Abstract
Penile squamous cell carcinoma (PSCC) is a rare cancer with orphan disease designation and a prevalence of 0.1–1 per 100,000 men in high-income countries, but it constitutes up to 10% of malignancies in men in some African, Asian and South American regions. Risk factors for PSCC include the absence of childhood circumcision, phimosis, chronic inflammation, poor penile hygiene, smoking, immunosuppression and infection with human papillomavirus (HPV). Several different subtypes of HPV-related and non-HPV-related penile cancers have been described, which also have different prognostic profiles. Localized disease can be effectively managed by topical therapy, surgery or radiotherapy. As PSCC is characterized by early lymphatic spread and imaging is inadequate for the detection of micrometastatic disease, correct and upfront surgical staging of the inguinal lymph nodes is crucial in disease management. Advanced stages of disease require multimodal management. Optimal sequencing of treatments and patient selection are still being investigated. Cisplatin-based chemotherapy regimens are the mainstay of systemic therapy for advanced PSCC, but they have poor and non-durable responses and high rates of toxic effects, indicating a need for the development of more effective and less toxic therapeutic options. Localized and advanced penile cancers and their treatment have profound physical and psychosexual effects on the quality of life of patients and survivors by altering sexual and urinary function and causing lymphoedema.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Douglawi, A. & Masterson, T. A. Penile cancer epidemiology and risk factors: a contemporary review. Curr. Opin. Urol. 29, 145–149 (2019).
Olesen, T. B. et al. Prevalence of human papillomavirus DNA and p16(INK4a) in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol. 20, 145–158 (2019).
Dräger, D. L., Milerski, S., Sievert, K. D. & Hakenberg, O. W. Psychosocial effects in patients with penile cancer: a systematic review. Urologe A 57, 444–452 (2018).
Novara, G., Galfano, A., De Marco, V., Artibani, W. & Ficarra, V. Prognostic factors in squamous cell carcinoma of the penis. Nat. Clin. Pract. Urol. 4, 140–146 (2007).
Woldu, S. L. et al. Usage and survival implications of surgical staging of inguinal lymph nodes in intermediate- to high-risk, clinical localized penile cancer: a propensity-score matched analysis. Urol. Oncol. 36, 159.e7–159.17 (2018).
Ross, G. L. et al. The learning curve for sentinel node biopsy in malignant melanoma. Br. J. Plast. Surg. 55, 298–301 (2002).
Flaig, T. W. et al. NCCN Guidelines Version 2.2020 Penile Cancer (NCCN, 2020).
Hakenberg, O. W. et al. EAU guidelines on penile cancer: 2014 update. Eur. Urol. 67, 142–150 (2015).
Pagliaro, L. C. et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J. Clin. Oncol. 28, 3851–3857 (2010).
Agarwal, G., Gupta, S. & Spiess, P. E. Novel targeted therapies for the treatment of penile cancer. Expert Opin. Drug Discov. 9, 959–968 (2014).
Montes Cardona, C. E. & García-Perdomo, H. A. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev. Panam. Salud Publica 41, e117 (2017).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Ferlay, J. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953 (2019).
Favorito, L. A. et al. Epidemiologic study on penile cancer in Brazil. Int. Braz. J. Urol. 34, 587–593 (2008).
Visser, O. et al. Incidence and survival of rare urogenital cancers in Europe. Eur. J. Cancer 48, 456–464 (2012).
Rando Sous, A. et al. A review of penile cancer. Adv. Urol. 2009, 415062 (2009).
Goodman, M. T., Hernandez, B. Y. & Shvetsov, Y. B. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol. Biomarkers Prev. 16, 1833–1839 (2007).
Djajadiningrat, R. S. et al. Contemporary management of regional nodes in penile cancer — improvement of survival? J. Urol. 191, 68–73 (2014).
Pagliaro, L. C. & Crook, J. Multimodality therapy in penile cancer: when and which treatments? World J. Urol. 27, 221–225 (2009).
Verhoeven, R. H. A. et al. Population-based survival of penile cancer patients in Europe and the United States of America: no improvement since 1990. Eur. J. Cancer 49, 1414–1421 (2013).
Ayres, B. et al. Has centralisation of penile cancer services in the United Kingdom improved survival? Eur. Urol. Suppl. 13, e50 (2014).
Vanthoor, J., Thomas, A., Tsaur, I. & Albersen, M. Making surgery safer by centralization of care: impact of case load in penile cancer. World J. Urol. 38, 1385–1390 (2020).
Hansen, B. T., Orumaa, M., Lie, A. K., Brennhovd, B. & Nygard, M. Trends in incidence, mortality and survival of penile squamous cell carcinoma in Norway 1956-2015. Int. J. Cancer 142, 1586–1593 (2018).
Emmanuel, A., Nettleton, J., Watkin, N. & Berney, D. M. The molecular pathogenesis of penile carcinoma-current developments and understanding. Virchows Archiv. 475, 397–405 (2019).
Leto, M., Santos Junior, G. F., Porro, A. M. & Tomimori, J. Human papillomavirus infection: etiopathogenesis, molecular biology and clinical manifestations. An. Bras. Dermatol. 86, 306–317 (2011).
Mosconi, A. M., Roila, F., Gatta, G. & Theodore, C. Cancer of the penis. Crit. Rev. Oncol. Hematol. 53, 165–177 (2005).
Spiess, P. E., Dhillon, J., Baumgarten, A. S., Johnstone, P. A. & Giuliano, A. R. Pathophysiological basis of human papillomavirus in penile cancer: key to prevention and delivery of more effective therapies. CA Cancer J. Clin. 66, 481–495 (2016).
Alemany, L. et al. Role of human papillomavirus in penile carcinomas worldwide. Eur. Urol. 69, 953–961 (2016).
Flaherty, A. et al. Implications for human papillomavirus in penile cancer. Urol. Oncol. 32, 53.e1–53.e8 (2014).
Ornellas, A. A. & Ornellas, P. Should routine neonatal circumcision be a police to prevent penile cancer? Opinion: Yes. Int. Braz. J. Urol. 43, 7–9 (2017).
Minhas, S., Manseck, A., Watya, S. & Hegarty, P. K. Penile cancer — prevention and premalignant conditions. Urology 76, S24–S35 (2010).
Larke, N. L., Thomas, S. L., dos Santos Silva, I. & Weiss, H. A. Male circumcision and penile cancer: a systematic review and meta-analysis. Cancer Causes Control 22, 1097–1110 (2011).
Clouston, D., Hall, A. & Lawrentschuk, N. Penile lichen sclerosus (balanitis xerotica obliterans). BJU Int. 108, 14–19 (2011).
Harish, K. & Ravi, R. The role of tobacco in penile carcinoma. Br. J. Urol. 75, 375–377 (1995).
Barnes, K. T. et al. Obesity is associated with increased risk of invasive penile cancer. BMC Urol. 16, 42 (2016).
Vieira, C. B. et al. Profile of patients with penile cancer in the region with the highest worldwide incidence. Sci. Rep. 10, 2965 (2020).
Clark, P. E. et al. Penile cancer. J. Natl Compr. Cancer Netw. 11, 594–615 (2013).
Stern, R. S., Bagheri, S. & Nichols, K. The persistent risk of genital tumors among men treated with psoralen plus ultraviolet A (PUVA) for psoriasis. J. Am. Acad. Dermatol. 47, 33–39 (2002).
Hoekstra, R. J., Trip, E. J., Ten Kate, F. J., Horenblas, S. & Lock, M. T. Penile intraepithelial neoplasia: nomenclature, incidence and progression to malignancy in the Netherlands. Int. J. Urol. 26, 353–357 (2019).
Ingles, D. J. et al. Human papillomavirus virus (HPV) genotype- and age-specific analyses of external genital lesions among men in the HPV Infection in Men (HIM) study. J. Infect. Dis. 211, 1060–1067 (2015).
Sudenga, S. L. et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur. Urol. 69, 166–173 (2016).
Akogbe, G. O. et al. Race and prevalence of human papillomavirus infection among men residing in Brazil, Mexico and the United States. Int. J. Cancer 131, E282–E291 (2012).
Kristiansen, S. et al. Risk factors for penile intraepithelial neoplasia: a population-based register study in Sweden, 2000-2012. Acta Derm. Venereol. 99, 315–320 (2019).
Yoon, C. S., Kim, K. D., Park, S. N. & Cheong, S. W. alpha(6) Integrin is the main receptor of human papillomavirus type 16 VLP. Biochem. Biophys. Res. Commun. 283, 668–673 (2001).
Yugawa, T. & Kiyono, T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev. Med. Virol. 19, 97–113 (2009).
Ferreux, E. et al. Evidence for at least three alternative mechanisms targeting the p16INK4A/cyclin D/Rb pathway in penile carcinoma, one of which is mediated by high-risk human papillomavirus. J. Pathol. 201, 109–118 (2003).
Steinestel, J. et al. The role of histologic subtype, p16(INK4a) expression, and presence of human papillomavirus DNA in penile squamous cell carcinoma. BMC Cancer 15, 220 (2015).
Klingelhutz, A. J., Foster, S. A. & McDougall, J. K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380, 79–82 (1996).
Gewin, L., Myers, H., Kiyono, T. & Galloway, D. A. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 18, 2269–2282 (2004).
Khansari, N., Shakiba, Y. & Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 3, 73–80 (2009).
De Paula, A. A. et al. The impact of cyclooxygenase-2 and vascular endothelial growth factor C immunoexpression on the prognosis of penile carcinoma. J. Urol. 187, 134–140 (2012).
Greenhough, A. et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30, 377–386 (2009).
Poetsch, M. et al. Alterations in the tumor suppressor gene p16(INK4A) are associated with aggressive behavior of penile carcinomas. Virchows Archiv. 458, 221–229 (2011).
Afonso, L. A. et al. Human papillomavirus, Epstein-Barr virus, and methylation status of p16(ink4a) in penile cancer. J. Med. Virol. 89, 1837–1843 (2017).
Lohneis, P. et al. Human papilloma virus status of penile squamous cell carcinoma is associated with differences in tumour-infiltrating T lymphocytes. Virchows Archiv. 466, 323–331 (2015).
Herbster, S., Paladino, A., de Freitas, S. & Boccardo, E. Alterations in the expression and activity of extracellular matrix components in HPV-associated infections and diseases. Clinics 73, e551s (2018).
Chu, C. et al. Immunophenotypes based on the tumor immune microenvironment allow for unsupervised penile cancer patient stratification. Cancers 12, 1796 (2020).
Gunia, S. et al. Diagnostic and prognostic impact of peritumoral stromal remodeling in patients with surgically treated invasive penile squamous cell cancer. Hum. Pathol. 45, 1169–1176 (2014).
Ottenhof, S. R. et al. The prognostic value of immune factors in the tumor microenvironment of penile squamous cell carcinoma. Front. Immunol. 9, 1253 (2018).
Aydin, A. M. et al. Understanding genomics and the immune environment of penile cancer to improve therapy. Nat. Rev. Urol. 17, 555–570 (2020).
Onywera, H. et al. The penile microbiota of Black South African men: relationship with human papillomavirus and HIV infection. BMC Microbiol. 20, 78 (2020).
Blank, C. U., Haanen, J. B., Ribas, A. & Schumacher, T. N. Cancer immunology. The “cancer immunogram”. Science 352, 658–660 (2016).
van dijk, N. et al. The cancer immunogram as a framework for personalized immunotherapy in urothelial cancer. Eur. Urol. 75, 435–444 (2019).
de Vries, H. M., Ottenhof, S. R., Horenblas, S., van der Heijden, M. S. & Jordanova, E. S. Defining the tumor microenvironment of penile cancer by means of the cancer immunogram. Eur. Urol. Focus. 5, 718–721 (2019).
Hernandez, B. Y. et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998–2003. Cancer 113, 2883–2891 (2008).
Barnholtz-Sloan, J. S., Maldonado, J. L., Pow-sang, J. & Giuliano, A. R. Incidence trends in primary malignant penile cancer. Urol. Oncol. 25, 361–367 (2007).
Favorito, L. A. et al. Epidemiologic study on penile cancer in Brazil. Int. Braz. J. Urol. 34, 587–591 (2008).
Dufour, J. F. et al. Urogenital manifestations in Wegener granulomatosis: a study of 11 cases and review of the literature. Medicine 91, 67–74 (2012).
Lee, D. K., Hinshaw, M., Cripps, D. & Jarrard, D. F. Pyoderma gangrenosum of penis. J. Urol. 170, 185–186 (2003).
Khan, D., Choudhary, A., Dutta, A. & Khan, I. Tuberculosis of the glans penis mimicking as carcinoma. Int. J. Mycobacteriol. 5, 341–342 (2016).
Tsaur, I., Ochsendorf, F. R., Bug, R. & Jonas, D. Primary syphilitic lesion mimicking penile cancer. Atypical manifestation with an unconventional diagnostic approach. Urologe A 48, 1210–1213 (2009).
Lont, A. P., Besnard, A. P., Gallee, M. P., van Tinteren, H. & Horenblas, S. A comparison of physical examination and imaging in determining the extent of primary penile carcinoma. BJU Int. 91, 493–495 (2003).
Shim, T. N., Ali, I., Muneer, A. & Bunker, C. B. Benign male genital dermatoses. Br. Med. J. 354, i4337 (2016).
Lucky, M. A., Rogers, B. & Parr, N. J. Referrals into a dedicated British penile cancer centre and sources of possible delay. Sex. Transm. Infect. 85, 527–530 (2009).
Van Poppel, H. et al. Penile cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24, vi115–vi124 (2013).
Maden, C. et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J. Natl Cancer Inst. 85, 19–24 (1993).
Daling, J. R. et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int. J. Cancer 116, 606–616 (2005).
Agrawal, A., Pai, D., Ananthakrishnan, N., Smile, S. R. & Ratnakar, C. Clinical and sonographic findings in carcinoma of the penis. J. Clin. Ultrasound 28, 399–406 (2000).
Horenblas, S., Kröger, R., Gallee, M. P., Newling, D. W. & van Tinteren, H. Ultrasound in squamous cell carcinoma of the penis; a useful addition to clinical staging? A comparison of ultrasound with histopathology. Urology 43, 702–707 (1994).
Kayes, O. et al. The role of magnetic resonance imaging in the local staging of penile cancer. Eur. Urol. 51, 1313–1318 (2007).
Petralia, G. et al. Local staging of penile cancer using magnetic resonance imaging with pharmacologically induced penile erection. Radiol. Med. 113, 517–528 (2008).
Rosevear, H. M. et al. Utility of ¹-F-FDG PET/CT in identifying penile squamous cell carcinoma metastatic lymph nodes. Urol. Oncol. 30, 723–726 (2012).
Sadeghi, R., Gholami, H., Zakavi, S. R., Kakhki, V. R. & Horenblas, S. Accuracy of 18F-FDG PET/CT for diagnosing inguinal lymph node involvement in penile squamous cell carcinoma: systematic review and meta-analysis of the literature. Clin. Nucl. Med. 37, 436–441 (2012).
Ottenhof, S. R. & Vegt, E. The role of PET/CT imaging in penile cancer. Transl. Androl. Urol. 6, 833–838 (2017).
Shabbir, M. et al. Glans resurfacing for the treatment of carcinoma in situ of the penis: surgical technique and outcomes. Eur. Urol. 59, 142–147 (2011).
Moch, H., Cubilla, A. L., Humphrey, P. A., Reuter, V. E. & Ulbright, T. M. The 2016 WHO classification of tumours of the urinary system and male genital organs — part a: renal, penile, and testicular tumours. Eur. Urol. 70, 93–105 (2016).
Chaux, A., Velazquez, E. F., Algaba, F., Ayala, G. & Cubilla, A. L. Developments in the pathology of penile squamous cell carcinomas. Urology 76, S7–S14 (2010).
Velazquez, E. F. et al. Protocol for the examination of specimens from patients with carcinoma of the penis. Arch. Pathol. Lab. Med. 134, 923–929 (2010).
Paner, G. P. et al. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur. Urol. 73, 560–569 (2018).
Amin, M. B. et al. AJCC Cancer Staging Manual 8th edn (Springer, 2017).
Dadar, M. et al. Advances in designing and developing vaccines, drugs and therapeutic approaches to counter human papilloma virus. Front. Immunol. 9, 2478 (2018).
Schiffman, M. et al. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2, 16086 (2016).
Stanley, M. HPV vaccination in boys and men. Hum. Vaccin. Immunother. 10, 2109–2111 (2014).
Ng, S. S., Hutubessy, R. & Chaiyakunapruk, N. Systematic review of cost-effectiveness studies of human papillomavirus (HPV) vaccination: 9-valent vaccine, gender-neutral and multiple age cohort vaccination. Vaccine 36, 2529–2544 (2018).
Powell, N., Hibbitts, S. & Evans, M. Gender neutral vaccination against HPV. Br. Med. J. 362, k3837 (2018).
Markowitz, L. E. et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J. Infect. Dis. 208, 385–393 (2013).
Schiller, J. T., Castellsagué, X. & Garland, S. M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 30, F123–F138 (2012).
Mesher, D., Panwar, K., Thomas, S. L., Beddows, S. & Soldan, K. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: an ongoing cross-sectional study. BMJ Open 6, e009915 (2016).
Giuliano, A. R. et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N. Engl. J. Med. 364, 401–411 (2011).
Palefsky, J. M. et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N. Engl. J. Med. 365, 1576–1585 (2011).
Shabbir, M., Barod, R., Hegarty, P. K. & Minhas, S. Primary prevention and vaccination for penile cancer. Ther. Adv. Urol. 5, 161–169 (2013).
Olsen, J. & Jørgensen, T. R. Revisiting the cost-effectiveness of universal HPV-vaccination in Denmark accounting for all potentially vaccine preventable HPV-related diseases in males and females. Cost. Eff. Resour. Alloc. 13, 4 (2015).
Canfell, K. et al. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine 30, F157–F167 (2012).
Raskin, Y., Vanthoor, J., Milenkovic, U., Muneer, A. & Albersen, M. Organ-sparing surgical and nonsurgical modalities in primary penile cancer treatment. Curr. Opin. Urol. 29, 156–164 (2019).
Crook, J. Contemporary role of radiotherapy in the management of primary penile tumors and metastatic disease. Urol. Clin. North Am. 43, 435–448 (2016).
Chipollini, J., Necchi, A. & Spiess, P. E. Outcomes for patients with node-positive penile cancer: impact of perioperative systemic therapies and the importance of surgical intervention. Eur. Urol. 74, 241–242 (2018).
Bandini, M., Pederzoli, F. & Necchi, A. Neoadjuvant chemotherapy for lymph node-positive penile cancer: current evidence and knowledge. Curr. Opin. Urol. 30, 218–222 (2020).
Robinson, R. et al. Risks and benefits of adjuvant radiotherapy after inguinal lymphadenectomy in node-positive penile cancer: a systematic review by the european association of urology penile cancer guidelines panel. Eur. Urol. 74, 76–83 (2018).
Ashley, S. et al. Human papilloma virus (HPV) status may impact treatment outcomes in patients with pre-cancerous penile lesions (an eUROGEN Study). Int. J. Impot. Res. https://doi.org/10.1038/s41443-020-0327-4 (2020).
Kravvas, G. et al. The management of penile intraepithelial neoplasia (PeIN): clinical and histological features and treatment of 345 patients and a review of the literature. J. Dermatol. Treat. https://doi.org/10.1080/09546634.2020.1800574 (2020).
Manjunath, A., Brenton, T., Wylie, S., Corbishley, C. M. & Watkin, N. A. Topical Therapy for non-invasive penile cancer (Tis)-updated results and toxicity. Transl. Androl. Urol. 6, 803–808 (2017).
Alnajjar, H. M. et al. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. Eur. Urol. 62, 923–928 (2012).
Deen, K. & Burdon-Jones, D. Imiquimod in the treatment of penile intraepithelial neoplasia: an update. Australas. J. Dermatol. 58, 86–92 (2017).
Bandieramonte, G. et al. Peniscopically controlled CO2 laser excision for conservative treatment of in situ and T1 penile carcinoma: report on 224 patients. Eur. Urol. 54, 875–882 (2008).
Tang, D. H. et al. Laser ablation as monotherapy for penile squamous cell carcinoma: a multi-center cohort analysis. Urol. Oncol. 36, 147–152 (2018).
van Bezooijen, B. P., Horenblas, S., Meinhardt, W. & Newling, D. W. Laser therapy for carcinoma in situ of the penis. J. Urol. 166, 1670–1671 (2001).
Protzel, C. & Hakenberg, O. W. Local treatment of penile cancer. Urologe A 57, 423–427 (2018).
Shaw, K. S., Nguyen, G. H., Lacouture, M. & Deng, L. Combination of imiquimod with cryotherapy in the treatment of penile intraepithelial neoplasia. JAAD Case Rep. 3, 546–549 (2017).
Paoli, J. et al. Penile intraepithelial neoplasia: results of photodynamic therapy. Acta Derm. Venereol. 86, 418–421 (2006).
Chipollini, J. et al. Surgical management of penile carcinoma in situ: results from an international collaborative study and review of the literature. BJU Int. 121, 393–398 (2018).
Sri, D. et al. A study into the association between local recurrence rates and surgical resection margins in organ-sparing surgery for penile squamous cell cancer. BJU Int. 122, 576–582 (2018).
Agrawal, A., Pai, D., Ananthakrishnan, N., Smile, S. R. & Ratnakar, C. The histological extent of the local spread of carcinoma of the penis and its therapeutic implications. BJU Int. 85, 299–301 (2000).
Minhas, S. et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 96, 1040–1043 (2005).
Philippou, P. et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J. Urol. 188, 803–808 (2012).
Djajadiningrat, R. S. et al. Penile sparing surgery for penile cancer — does it affect survival? J. Urol. 192, 120–125 (2014).
Roussel, E. et al. Predictors of local recurrence and its impact on survival after glansectomy for penile cancer: time to challenge the dogma? BJU Int. https://doi.org/10.1111/bju.15297 (2020).
Kamel, M. H. et al. Survival outcomes of organ sparing surgery, partial penectomy, and total penectomy in pathological T1/T2 penile cancer: Report from the National Cancer Data Base. Urol. Oncol. 36, 82.e87–82.e15 (2018).
Baumgarten, A. et al. Penile sparing surgery for penile cancer: a multicenter international retrospective cohort. J. Urol. 199, 1233–1237 (2018).
Albersen, M. et al. Predictive factors for local recurrence after glansectomy and neoglans reconstruction for penile squamous cell carcinoma. Urol. Oncol. 36, 141–146 (2018).
Alnajjar, H. M., Randhawa, K. & Muneer, A. Localized disease: types of reconstruction/plastic surgery techniques after glans resurfacing/glansectomy/partial/total penectomy. Curr. Opin. Urol. 30, 213–217 (2020).
Pérez, J. et al. Oncological and functional outcomes after organ-sparing plastic reconstructive surgery for penile cancer. Urology 142, 161–165.e1 (2020).
Burnett, A. L. Penile preserving and reconstructive surgery in the management of penile cancer. Nat. Rev. Urol. 13, 249–257 (2016).
Kitamura, Y. et al. Penile-preserving surgery for male distal urethral carcinoma followed by buccal mucosa urethroplasty. IJU Case Rep. 2, 198–201 (2019).
Ficarra, V. et al. General state of health and psychological well-being in patients after surgery for urological malignant neoplasms. Urol. Int. 65, 130–134 (2000).
Sarin, R., Norman, A. R., Steel, G. G. & Horwich, A. Treatment results and prognostic factors in 101 men treated for squamous carcinoma of the penis. Int. J. Radiat. Oncol. Biol. Phys. 38, 713–722 (1997).
Rozan, R. et al. Interstitial brachytherapy for penile carcinoma: a multicentric survey (259 patients). Radiother. Oncol. 36, 83–93 (1995).
Crook, J., Ma, C. & Grimard, L. Radiation therapy in the management of the primary penile tumor: an update. World J. Urol. 27, 189–196 (2009).
de Crevoisier, R. et al. Long-term results of brachytherapy for carcinoma of the penis confined to the glans (N- or NX). Int. J. Radiat. Oncol. Biol. Phys. 74, 1150–1156 (2009).
McLean, M. et al. The results of primary radiation therapy in the management of squamous cell carcinoma of the penis. Int. J. Radiat. Oncol. Biol. Phys. 25, 623–628 (1993).
Neave, F., Neal, A. J., Hoskin, P. J. & Hope-Stone, H. F. Carcinoma of the penis: a retrospective review of treatment with iridium mould and external beam irradiation. Clin. Oncol. 5, 207–210 (1993).
Gotsadze, D., Matveev, B., Zak, B. & Mamaladze, V. Is conservative organ-sparing treatment of penile carcinoma justified? Eur. Urol. 38, 306–312 (2000).
Zouhair, A. et al. Radiation therapy alone or combined surgery and radiation therapy in squamous-cell carcinoma of the penis? Eur. J. Cancer 37, 198–203 (2001).
Hasan, S. et al. The role of brachytherapy in organ preservation for penile cancer: a meta-analysis and review of the literature. Brachytherapy 14, 517–524 (2015).
Crook, J. Radiotherapy approaches for locally advanced penile cancer: neoadjuvant and adjuvant. Curr. Opin. Urol. 27, 62–67 (2017).
Korzeniowski, M. A. & Crook, J. M. Contemporary role of radiotherapy in the management of penile cancer. Transl. Androl. Urol. 6, 855–867 (2017).
Leone, A., Diorio, G. J., Pettaway, C., Master, V. & Spiess, P. E. Contemporary management of patients with penile cancer and lymph node metastasis. Nat. Rev. Urol. 14, 335–347 (2017).
Ficarra, V., Akduman, B., Bouchot, O., Palou, J. & Tobias-Machado, M. Prognostic factors in penile cancer. Urology 76, S66–S73 (2010).
Horenblas, S. & van Tinteren, H. Squamous cell carcinoma of the penis. IV. Prognostic factors of survival: analysis of tumor, nodes and metastasis classification system. J. Urol. 151, 1239–1243 (1994).
Djajadiningrat, R. S. et al. Contemporary management of regional nodes in penile cancer-improvement of survival? J. Urol. 191, 68–73 (2014).
Srinivas, V., Morse, M. J., Herr, H. W., Sogani, P. C. & Whitmore, W. F. Jr. Penile cancer: relation of extent of nodal metastasis to survival. J. Urol. 137, 880–882 (1987).
Kroon, B. K., Horenblas, S., Deurloo, E. E., Nieweg, O. E. & Teertstra, H. J. Ultrasonography-guided fine-needle aspiration cytology before sentinel node biopsy in patients with penile carcinoma. BJU Int. 95, 517–521 (2005).
Graafland, N. M., Teertstra, H. J., Besnard, A. P., van Boven, H. H. & Horenblas, S. Identification of high risk pathological node positive penile carcinoma: value of preoperative computerized tomography imaging. J. Urol. 185, 881–887 (2011).
Hughes, B. E. et al. Lymph node metastasis in intermediate-risk penile squamous cell cancer: a two-centre experience. Eur. Urol. 57, 688–692 (2010).
Graafland, N. M. et al. Prognostic factors for occult inguinal lymph node involvement in penile carcinoma and assessment of the high-risk EAU subgroup: a two-institution analysis of 342 clinically node-negative patients. Eur. Urol. 58, 742–747 (2010).
Hegarty, P. K. et al. A prospective study of 100 cases of penile cancer managed according to European Association of Urology guidelines. BJU Int. 98, 526–531 (2006).
Ercole, C. E., Pow-Sang, J. M. & Spiess, P. E. Update in the surgical principles and therapeutic outcomes of inguinal lymph node dissection for penile cancer. Urol. Oncol. 31, 505–516 (2013).
Kroon, B. K. et al. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J. Urol. 173, 816–819 (2005).
Ornellas, A. A. et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J. Urol. 180, 1354–1359 (2008).
Kirrander, P., Sherif, A., Friedrich, B., Lambe, M. & Håkansson, U. Swedish National Penile Cancer Register: incidence, tumour characteristics, management and survival. BJU Int. 117, 287–292 (2016).
Lam, W. et al. Dynamic sentinel lymph node biopsy in patients with invasive squamous cell carcinoma of the penis: a prospective study of the long-term outcome of 500 inguinal basins assessed at a single institution. Eur. Urol. 63, 657–663 (2013).
Brouwer, O. R. et al. Comparing the hybrid fluorescent-radioactive tracer indocyanine green-99mTc-nanocolloid with 99mTc-nanocolloid for sentinel node identification: a validation study using lymphoscintigraphy and SPECT/CT. J. Nucl. Med. 53, 1034–1040 (2012).
Dell’Oglio, P. et al. Hybrid indocyanine green–(99m)Tc–nanocolloid for single-photon emission computed tomography and combined radio- and fluorescence-guided sentinel node biopsy in penile cancer: results of 740 inguinal basins assessed at a single institution. Eur. Urol. 78, 865–872 (2020).
Kroon, B. K. et al. How to avoid false-negative dynamic sentinel node procedures in penile carcinoma. J. Urol. 171, 2191–2194 (2004).
Jakobsen, J. K. et al. DaPeCa-3: promising results of sentinel node biopsy combined with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in clinically lymph node-negative patients with penile cancer – a national study from Denmark. BJU Int. 118, 102–111 (2016).
Kroon, B. K., Lont, A. P., Valdés Olmos, R. A., Nieweg, O. E. & Horenblas, S. Morbidity of dynamic sentinel node biopsy in penile carcinoma. J. Urol. 173, 813–815 (2005).
Leijte, J. A. et al. Two-center evaluation of dynamic sentinel node biopsy for squamous cell carcinoma of the penis. J. Clin. Oncol. 27, 3325–3329 (2009).
Spiess, P. E. et al. Preoperative lymphoscintigraphy and dynamic sentinel node biopsy for staging penile cancer: results with pathological correlation. J. Urol. 177, 2157–2161 (2007).
Nabavizadeh, R. et al. Inguinal lymph node dissection in the era of minimally invasive surgical technology. Urol. Oncol. https://doi.org/10.1016/j.urolonc.2020.07.026 (2020).
Nabavizadeh, R. et al. Utility of minimally invasive technology for inguinal lymph node dissection in penile cancer. J. Clin. Med. 9, 2501 (2020).
Protzel, C. et al. Lymphadenectomy in the surgical management of penile cancer. Eur. Urol. 55, 1075–1088 (2009).
Catalona, W. J. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: technique and preliminary results. J. Urol. 140, 306–310 (1988).
Niyogi, D., Noronha, J., Pal, M., Bakshi, G. & Prakash, G. Management of clinically node-negative groin in patients with penile cancer. Indian J. Urol. 36, 8–15 (2020).
Tobias-Machado, M. et al. Single-site video endoscopic inguinal lymphadenectomy: initial report. J. Endourol. 25, 607–610 (2011).
Tobias-Machado, M. et al. Can video endoscopic inguinal lymphadenectomy achieve a lower morbidity than open lymph node dissection in penile cancer patients? J. Endourol. 22, 1687–1691 (2008).
Kumar, V. & Sethia, K. K. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU Int. 119, 530–534 (2017).
Russell, C. M. et al. Minimally invasive inguinal lymphadenectomy in the management of penile carcinoma. Urology 106, 113–118 (2017).
Tobias-Machado, M. et al. Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J. Urol. 177, 953–957 (2007).
Hu, J. et al. Comparison of clinical feasibility and oncological outcomes between video endoscopic and open inguinal lymphadenectomy for penile cancer: a systematic review and meta-analysis. Medicine 98, e15862 (2019).
Yao, K. et al. Fascia lata preservation during inguinal lymphadenectomy for penile cancer: rationale and outcome. Urology 82, 642–647 (2013).
Ottenhof, S. R. et al. Surgical and oncological outcomes in patients after vascularised flap reconstruction for locoregionally advanced penile cancer. Eur. Urol. Focus. 5, 867–874 (2019).
Alnajjar, H. M. et al. Long-term outcomes for penile cancer patients presenting with advanced N3 disease requiring a myocutaneous flap reconstruction or primary closure-a retrospective single centre study. Transl. Androl. Urol. 8 (Suppl. 1), S13–S21 (2019).
Yegiyants, S., Romero, L. M., Haigh, P. I. & DiFronzo, L. A. Completion axillary lymph node dissection not required for regional control in patients with breast cancer who have micrometastases in a sentinel node. Arch. Surg. 145, 564–569 (2010).
Fournier, K., Schiller, A., Perry, R. R. & Laronga, C. Micrometastasis in the sentinel lymph node of breast cancer does not mandate completion axillary dissection. Ann. Surg. 239, 859–863 (2004).
Lont, A. P. et al. Pelvic lymph node dissection for penile carcinoma: extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J. Urol. 177, 947–952 (2007).
Necchi, A. et al. Clinical outcomes of perioperative chemotherapy in patients with locally advanced penile squamous-cell carcinoma: results of a multicenter analysis. Clin. Genitourin. Cancer 15, 548–555.e543 (2017).
Nicolai, N. et al. A combination of cisplatin and 5-fluorouracil with a taxane in patients who underwent lymph node dissection for nodal metastases from squamous cell carcinoma of the penis: treatment outcome and survival analyses in neoadjuvant and adjuvant settings. Clin. Genitourin. Cancer 14, 323–330 (2016).
Nicholson, S. et al. Phase II trial of docetaxel, cisplatin and 5FU chemotherapy in locally advanced and metastatic penis cancer (CRUK/09/001). Br. J. Cancer 109, 2554–2559 (2013).
Djajadiningrat, R. S., Bergman, A. M., van Werkhoven, E., Vegt, E. & Horenblas, S. Neoadjuvant taxane-based combination chemotherapy in patients with advanced penile cancer. Clin. Genitourin. Cancer 13, 44–49 (2015).
Azizi, M. et al. Systematic review and meta-analysis-is there a benefit in using neoadjuvant systemic chemotherapy for locally advanced penile squamous cell carcinoma? J. Urol. 203, 1147–1155 (2020).
Necchi, A. et al. Nomogram-based prediction of overall survival after regional lymph node dissection and the role of perioperative chemotherapy in penile squamous cell carcinoma: a retrospective multicenter study. Urol. Oncol. 37, 531.e7–531.e15 (2019).
Homesley, H. D., Bundy, B. N., Sedlis, A. & Adcock, L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet. Gynecol. 68, 733–740 (1986).
Kunos, C., Simpkins, F., Gibbons, H., Tian, C. & Homesley, H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstet. Gynecol. 114, 537–546 (2009).
Tang, D. H. et al. Adjuvant pelvic radiation is associated with improved survival and decreased disease recurrence in pelvic node-positive penile cancer after lymph node dissection: a multi-institutional study. Urol. Oncol. 35, 605.e17–605.e23 (2017).
Kulkarni, J. N. & Kamat, M. R. Prophylactic bilateral groin node dissection versus prophylactic radiotherapy and surveillance in patients with N0 and N1-2A carcinoma of the penis. Eur. Urol. 26, 123–128 (1994).
Graafland, N. M. et al. Inguinal recurrence following therapeutic lymphadenectomy for node positive penile carcinoma: outcome and implications for management. J. Urol. 185, 888–893 (2011).
Franks, K. N. et al. Radiotherapy for node positive penile cancer: experience of the Leeds teaching hospitals. J. Urol. 186, 524–529 (2011).
Winters, B. R. et al. Is there a benefit to adjuvant radiation in stage III penile cancer after lymph node dissection? Findings from the National Cancer Database. Urol. Oncol. 36, 92.e11–92.e16 (2018).
Giannatempo, P. et al. Impact of human papillomavirus (HPV) infection on the outcome of perioperative treatments for penile squamous-cell carcinoma (PSCC). J. Clin. Oncol. 38, 5088–5088 (2020).
Canter, D. J., Nicholson, S., Watkin, N., Hall, E. & Pettaway, C. The International Penile advanced cancer trial (InPACT): rationale and current status. Eur. Urol. Focus. 5, 706–709 (2019).
Pickering, L. M. et al. VinCaP: a phase II trial of vinflunine chemotherapy in locally-advanced and metastatic carcinoma of the penis (CRUK/12/021). J. Clin. Oncol. 36, 4514–4514 (2018).
Theodore, C. et al. A phase II multicentre study of irinotecan (CPT 11) in combination with cisplatin (CDDP) in metastatic or locally advanced penile carcinoma (EORTC PROTOCOL 30992). Ann. Oncol. 19, 1304–1307 (2008).
Di Lorenzo, G. et al. Paclitaxel in pretreated metastatic penile cancer: final results of a phase 2 study. Eur. Urol. 60, 1280–1284 (2011).
Brown, J. C., Chu, C. S., Cheville, A. L. & Schmitz, K. H. The prevalence of lymphedema symptoms among survivors of long-term cancer with or at risk for lower limb lymphedema. Am. J. Phys. Med. Rehabil. 92, 223–231 (2013).
Dräger, D. L., Protzel, C. & Hakenberg, O. W. Identifying psychosocial distress and stressors using distress-screening instruments in patients with localized and advanced penile cancer. Clin. Genitourin. Cancer 15, 605–609 (2017).
Maddineni, S. B., Lau, M. M. & Sangar, V. K. Identifying the needs of penile cancer sufferers: a systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol. 9, 8 (2009).
Parnham, A. S. et al. Glansectomy and split-thickness skin graft for penile cancer. Eur. Urol. 73, 284–289 (2018).
Yang, J. et al. Glans preservation contributes to postoperative restoration of male sexual function: a multicenter clinical study of glans preserving surgery. J. Urol. 192, 1410–1417 (2014).
Yu, C. et al. Sexual function after partial penectomy: a prospectively study from China. Sci. Rep. 6, 21862 (2016).
Alnajjar, H. M. et al. A novel ‘Batman’ scrotectomy technique for the management of scrotal lymphoedema following treatment for penile cancer. Transl. Androl. Urol. 8, 448–456 (2019).
Kayes, O., Ahmed, H. U., Arya, M. & Minhas, S. Molecular and genetic pathways in penile cancer. Lancet Oncol. 8, 420–429 (2007).
Sand, F. L., Rasmussen, C. L., Frederiksen, M. H., Andersen, K. K. & Kjaer, S. K. Prognostic significance of HPV and p16 status in men diagnosed with penile cancer: a systematic review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 27, 1123–1132 (2018).
Jacob, J. M. et al. Comparative genomic profiling of refractory and metastatic penile and nonpenile cutaneous squamous cell carcinoma: implications for selection of systemic therapy. J. Urol. 201, 541–548 (2019).
Necchi, A. et al. Gene expression profiling of advanced penile squamous cell carcinoma receiving cisplatin-based chemotherapy improves prognostication and identifies potential therapeutic targets. Eur. Urol. Focus. 4, 733–736 (2018).
De Bacco, M. W. et al. PD-L1 and p16 expression in penile squamous cell carcinoma from an endemic region. Clin. Genitourin. Cancer 18, e254–e259 (2020).
Ahmed, M. E., Falasiri, S., Hajiran, A., Chahoud, J. & Spiess, P. E. The immune microenvironment in penile cancer and rationale for immunotherapy. J. Clin. Med. 9, 3334 (2020).
Davidsson, S. et al. PD-L1 expression in men with penile cancer and its association with clinical outcomes. Eur. Urol. Oncol. 2, 214–221 (2019).
Ottenhof, S. R. et al. Expression of programmed death ligand 1 in penile cancer is of prognostic value and associated with HPV status. J. Urol. 197, 690–697 (2017).
Cocks, M. et al. Immune-checkpoint status in penile squamous cell carcinoma: a North American cohort. Hum. Pathol. 59, 55–61 (2017).
Necchi, A. et al. Prognostic factors of adjuvant taxane, cisplatin, and 5-fluorouracil chemotherapy for patients with penile squamous cell carcinoma after regional lymphadenectomy. Clin. Genitourin. Cancer 14, 518–523 (2016).
Thomas, A., Vanthoor, J., Vos, G., Tsaur, I. & Albersen, M. Risk factors and molecular characterization of penile cancer: impact on prognosis and potential targets for systemic therapy. Curr. Opin. Urol. 30, 202–207 (2020).
McDaniel, A. S. et al. Genomic profiling of penile squamous cell carcinoma reveals new opportunities for targeted therapy. Cancer Res. 75, 5219–5227 (2015).
Huang, T. et al. Effective combinatorial immunotherapy for penile squamous cell carcinoma. Nat. Commun. 11, 2124 (2020).
Muñoz, J. J. et al. A comprehensive characterization of cell cultures and xenografts derived from a human verrucous penile carcinoma. Tumour Biol. 37, 11375–11384 (2016).
Chen, J. et al. Establishment and characterization of a penile cancer cell line, penl1, with a deleterious TP53 mutation as a paradigm of HPV-negative penile carcinogenesis. Oncotarget 7, 51687–51698 (2016).
Zhou, Q. H. et al. Molecular characterization and integrative genomic analysis of a panel of newly established penile cancer cell lines. Cell Death Dis. 9, 684 (2018).
Thomas, A. et al. Establishment, characterization, and imaging of a first platinum-resistant penile cancer patient-derived xenograft in nude mice: a eUROGEN project. Eur. Urol. 78, 294–296 (2020).
McGregor, B. A. et al. Phase II study of nivolumab and ipilimumab for advanced rare genitourinary cancers. J. Clin. Oncol. 38, 5018–5018 (2020).
Norberg, S. et al. Safety and clinical activity of gene-engineered T-cell therapy targeting HPV-16 E7 for epithelial cancers. J. Clin. Oncol. 38, 101–101 (2020).
Campbell, R. A. et al. Disparity between pre-existing management of penile cancer and NCCN guidelines. Urol. Oncol. 35, 531.e9–531.e14 (2017).
Bada, M. et al. Adherence to the EAU guidelines on penile cancer treatment: European, multicentre, retrospective study. J. Cancer Res. Clin. Oncol. 145, 921–926 (2019).
Tang, V. et al. Should centralized histopathological review in penile cancer be the global standard? BJU Int. 114, 340–343 (2014).
Bayles, A. C. & Sethia, K. K. The impact of improving outcomes guidance on the management and outcomes of patients with carcinoma of the penis. Ann. R. Coll. Surg. Engl. 92, 44–45 (2010).
Breen, K. J. et al. Penile cancer — guideline adherence produces optimum results. Surgeon 13, 200–206 (2015).
Joshi, S. S. et al. Treatment trends and outcomes for patients with lymph node-positive cancer of the penis. JAMA Oncol. 4, 643–649 (2018).
Williams, S. B. et al. Impact of centralizing care for genitourinary malignancies to high-volume providers: a systematic review. Eur. Urol. Oncol. 2, 265–273 (2019).
Kamel, M. H. Should the care of penile cancer be confined to centralized centers of excellence? Eur. Urol. Focus. 5, 735–736 (2019).
Hakenberg, O. W. et al. The diagnosis and treatment of penile cancer. Dtsch. Arztebl. Int. 115, 646–652 (2018).
Chaux, A. & Cubilla, A. L. Advances in the pathology of penile carcinomas. Hum. Pathol. 43, 771–789 (2012).
Cubilla, A. L. et al. Basaloid squamous cell carcinoma of the penis with papillary features: a clinicopathologic study of 12 cases. Am. J. Surg. Pathol. 36, 869–875 (2012).
Cubilla, A. L. et al. The World Health Organisation 2016 classification of penile carcinomas: a review and update from the International Society of Urological Pathology expert-driven recommendations. Histopathology 72, 893–904 (2018).
Boulet, G., Horvath, C., Vanden Broeck, D., Sahebali, S. & Bogers, J. Human papillomavirus: E6 and E7 oncogenes. Int. J. Biochem. Cell Biol. 39, 2006–2011 (2007).
Summerton, D. J., Campbell, A., Minhas, S. & Ralph, D. J. Reconstructive surgery in penile trauma and cancer. Nat. Clin. Pract. Urol. 2, 391–397 (2005).
Hadway, P., Sahdev, V., Arya, M. & Muneer, A. Recent developments and current management of penile cancer. Clin. Pract. 11, 169–181 (2014).
Kirkham, A. P. S. in Textbook of Penile Cancer (eds Muneer, A. & Horenblas, S.) 89-114 (Springer, 2016).
Crook, J. Organ preserving radiation strategies for penile cancer. Urol. Oncol. https://doi.org/10.1016/j.urolonc.2020.06.025 (2020).
Dodge, O. G., Linsell, C. A. & Davies, J. N. Circumcision and the incidence of carcinoma of the penis and the cervix. a study in Kenya and Uganda Africans. East Afr. Med. J. 40, 440–444 (1963).
Wolbarst, A. Circumcision and penile cancer. Lancet 219, 150–153 (1932).
Schrek, R. & Lenowitz, H. Etiologic factors in carcinoma of the penis. Cancer Res. 7, 180–187 (1947).
Reuter, S., Gupta, S. C., Chaturvedi, M. M. & Aggarwal, B. B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49, 1603–1616 (2010).
Elinav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771 (2013).
Dave, S., Afshar, K., Braga, L. H. & Anderson, P. Canadian Urological Association guideline on the care of the normal foreskin and neonatal circumcision in Canadian infants (full version). Can. Urol. Assoc. J. 12, E76–E99 (2018).
Castellsagué, X. et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N. Engl. J. Med. 346, 1105–1112 (2002).
Doyle, S. M., Kahn, J. G., Hosang, N. & Carroll, P. R. The impact of male circumcision on HIV transmission. J. Urol. 183, 21–26 (2010).
Schenker, I. Cutting-edge success in preventing heterosexual HIV transmission in Africa: voluntary medical male circumcision has reached 15 million men. AIDS Educ. Prev. 30, 232–242 (2018).
Amin, M. B. et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67, 93–99 (2017).
Acknowledgements
The authors thank a patient who kindly contributed with his disease experience to this article. M.A. holds a mandate of the Foundation Against Cancer.
Author information
Authors and Affiliations
Contributions
Introduction (M.A., A.Th. and A.-S.V.R.); Epidemiology (M.A. and A.Th.); Mechanisms/Pathophysiology (M.A., A.Th. and P.E.S.); Diagnosis, screening and prevention (M.A., A.Th., A.M. and A.-S.V.R.); Management (M.A., A.Th., A.N., A.M., M.T.-M. and A.Tr.); Quality of life (M.A., A.Th. and A.M.); Outlook (M.A., A.Th., A.N. and A.M.).
Corresponding author
Ethics declarations
Competing interests
M.A.: eUROGEN clinical lead on rare urogenital cancer workstream (WS3); Nature Reviews Urology advisory board member; European Urology Associate Editor; EAU Scientific Office member; EAU–ASCO penile cancer guideline panel member. A.Th.: EUSP scholar. A.M.: eUROGEN research lead on rare urogenital cancer workstream (WS3); ESMO penile cancer guideline panel member; editorial board BJU International. A.N.: EAU–ASCO penile cancer guideline panel member, penile cancer research funding to institute by Ipsen and Pfizer. P.E.S.: NCCN penile cancer guideline discussion writing committee member; EAU–ASCO penile cancer guideline panel member; Nature Reviews Urology advisory board member. M.T.-M.: coordinator of Penile Cancer Collaborative Coalition – Latin America. All other authors declare no competing interests.
Additional information
Informed consent
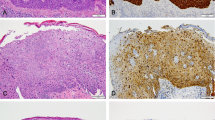
The authors affirm that human research participants provided informed consent for publication of the images in Figure 3 and Figure 6.
Peer review information
Nature Reviews Disease Primers thanks O. Brouwer, J. Crook, O. Hakenberg, G. Netto, F. Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thomas, A., Necchi, A., Muneer, A. et al. Penile cancer. Nat Rev Dis Primers 7, 11 (2021). https://doi.org/10.1038/s41572-021-00246-5
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-021-00246-5
This article is cited by
-
Long-term outcomes of penile squamous cell carcinoma in men age ≤50 years old compared with men >50 years old from a single tertiary referral centre: a propensity score matched analysis
International Journal of Impotence Research (2024)
-
Accuracy of dynamic sentinel lymph node biopsy for inguinal lymph node staging in cN0 penile cancer
EJNMMI Research (2023)
-
Analysis of quality information provided by “Dr. YouTubeTM” on Phimosis
International Journal of Impotence Research (2023)
-
Selinexor demonstrates anti-tumor efficacy in paired patient-derived xenograft models and hydrogel-embedded histoculture drug sensitivity test of penile cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Exploring the role of natural bioactive molecules in genitourinary cancers: how far has research progressed?
Natural Products and Bioprospecting (2023)