytotoxic and biological therapies of colorectal liver metastases: expert consensus statement. HPB 15, 106–115 (2013).
Margonis, G. A. et al. Association between specific mutations in KRAS codon 12 and colorectal liver metastasis. JAMA Surg. 150, 722–729 (2015).
Margonis, G. A. et al. KRAS mutation status dictates optimal surgical margin width in patients undergoing resection of colorectal liver metastases. Ann. Surg. Oncol. 24, 264–271 (2017).
Margonis, G. A. et al. Codon 13 KRAS mutation predicts patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Cancer 122, 2698–2707 (2016).
Wu, X. Z., Ma, F. & Wang, X. L. Serological diagnostic factors for liver metastasis in patients with colorectal cancer. World J. Gastroenterol. 16, 4084–4088 (2010).
Locker, G. Y. et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 24, 5313–5327 (2006).
Fong, Y., Fortner, J., Sun, R. L., Brennan, M. F. & Blumgart, L. H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann. Surg. 230, 309–318; discussion 318-321 (1999).
Pantel, K. & Alix-Panabieres, C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 73, 6384–6388 (2013).
Zhang, W. et al. Liquid biopsy for cancer: circulating tumor cells, circulating free DNA or exosomes? Cell Physiol. Biochem. 41, 755–768 (2017).
Alix-Panabieres, C., Schwarzenbach, H. & Pantel, K. Circulating tumor cells and circulating tumor DNA. Annu. Rev. Med. 63, 199–215 (2012).
Alix-Panabieres, C. & Pantel, K. Circulating tumor cells: liquid biopsy of cancer. Clin. Chem. 59, 110–118 (2013).
Cohen, S. J. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008).
Seeberg, L. T. et al. Circulating tumor cells in patients with colorectal liver metastasis predict impaired survival. Ann. Surg. 261, 164–171 (2015).
Tien, Y. W. et al. A high circulating tumor cell count in portal vein predicts liver metastasis from periampullary or pancreatic cancer: a high portal venous CTC count predicts liver metastases. Medicine 95, e3407 (2016).
Crowley, E., Di Nicolantonio, F., Loupakis, F. & Bardelli, A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 10, 472–484 (2013).
Siravegna, G., Marsoni, S., Siena, S. & Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 14, 531–548 (2017).
Thierry, A. R. et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat. Med. 20, 430–435 (2014).
Misale, S. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486, 532–536 (2012).
Heitzer, E. et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 73, 2965–2975 (2013).
Li, G., Tang, W. & Yang, F. Cancer liquid biopsy using integrated microfluidic exosome analysis platforms. Biotechnol. J. 15, e1900225 (2020).
Merker, J. D. et al. Circulating tumor DNA analysis in patients with cancer: american society of clinical oncology and college of american pathologists joint review. J. Clin. Oncol. 36, 1631–1641 (2018).
Huang, S. et al. microRNA biomarkers in colorectal cancer liver metastasis. J. Cancer 9, 3867–3873 (2018).
Wang, L. G. & Gu, J. Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol. 36, e61–e67 (2012).
Kingham, T. P. et al. MicroRNA-203 predicts human survival after resection of colorectal liver metastasis. Oncotarget 8, 18821–18831 (2017).
Geng, L. et al. MicroRNA-192 suppresses liver metastasis of colon cancer. Oncogene 33, 5332–5340 (2014).
Costas-Chavarri, A. et al. Treatment of patients with early-stage colorectal cancer: ASCO resource-stratified guideline. J. Glob. Oncol. 5, 1–19 (2019).
Pawlik, T. M. & Choti, M. A. Surgical therapy for colorectal metastases to the liver. J. Gastrointest. Surg. 11, 1057–1077 (2007).
Altendorf-Hofmann, A. & Scheele, J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg. Oncol. Clin. N. Am. 12, 165–192 (2003).
Pulitano, C. et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann. Surg. Oncol. 18, 1380–1388 (2011).
Cloyd, J. M., Wiseman, J. T. & Pawlik, T. M. Surgical management of pancreatic neuroendocrine liver metastases. J. Gastrointest. Oncol. 11, 590–600 (2020).
Kunz, P. L. et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 42, 557–577 (2013).
Pavel, M. et al. ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 95, 157–176 (2012).
Jin, K. et al. Surgical management for non-functional pancreatic neuroendocrine neoplasms with synchronous liver metastasis: a consensus from the Chinese Study Group for Neuroendocrine Tumors (CSNET). Int. J. Oncol. 49, 1991–2000 (2016).
Singh, S. et al. Consensus recommendations for the diagnosis and management of pancreatic neuroendocrine tumors: guidelines from a Canadian National Expert Group. Ann. Surg. Oncol. 22, 2685–2699 (2015).
Saxena, A., Chua, T. C., Perera, M., Chu, F. & Morris, D. L. Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surgical Oncol. 21, e131–e141 (2012).
Mayo, S. C. et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann. Surg. Oncol. 18, 3657–3665 (2011).
Scott, A. T. et al. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs). Surgery 165, 166–175 (2019).
Ejaz, A. et al. Cytoreductive debulking surgery among patients with neuroendocrine liver metastasis: a multi-institutional analysis. HPB 20, 277–284 (2018).
Adam, R. et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann. Surg. 244, 524–535 (2006).
Groeschl, R. T. et al. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: a multi-institutional analysis. J. Am. Coll. Surg. 214, 769–777 (2012).
Gaujoux, S. et al. Resection of adrenocortical carcinoma liver metastasis: is it justified? Ann. Surg. Oncol. 19, 2643–2651 (2012).
Gleisner, A. L. et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 110, 2484–2492 (2007).
Pawlik, T. M. et al. Hepatic resection for metastatic melanoma: distinct patterns of recurrence and prognosis for ocular versus cutaneous disease. Ann. Surg. Oncol. 13, 712–720 (2006).
Muhlbacher, F. et al. Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transpl. Proc. 23, 1567–1568 (1991).
Hagness, M. et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann. Surg. 257, 800–806 (2013).
Dueland, S. et al. Survival following liver transplantation for patients with nonresectable liver-only colorectal metastases. Ann. Surg. 271, 212–218 (2020).
Moris, D. et al. Liver transplantation for unresectable colorectal liver metastases: a systematic review. J. Surg. Oncol. 116, 288–297 (2017).
Le Treut, Y. P. et al. Liver transplantation for neuroendocrine tumors in Europe — results and trends in patient selection: a 213-case European liver transplant registry study. Ann. Surg. 257, 807–815 (2013).
Moris, D. et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: a systematic review. Surgery 162, 525–536 (2017).
Crocetti, L., de Baere, T., Pereira, P. L. & Tarantino, F. P. CIRSE standards of practice on thermal ablation of liver tumours. Cardiovasc. Intervent. Radiol. 43, 951–962 (2020).
Ruers, T. et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann. Oncol. 23, 2619–2626 (2012).
Ruers, T. et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J. Natl Cancer Inst. 109, djx015 (2017).
Otto, G. et al. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann. Surg. 251, 796–803 (2010).
Lubner, M. G., Brace, C. L., Ziemlewicz, T. J., Hinshaw, J. L. & Lee, F. T. Jr. Microwave ablation of hepatic malignancy. Semin. Intervent. Radiol. 30, 56–66 (2013).
Correa-Gallego, C. et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann. Surg. Oncol. 21, 4278–4283 (2014).
Meijerink, M. R. et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc. Intervent. Radiol. 41, 1189–1204 (2018).
Shibata, T., Niinobu, T., Ogata, N. & Takami, M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 89, 276–284 (2000).
Meijerink, M. R., Puijk, R. S. & van den Tol, P. M. P. COLLISION trial seeks to answer time-honored question: “Thermal ablation or surgery for colorectal liver metastases?” Cardiovasc. Intervent. Radiol. 42, 1059–1061 (2019).
Gurusamy, K. et al. Liver resection surgery versus thermal ablation for colorectal LiVer MetAstases (LAVA): study protocol for a randomised controlled trial. Trials 19, 105 (2018).
Ruarus, A. H. et al. Conductivity rise during irreversible electroporation: true permeabilization or heat? Cardiovasc. Intervent. Radiol. 41, 1257–1266 (2018).
Hosein, P. J. et al. Percutaneous irreversible electroporation for the treatment of colorectal cancer liver metastases with a proposal for a new response evaluation system. J. Vasc. Interv. Radiol. 25, 1233–1239 e1232 (2014).
Kelly, C. & Cassidy, J. Chemotherapy in metastatic colorectal cancer. Surgical Oncol. 16, 65–70 (2007).
Giacchetti, S. et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 18, 136–147 (2000).
Tol, J. et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N. Engl. J. Med. 360, 563–572 (2009).
Saltz, L. B. et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin. Oncol. 26, 2013–2019 (2008).
Gordon, M. S. et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J. Clin. Oncol. 19, 843–850 (2001).
El Zouhairi, M., Charabaty, A. & Pishvaian, M. J. Molecularly targeted therapy for metastatic colon cancer: proven treatments and promising new agents. Gastrointest. Cancer Res. 4, 15–21 (2011).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Ohhara, Y. et al. Comparison between three oxaliplatin-based regimens with bevacizumab in patients with metastatic colorectal cancer. Onco Targets Ther. 8, 529–537 (2015).
Kim, S. A. et al. Conversion surgery after cetuximab or bevacizumab plus FOLFIRI chemotherapy in colorectal cancer patients with liver- and/or lung-limited metastases. J. Cancer Res. Clin. Oncol. 146, 2399–2410 (2020).
Sobrero, A. F. et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 2311–2319 (2008).
Schwartzberg, L. S. et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 32, 2240–2247 (2014).
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008).
Bolhuis, K., Kos, M., van Oijen, M. G. H., Swijnenburg, R. J. & Punt, C. J. A. Conversion strategies with chemotherapy plus targeted agents for colorectal cancer liver-only metastases: a systematic review. Eur. J. Cancer 141, 225–238 (2020).
Van Cutsem, E. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016). Guidelines on the management of patients with metastatic colorectal cancer.
Adam, R. & Kitano, Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann. Gastroenterol. Surg. 3, 50–56 (2019).
Yao, J. C. et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 514–523 (2011).
Raymond, E. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 501–513 (2011).
Yao, J. C. et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387, 968–977 (2016).
Cloyd, J. M., Konda, B., Shah, M. H. & Pawlik, T. M. The emerging role of targeted therapies for advanced well-differentiated gastroenteropancreatic neuroendocrine tumors. Expert Rev. Clin. Pharmacol. 12, 101–108 (2019).
Moertel, C. G., Hanley, J. A. & Johnson, L. A. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 303, 1189–1194 (1980).
Kunz, P. L. et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: a trial of the ECOG-ACRIN Cancer Research Group (E2211). J. Clin. Oncol. 36, 4004–4004 (2018).
Strosberg, J. et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017).
Zhu, J., Yang, Y., Zhou, L., Jiang, M. & Hou, M. A long-term follow-up of the imatinib mesylate treatment for the patients with recurrent gastrointestinal stromal tumor (GIST): the liver metastasis and the outcome. BMC Cancer 10, 199 (2010).
Kim, E. J. & Zalupski, M. M. Systemic therapy for advanced gastrointestinal stromal tumors: beyond imatinib. J. Surg. Oncol. 104, 901–906 (2011).
Vokes, E. E. et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann. Oncol. 29, 959–965 (2018).
Peters, S. et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 377, 829–838 (2017).
Asahara, T. et al. Studies of postoperative transarterial infusion chemotherapy for liver metastasis of colorectal carcinoma after hepatectomy. Hepatogastroenterology 45, 805–811 (1998).
Lorenz, M. et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann. Surg. 228, 756–762 (1998).
Kemeny, N. et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N. Engl. J. Med. 341, 2039–2048 (1999).
Kemeny, N. E. & Gonen, M. Hepatic arterial infusion after liver resection. N. Engl. J. Med. 352, 734–735 (2005).
Bolton, J. S. et al. Hepatic arterial infusion and systemic chemotherapy after multiple metastasectomy in patients with colorectal carcinoma metastatic to the liver: a North Central Cancer Treatment Group (NCCTG) phase II study, 92-46-52. Clin. Colorectal Cancer 11, 31–37 (2012).
Boileve, A. et al. Hepatic arterial infusion of oxaliplatin plus systemic chemotherapy and targeted therapy for unresectable colorectal liver metastases. Eur. J. Cancer 138, 89–98 (2020).
D’Angelica, M. I. et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann. Surg. 261, 353–360 (2015).
Massmann, A. et al. Transarterial chemoembolization (TACE) for colorectal liver metastases — current status and critical review. Langenbecks Arch. Surg. 400, 641–659 (2015).
Richardson, A. J., Laurence, J. M. & Lam, V. W. Transarterial chemoembolization with irinotecan beads in the treatment of colorectal liver metastases: systematic review. J. Vasc. Interv. Radiol. 24, 1209–1217 (2013).
Fiorentini, G. et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 32, 1387–1395 (2012).
Lau, W. Y. et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int. J. Radiat. Oncol. Biol. Phys. 40, 583–592 (1998).
Yan, Z. P., Lin, G., Zhao, H. Y. & Dong, Y. H. An experimental study and clinical pilot trials on yttrium-90 glass microspheres through the hepatic artery for treatment of primary liver cancer. Cancer 72, 3210–3215 (1993).
Kucuk, O. N., Soydal, C., Lacin, S., Ozkan, E. & Bilgic, S. Selective intraarterial radionuclide therapy with yttrium-90 (Y-90) microspheres for unresectable primary and metastatic liver tumors. World J. Surg. Oncol. 9, 86 (2011).
Bienert, M. et al. 90Y microsphere treatment of unresectable liver metastases: changes in 18F-FDG uptake and tumour size on PET/CT. Eur. J. Nucl. Med. Mol. Imaging 32, 778–787 (2005).
Wasan, H. S. et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 18, 1159–1171 (2017).
Kang, J. I. et al. A phase I trial of proton stereotactic body radiation therapy for liver metastases. J. Gastrointest. Oncol. 10, 112–117 (2019).
Hong, T. S. et al. Phase II study of proton-based stereotactic body radiation therapy for liver metastases: importance of tumor genotype. J. Natl Cancer Inst. 109, djx031 (2017).
Mahadevan, A. et al. Stereotactic body radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch(R) Patient Registry. Radiat. Oncol. 13, 26 (2018).
Mogrovejo, E., Manickam, P., Amin, M. & Cappell, M. S. Characterization of the syndrome of acute liver failure caused by metastases from breast carcinoma. Dig. Dis. Sci. 59, 724–736 (2014).
Rahnemai-Azar, A. A. et al. Update on liver failure following hepatic resection: strategies for prediction and avoidance of post-operative liver insufficiency. J. Clin. Transl. Hepatol. 6, 97–104 (2018).
Sangro, B. et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer 112, 1538–1546 (2008).
Ito, K. et al. Biliary sclerosis after hepatic arterial infusion pump chemotherapy for patients with colorectal cancer liver metastasis: incidence, clinical features, and risk factors. Ann. Surg. Oncol. 19, 1609–1617 (2012).
King, P. D. & Perry, M. C. Hepatotoxicity of chemotherapy. Oncologist 6, 162–176 (2001).
Blazeby, J. M. et al. Validation of the European Organization for Research and Treatment of Cancer QLQ-LMC21 questionnaire for assessment of patient-reported outcomes during treatment of colorectal liver metastases. Br. J. Surg. 96, 291–298 (2009).
Adamowicz, K., Saad, E. D. & Jassem, J. Health-related quality of life assessment in contemporary phase III trials in advanced colorectal cancer. Cancer Treat. Rev. 50, 194–199 (2016).
Gong, J. et al. Moving beyond conventional clinical trial end points in treatment-refractory metastatic colorectal cancer: a composite quality-of-life and symptom control end point. Clin. Ther. 39, 2135–2145 (2017).
Rees, J. R. et al. The prognostic value of patient-reported outcome data in patients with colorectal hepatic metastases who underwent surgery. Clin. Colorectal Cancer 15, 74–81 e71 (2016).
Wolstenholme, J. et al. Quality of life in the FOXFIRE, SIRFLOX and FOXFIRE-global randomised trials of selective internal radiotherapy for metastatic colorectal cancer. Int. J. Cancer 147, 1078–1085 (2020).
Helou, J. et al. Quality of life changes after stereotactic ablative radiotherapy for liver metastases: a prospective cohort analysis. Radiother. Oncol. 129, 435–440 (2018).
Kemeny, N. E. et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J. Clin. Oncol. 24, 1395–1403 (2006).
Soveri, L. M. et al. Long-term neuropathy and quality of life in colorectal cancer patients treated with oxaliplatin containing adjuvant chemotherapy. Acta Oncol. 58, 398–406 (2019).
Lin, X., Hong, S., Chen, J., Chen, W. & Wu, Z. The potential targets for metastases: a study on altered circular RNA profile in breast cancer liver metastases. Epigenomics 11, 1237–1250 (2019).
Ejaz, A. et al. Associations between patient perceptions of communication, cure, and other patient-related factors regarding patient-reported quality of care following surgical resection of lung and colorectal cancer. J. Gastrointest. Surg. 20, 812–826 (2016).
Fretland, Å. A. et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann. Surg. 267, 199–207 (2018).
Rubenstein, J. H., Enns, R., Heidelbaugh, J., Barkun, A. & Clinical Guidelines Committee. American gastroenterological association institute guideline on the diagnosis and management of Lynch syndrome. Gastroenterology 149, 777–782; quiz e716-777 (2015).
Geiersbach, K. B. & Samowitz, W. S. Microsatellite instability and colorectal cancer. Arch. Pathol. Lab. Med. 135, 1269–1277 (2011).
Achrol, A. S., Rennert, R. C., Anders, C. et al. Brain metastases. Nat. Rev. Dis. Prim. 5, 5 (2019).
Author information
Authors and Affiliations
Contributions
Introduction (T.M.P. and D.I.T.); Epidemiology (D.I.T and P.-A.C.); Mechanisms/pathophysiology (P.B. and R.J.M.); Diagnosis, screening and prevention (I.E., E.d.S. and D.I.T.); Management (R.W.P., T.M.P. and D.I.T.); Quality of life (M.I.D.); Outlook (R.J.M. and M.D.); Overview of Primer (T.M.P.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks H. Baba, M. Ducreux, P. Galle, G. Poston, M. Schmid and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Left-sided tumours
-
Tumours that originate from the left descending colon.
- Right-sided tumours
-
Tumours that originate from the first part of the colon corresponding to the ascending colon.
- Conversion treatment
-
The use of cytotoxic drugs and/or chemotherapy that shrink the tumour to a level considered amenable to resection.
- Stage M1c melanoma
-
The cancer has spread to any other location that does not involve the central nervous system.
- Monosomy
-
The state of having a single copy of a chromosome pair in contrast to the usual two copies found in diploid cells.
- Intravasation
-
Invasion of cancer cells through the basement membrane into a blood or lymphatic vessel.
- Kupffer cells
-
Macrophages that are phagocytic and reside in sinusoids of the liver in proximity to the endothelial cells.
- Sinusoidal vessels
-
Low-pressure vascular channels that receive blood from terminal branches of the hepatic artery and portal vein at the periphery of lobules and deliver it into central veins.
- Space of Disse
-
The perisinusoidal space in the liver between a hepatocyte and a sinusoid.
- Myelopoiesis
-
Process in which innate immune cells, such as neutrophils, dendritic cells and monocytes, develop from a myeloid progenitor cell.
- Desmosomes
-
Specialized adhesive protein complexes that localize to intercellular junctions and are responsible for maintaining the mechanical integrity of tissues.
- Ring enhancement
-
Characteristic feature in CT, which represents a zonal area of viable tumour cells.
- Cytoreduction
-
Approach to cancer treatment that aims to reduce the number of cancer cells via resection of the primary tumour or metastatic deposits.
- Future liver remnant
-
Volume of functional liver after resection
- Cytokeratin
-
Type of structural protein that is expressed by the epithelial cells.
- Microsatellite instability
-
The condition of genetic hypermutability (predisposition to mutation) that results from impaired DNA mismatch repair.
- Hilar lymphadenopathy
-
Enlargement of lymph nodes at the hepatic hilum.
- Disease-free interval
-
Interval from the treatment of the primary tumour to the detection of metastases.
- Metachronous metastasis
-
Metastasis developed after a period of time from diagnosis of the primary tumour.
- Hepatic hilum
-
Anatomical region where bile ducts, hepatic arterial branches, portal vein branches, lymphatics and nerves enter or leave the liver.
- Heat-sink phenomenon
-
Phenomenon that limits ablation effectiveness when the target lesion is close (<1 cm) to a large blood vessel (≥3 mm diameter); the flowing blood causes a cooling effect, thereby reducing the ablation volume.
- Local control rate
-
Rate of controlling cancer growth at the local site of origin.
Rights and permissions
About this article
Cite this article
Tsilimigras, D.I., Brodt, P., Clavien, PA. et al. Liver metastases. Nat Rev Dis Primers 7, 27 (2021). https://doi.org/10.1038/s41572-021-00261-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-021-00261-6
This article is cited by
-
Efferocytosis reprograms the tumor microenvironment to promote pancreatic cancer liver metastasis
Nature Cancer (2024)
-
Targeting initial tumour–osteoclast spatiotemporal interaction to prevent bone metastasis
Nature Nanotechnology (2024)
-
Discovery of novel DNA methylation biomarker panels for the diagnosis and differentiation between common adenocarcinomas and their liver metastases
Scientific Reports (2024)
-
Periostin–TGF-β feedforward loop contributes to tumour-stroma crosstalk in liver metastatic outgrowth of colorectal cancer
British Journal of Cancer (2024)
-
Patient vulnerability is associated with poor prognosis following upfront hepatectomy for colorectal liver metastasis
International Journal of Clinical Oncology (2024)