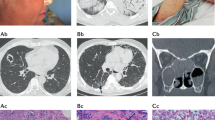
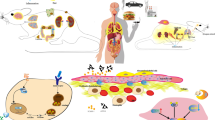
Abstract
The anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAVs) are a group of disorders involving severe, systemic, small-vessel vasculitis and are characterized by the development of autoantibodies to the neutrophil proteins leukocyte proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA). The three AAV subgroups, namely granulomatosis with polyangiitis (GPA), microscopic polyangiitis and eosinophilic GPA (EGPA), are defined according to clinical features. However, genetic and other clinical findings suggest that these clinical syndromes may be better classified as PR3-positive AAV (PR3-AAV), MPO-positive AAV (MPO-AAV) and, for EGPA, by the presence or absence of ANCA (ANCA+ or ANCA–, respectively). Although any tissue can be involved in AAV, the upper and lower respiratory tract and kidneys are most commonly and severely affected. AAVs have a complex and unique pathogenesis, with evidence for a loss of tolerance to neutrophil proteins, which leads to ANCA-mediated neutrophil activation, recruitment and injury, with effector T cells also involved. Without therapy, prognosis is poor but treatments, typically immunosuppressants, have improved survival, albeit with considerable morbidity from glucocorticoids and other immunosuppressive medications. Current challenges include improving the measures of disease activity and risk of relapse, uncertainty about optimal therapy duration and a need for targeted therapies with fewer adverse effects. Meeting these challenges requires a more detailed knowledge of the fundamental biology of AAV as well as cooperative international research and clinical trials with meaningful input from patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Watts, R. A. et al. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol. Dial. Transplant. 30 (Suppl. 1), i14–i22 (2015). A granular and comprehensive review of contemporary AAV epidemiology.
Berti, A., Cornec, D., Crowson, C. S., Specks, U. & Matteson, E. L. The epidemiology of antineutrophil cytoplasmic autoantibody-associated vasculitis in Olmsted County, Minnesota: a twenty-year US population-based study. Arthritis Rheumatol. 69, 2338–2350 (2017).
Mohammad, A. J., Jacobsson, L. T., Mahr, A. D., Sturfelt, G. & Segelmark, M. Prevalence of Wegener’s granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg-Strauss syndrome within a defined population in southern Sweden. Rheumatology 46, 1329–1337 (2007).
Tan, J. A. et al. Mortality in ANCA-associated vasculitis: a meta-analysis of observational studies. Ann. Rheum. Dis. 76, 1566–1574 (2017).
Basu, N. et al. The characterisation and determinants of quality of life in ANCA associated vasculitis. Ann. Rheum. Dis. 73, 207–211 (2014).
Raimundo, K., Farr, A. M., Kim, G. & Duna, G. Clinical and economic burden of antineutrophil cytoplasmic antibody-associated vasculitis in the United States. J. Rheumatol. 42, 2383–2391 (2015).
Knight, A., Ekbom, A., Brandt, L. & Askling, J. Increasing incidence of Wegener’s granulomatosis in Sweden, 1975–2001. J. Rheumatol. 33, 2060–2063 (2006).
Liu, L. J., Chen, M., Yu, F., Zhao, M. H. & Wang, H. Y. Evaluation of a new algorithm in classification of systemic vasculitis. Rheumatology 47, 708–712 (2008).
Watts, R. A. et al. Renal vasculitis in Japan and the UK — are there differences in epidemiology and clinical phenotype? Nephrol. Dial. Transpl. 23, 3928–3931 (2008).
Watts, R. A. et al. Geoepidemiology of systemic vasculitis: comparison of the incidence in two regions of Europe. Ann. Rheum. Dis. 60, 170–172 (2001).
O’Donnell, J. L., Stevanovic, V. R., Frampton, C., Stamp, L. K. & Chapman, P. T. Wegener’s granulomatosis in New Zealand: evidence for a latitude-dependent incidence gradient. Intern. Med. J. 37, 242–246 (2007).
Cao, Y. et al. DRB1*15 allele is a risk factor for PR3-ANCA disease in African Americans. J. Am. Soc. Nephrol. 22, 1161–1167 (2011).
Mahr, A., Guillevin, L., Poissonnet, M. & Ayme, S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener’s granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheumatol. 51, 92–99 (2004).
Pearce, F. A. et al. Incidence of ANCA-associated vasculitis in a UK mixed ethnicity population. Rheumatology 55, 1656–1663 (2016).
Iudici, M. et al. Childhood-onset granulomatosis with polyangiitis and microscopic polyangiitis: systematic review and meta-analysis. Orphanet J. Rare Dis. 11, 141 (2016).
Falk, R. J., Hogan, S., Carey, T. S. & Jennette, J. C. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The Glomerular Disease Collaborative Network. Ann. Intern. Med. 113, 656–663 (1990).
Tidman, M., Olander, R., Svalander, C. & Danielsson, D. Patients hospitalized because of small vessel vasculitides with renal involvement in the period 1975-95: organ involvement, anti-neutrophil cytoplasmic antibodies patterns, seasonal attack rates and fluctuation of annual frequencies. J. Intern. Med. 244, 133–141 (1998).
Watts, R. A., Mooney, J., Skinner, J., Scott, D. G. & Macgregor, A. J. The contrasting epidemiology of granulomatosis with polyangiitis (Wegener’s) and microscopic polyangiitis. Rheumatology 51, 926–931 (2012).
Draibe, J. et al. Seasonal variations in the onset of positive and negative renal ANCA-associated vasculitis in Spain. Clin. Kidney J. 11, 468–473 (2018).
Mahr, A. et al. Seasonal variations in onset of Wegener’s granulomatosis: increased in summer? J. Rheumatol. 33, 1615–1622 (2006).
Aries, P. M., Herlyn, K., Reinhold-Keller, E. & Latza, U. No seasonal variation in the onset of symptoms of 445 patients with ‘Wegener’s granulomatosis. Arthritis Rheumatol. 59, 904–904 (2008).
Stegeman, C. A. et al. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann. Intern. Med. 120, 12–17 (1994). A study that provides evidence for a putative causal role for infection in the relapse of GPA.
Laudien, M. et al. Nasal carriage of Staphylococcus aureus and endonasal activity in Wegener’s granulomatosis as compared to rheumatoid arthritis and chronic rhinosinusitis with nasal polyps. Clin. Exp. Rheumatol. 28 (Suppl. 57), 51–55 (2010).
Lane, S. E., Watts, R. A., Bentham, G., Innes, N. J. & Scott, D. G. Are environmental factors important in primary systemic vasculitis? A case-control study. Arthritis Rheumatol. 48, 814–823 (2003).
Nuyts, G. D. et al. Wegener granulomatosis is associated to exposure to silicon-compounds-a case-control study. Nephrol. Dial. Transpl. 10, 1162–1165 (1995).
Yashiro, M. et al. Significantly high regional morbidity of MPO-ANCA-related angitis and/or nephritis with respiratory tract involvement after the 1995 Great Earthquake in Kobe (Japan). Am. J. Kidney Dis. 35, 889–895 (2000).
Takeuchi, Y. et al. The influence of the Great East Japan earthquake on microscopic polyangiitis: a retrospective observational study. PLoS ONE 12, e0177482 (2017).
Farquhar, H. J. et al. Incidence of anti-neutrophil cytoplasmic antibody-associated vasculitis before and after the February 2011 Christchurch Earthquake. Intern. Med. J. 47, 57–61 (2017).
Cotch, M. F. et al. The epidemiology of Wegener’s granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheumatol. 39, 87–92 (1996).
Li, J. et al. The frequency of ANCA-associated vasculitis in a national database of hospitalized patients in China. Arthritis Res. Ther. 20, 226 (2018).
Gatenby, P. A., Lucas, R. M., Engelsen, O., Ponsonby, A. L. & Clements, M. Antineutrophil cytoplasmic antibody-associated vasculitides: could geographic patterns be explained by ambient ultraviolet radiation? Arthritis Rheumatol. 61, 1417–1424 (2009).
McDermott, G. et al. Association of cigarette smoking with antineutrophil cytoplasmic antibody-associated vasculitis. JAMA Intern. Med. 180, 1–7 (2020).
Hutton, H. L., Holdsworth, S. R. & Kitching, A. R. ANCA-associated vasculitis: pathogenesis, models, and preclinical testing. Sem. Nephrol. 37, 418–435 (2017).
Knight, A., Sandin, S. & Askling, J. Risks and relative risks of Wegener’s granulomatosis among close relatives of patients with the disease. Arthritis Rheumatol. 58, 302–307 (2008).
Jagiello, P. et al. New genomic region for Wegener’s granulomatosis as revealed by an extended association screen with 202 apoptosis-related genes. Hum. Genet. 114, 468–477 (2004).
Lyons, P. A. et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 367, 214–223 (2012).
Xie, G. et al. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheumatol. 65, 2457–2468 (2013).
Merkel, P. A. et al. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. 69, 1054–1066 (2017). Lyons et al. (2012), Xie et al. (2013) and Merkel et al. (2017) provide clear evidence for a genetic contribution to differences between PR3-AAV and MPO-AAV and link PR3-AAV to variation in the autoantigen itself.
Siminovitch, K. A. PTPN22 and autoimmune disease. Nat. Genet. 36, 1248–1249 (2004).
Sun, B. B. et al. Genomic atlas of the human plasma proteome. Nature 558, 73–79 (2018).
Niehrs, A. et al. A subset of HLA-DP molecules serve as ligands for the natural cytotoxicity receptor NKp44. Nat. Immunol. 20, 1129–1137 (2019).
Lyons, P. A. et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat. Commun. 10, 5120 (2019).
Sablé-Fourtassou, R. et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann. Intern. Med. 143, 632–638 (2005).
Sinico, R. A. et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheumatol. 52, 2926–2935 (2005).
Nakazawa, D. et al. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J. Am. Soc. Nephrol. 25, 990–997 (2014).
Ooi, J. D. et al. A plasmid-encoded peptide from Staphylococcus aureus induces anti-myeloperoxidase nephritogenic autoimmunity. Nat. Comm. 10, 3392 (2019).
Jones, B. E. et al. Gene-specific DNA methylation changes predict remission in patients with ANCA-associated vasculitis. J. Am. Soc. Nephrol. 28, 1175–1187 (2017).
Kessenbrock, K. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15, 623–625 (2009).
Martin, K. R. & Witko-Sarsat, V. Proteinase 3: the odd one out that became an autoantigen. J. Leuk. Biol. 102, 689–698 (2017).
Witko-Sarsat, V. et al. A large subset of neutrophils expressing membrane proteinase 3 is a risk factor for vasculitis and rheumatoid arthritis. J. Am. Soc. Nephrol. 10, 1224–1233 (1999).
Jerke, U. et al. Complement receptor Mac-1 is an adaptor for NB1 (CD177)-mediated PR3-ANCA neutrophil activation. J. Biol. Chem. 286, 7070–7081 (2011).
Odobasic, D., Kitching, A. R. & Holdsworth, S. R. Neutrophil-mediated regulation of innate and adaptive immunity: the role of myeloperoxidase. J. Immunol. Res. 2016, 2349817 (2016).
Reiding, K. R. et al. Neutrophil myeloperoxidase harbors distinct site-specific peculiarities in its glycosylation. J. Biol. Chem. 294, 20233–20245 (2019).
Kain, R. et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat. Med. 14, 1088–1096 (2008).
Pendergraft, W. F. et al. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat. Med. 10, 72–79 (2004).
Yang, J. et al. ANCA patients have T cells responsive to complementary PR-3 antigen. Kidney Int. 74, 1159–1169 (2008).
Suzuki, K. et al. A novel autoantibody against moesin in the serum of patients with MPO-ANCA-associated vasculitis. Nephrol. Dial. Transpl. 29, 1168–1177 (2014).
Bautz, D. J. et al. Antibodies with dual reactivity to plasminogen and complementary PR3 in PR3-ANCA vasculitis. J. Am. Soc. Nephrol. 19, 2421–2429 (2008).
Berden, A. E. et al. Anti-plasminogen antibodies compromise fibrinolysis and associate with renal histology in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 21, 2169–2179 (2010).
McCall, A. S. et al. Inhibitory anti-peroxidasin antibodies in pulmonary-renal syndromes. J. Am. Soc. Nephrol. 29, 2619–2625 (2018).
Simon, A. et al. Detection of anti-pentraxin-3 autoantibodies in ANCA-associated vasculitis. PLoS ONE 11, e0147091 (2016).
Roth, A. J. et al. Anti-LAMP-2 antibodies are not prevalent in patients with antineutrophil cytoplasmic autoantibody glomerulonephritis. J. Am. Soc. Nephrol. 23, 545–555 (2012).
Tadema, H., Kallenberg, C. G., Stegeman, C. A. & Heeringa, P. Reactivity against complementary proteinase-3 is not increased in patients with PR3-ANCA-associated vasculitis. PLoS ONE 6, e17972 (2011).
Olson, S. W. et al. Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J. Am. Soc. Nephrol. 22, 1946–1952 (2011).
Cui, Z., Zhao, M. H., Segelmark, M. & Hellmark, T. Natural autoantibodies to myeloperoxidase, proteinase 3, and the glomerular basement membrane are present in normal individuals. Kidney Int. 78, 590–597 (2010).
Tan, D. S. et al. Thymic deletion and regulatory T cells prevent antimyeloperoxidase GN. J. Am. Soc. Nephrol. 24, 573–585 (2013).
Abdulahad, W. H. et al. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener’s granulomatosis in remission. Arthritis Rheumatol. 56, 2080–2091 (2007).
Free, M. E. et al. Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheumatol. 65, 1922–1933 (2013).
Bunch, D. O. et al. Decreased CD5+ B cells in active ANCA vasculitis and relapse after rituximab. Clin. J. Am. Soc. Nephrol. 8, 382–391 (2013).
Wilde, B. et al. Regulatory B cells in ANCA-associated vasculitis. Ann. Rheum. Dis. 72, 1416–1419 (2013).
Free, M. E. et al. Restricted myeloperoxidase epitopes drive the adaptive immune response in MPO-ANCA vasculitis. J. Autoimm. 106, 102306 (2020).
Ooi, J. D. et al. The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc. Natl Acad. Sci. USA 109, E2615–E2624 (2012). This study uses experimental models to demonstrate the role of MPO-specific CD4 + T cells in effector responses and defines a nephritogenic MPO T cell epitope.
Roth, A. J. et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J. Clin. Invest. 123, 1773–1783 (2013).
Chang, J. et al. CD8+ T cells effect glomerular injury in experimental anti-myeloperoxidase GN. J. Am. Soc. Nephrol. 28, 47–55 (2017).
Falk, R. J., Becker, M., Terrell, R. & Jennette, J. C. Anti-myeloperoxidase autoantibodies react with native but not denatured myeloperoxidase. Clin. Exp. Immunol. 89, 274–278 (1992).
Bini, P. et al. Antineutrophil cytoplasmic autoantibodies in Wegener’s granulomatosis recognize conformational epitope(s) on proteinase 3. J. Immunol. 149, 1409–1415 (1992).
Audrain, M. A. et al. Anti-native and recombinant myeloperoxidase monoclonals and human autoantibodies. Clin. Exp. Immunol. 107, 127–134 (1997).
Nagai, M. et al. Serum levels of BAFF and APRIL in myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis: association with disease activity. Nephron Clin. Pract. 118, c339–c345 (2011).
Holden, N. J. et al. ANCA-stimulated neutrophils release BLyS and promote B cell survival: a clinically relevant cellular process. Ann. Rheum. Dis. 70, 2229–2233 (2011).
Oleinika, K., Mauri, C. & Salama, A. D. Effector and regulatory B cells in immune-mediated kidney disease. Nat. Rev. Nephrol. 15, 11–26 (2019).
Steinmetz, O. M. et al. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 74, 448–457 (2008).
Kelley, J. M. et al. IgA and IgG antineutrophil cytoplasmic antibody engagement of Fc receptor genetic variants influences granulomatosis with polyangiitis. Proc. Natl Acad. Sci. USA 108, 20736–20741 (2011).
Jayne, D. R. et al. Severe pulmonary hemorrhage and systemic vasculitis in association with circulating anti-neutrophil cytoplasm antibodies of IgM class only. Clin. Nephrol. 32, 101–106 (1989).
Falk, R. J., Terrell, R. S., Charles, L. A. & Jennette, J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl Acad. Sci. USA 87, 4115–4119 (1990). This study links ANCAs to the pathogenesis of AAV by demonstrating that ANCAs activate neutrophils in vitro.
Williams, J. M. et al. Activation of the G(i) heterotrimeric G protein by ANCA IgG F(ab’)2 fragments is necessary but not sufficient to stimulate the recruitment of those downstream mediators used by intact ANCA IgG. J. Am. Soc. Nephrol. 14, 661–669 (2003).
Hewins, P., Williams, J. M., Wakelam, M. J. & Savage, C. O. Activation of Syk in neutrophils by antineutrophil cytoplasm antibodies occurs via Fcγ receptors and CD18. J. Am. Soc. Nephrol. 15, 796–808 (2004).
Johnson, P. A., Alexander, H. D., McMillan, S. A. & Maxwell, A. P. Up-regulation of the granulocyte adhesion molecule Mac-1 by autoantibodies in autoimmune vasculitis. Clin. Exp. Immunol. 107, 513–519 (1997).
Kuligowski, M. P. et al. Antimyeloperoxidase antibodies rapidly induce alpha-4-integrin-dependent glomerular neutrophil adhesion. Blood 113, 6485–6494 (2009).
Tse, W. Y., Nash, G. B., Hewins, P., Savage, C. O. & Adu, D. ANCA-induced neutrophil F-actin polymerization: implications for microvascular inflammation. Kidney Int. 67, 130–139 (2005).
Jennette, J. C. & Nachman, P. H. ANCA glomerulonephritis and vasculitis. Clin. J. Am. Soc. Nephrol. 12, 1680–1691 (2017).
Hong, Y. et al. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J. Am. Soc. Nephrol. 23, 49–62 (2012).
Xiao, H. et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Invest. 110, 955–963 (2002). A key study that uses experimental models to demonstrate the pathogenicity of anti-MPO antibodies in vivo.
Bansal, P. J. & Tobin, M. C. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann. Allergy Asthma Immunol. 93, 398–401 (2004).
Xiao, H. et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am. J. Pathol. 167, 39–45 (2005).
Pfister, H. et al. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood 104, 1411–1418 (2004).
Little, M. A. et al. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS ONE 7, e28626 (2012).
Charles, L. A., Falk, R. J. & Jennette, J. C. Reactivity of antineutrophil cytoplasmic autoantibodies with mononuclear phagocytes. J. Leuk. Biol. 51, 65–68 (1992).
O’Brien, E. C. et al. Intermediate monocytes in ANCA vasculitis: increased surface expression of ANCA autoantigens and IL-1β secretion in response to anti-MPO antibodies. Sci. Rep. 5, 11888 (2015).
Peschel, A. et al. Autoantibodies to hLAMP-2 in ANCA-negative pauci-immune focal necrotizing GN. J. Am. Soc. Nephrol. 25, 455–463 (2014).
Espy, C. et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s). Arthritis Rheumatol. 63, 2105–2115 (2011).
Lardinois, O. M. et al. Immunoglobulins G from patients with ANCA-associated vasculitis are atypically glycosylated in both the Fc and Fab regions and the relation to disease activity. PLoS ONE 14, e0213215 (2019).
Ciavatta, D. J. et al. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J. Clin. Invest. 120, 3209–3219 (2010).
Ohlsson, S. M. et al. Neutrophils from vasculitis patients exhibit an increased propensity for activation by anti-neutrophil cytoplasmic antibodies. Clin. Exp. Immunol. 176, 363–372 (2014).
Ohlsson, S. et al. Neutrophils from ANCA-associated vasculitis patients show an increased capacity to activate the complement system via the alternative pathway after ANCA stimulation. PLoS ONE 14, e0218272 (2019).
Summers, S. A. et al. Intrinsic renal cell and leukocyte-derived TLR4 aggravate experimental anti-MPO glomerulonephritis. Kidney Int. 78, 1263–1274 (2010).
Tadema, H. et al. Bacterial DNA motifs trigger ANCA production in ANCA-associated vasculitis in remission. Rheumatology 50, 689–696 (2011).
Holle, J. U. et al. Toll-like receptor TLR2 and TLR9 ligation triggers neutrophil activation in granulomatosis with polyangiitis. Rheumatology 52, 1183–1189 (2013).
Wang, C. et al. High mobility group box 1 contributes to anti-neutrophil cytoplasmic antibody-induced neutrophils activation through receptor for advanced glycation end products (RAGE) and Toll-like receptor 4. Arthritis Res. Ther. 17, 64 (2015).
Xiao, H. et al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J. Am. Soc. Nephrol. 25, 225–231 (2014).
Jayne, D. R. W. et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 28, 2756–2767 (2017).
Merkel, P. A. et al. A randomised, double-blind, active-controlled study of Avacopan in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Ann. Rheum. Dis. 79, 8 (2020).
Xiao, H., Schreiber, A., Heeringa, P., Falk, R. J. & Jennette, J. C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 170, 52–64 (2007).
Huugen, D. et al. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 71, 646–654 (2007).
Hao, J., Meng, L. Q., Xu, P. C., Chen, M. & Zhao, M. H. p38MAPK, ERK and PI3K signaling pathways are involved in C5a-primed neutrophils for ANCA-mediated activation. PLoS ONE 7, e38317 (2012).
Dick, J. et al. C5a receptor 1 promotes autoimmunity, neutrophil dysfunction and injury in experimental anti-myeloperoxidase glomerulonephritis. Kidney Int. 93, 615–625 (2018).
Freeley, S. J. et al. Experimentally-induced anti-myeloperoxidase vasculitis does not require properdin, MASP-2 or bone marrow-derived C5. J. Pathol. 240, 61–71 (2016).
Gou, S. J., Yuan, J., Wang, C., Zhao, M. H. & Chen, M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin. J. Am. Soc. Nephrol. 8, 1884–1891 (2013).
Chen, M., Jayne, D. R. W. & Zhao, M. H. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat. Rev. Nephrol. 13, 359–367 (2017).
Manenti, L. et al. Association of serum C3 concentration and histologic signs of thrombotic microangiopathy with outcomes among patients with ANCA-associated renal vasculitis. Clin. J. Am. Soc. Nephrol. 10, 2143–2151 (2015).
Augusto, J. F. et al. Low serum complement C3 levels at diagnosis of renal ANCA-associated vasculitis is associated with poor prognosis. PLoS ONE 11, e0158871 (2016).
Calderwood, J. W., Williams, J. M., Morgan, M. D., Nash, G. B. & Savage, C. O. ANCA induces β2 integrin and CXC chemokine-dependent neutrophil-endothelial cell interactions that mimic those of highly cytokine-activated endothelium. J. Leuk. Biol. 77, 33–43 (2005).
Little, M. A. et al. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood 106, 2050–2058 (2005).
Nolan, S. L. et al. Mechanisms of ANCA-mediated leukocyte-endothelial cell interactions in vivo. J. Am. Soc. Nephrol. 19, 973–984 (2008).
Brouwer, E. et al. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener’s granulomatosis and clinically related disorders. Clin. Exp. Immunol. 83, 379–386 (1991).
Abdulahad, W. H. et al. Increased frequency of circulating IL-21 producing Th-cells in patients with granulomatosis with polyangiitis (GPA). Arthritis Res. Ther. 15, R70 (2013).
Abdulahad, W. H., Kallenberg, C. G., Limburg, P. C. & Stegeman, C. A. Urinary CD4+effector memory T cells reflect renal disease activity in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 60, 2830–2838 (2009).
Gephardt, G. N., Ahmad, M. & Tubbs, R. R. Pulmonary vasculitis (Wegener’s granulomatosis). Immunohistochemical study of T and B cell markers. Am. J. Med. 74, 700–704 (1983).
Weidner, S., Carl, M., Riess, R. & Rupprecht, H. D. Histologic analysis of renal leukocyte infiltration in antineutrophil cytoplasmic antibody-associated vasculitis: importance of monocyte and neutrophil infiltration in tissue damage. Arthritis Rheumatol. 50, 3651–3657 (2004).
O’Sullivan, K. M. et al. Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney Int. 88, 1030–1046 (2015).
Ludviksson, B. R. et al. Active Wegener’s granulomatosis is associated with HLA-DR+CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: reversal with IL-10. J. Immunol. 160, 3602–3609 (1998).
Nogueira, E. et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol. Dial. Transplant. 25, 2209–2217 (2010).
Csernok, E. et al. Cytokine profiles in Wegener’s granulomatosis: predominance of type 1 (Th1) in the granulomatous inflammation. Arthritis Rheumatol. 42, 742–750 (1999).
Chanouzas, D. et al. The host cellular immune response to cytomegalovirus targets the endothelium and is associated with increased arterial stiffness in ANCA-associated vasculitis. Arthritis Res. Ther. 20, 194 (2018).
Chanouzas, D. et al. Subclinical reactivation of cytomegalovirus drives CD4+CD28null T-cell expansion and impaired immune response to pneumococcal vaccination in antineutrophil cytoplasmic antibody-associated vasculitis. J. Infect. Dis. 219, 234–244 (2019).
McKinney, E. F. et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat. Med. 16, 586–591 (2010). This study identifies CD8 + T cell transcription signatures that correlate with risk of remaining in remission or to flare in AAV, leading to prospective biomarker studies.
McKinney, E. F., Lee, J. C., Jayne, D. R., Lyons, P. A. & Smith, K. G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616 (2015).
Bajema, I. M., Hagen, E. C., de Heer, E., van der Woude, F. J. & Bruijn, J. A. Colocalization of ANCA-antigens and fibrinoid necrosis in ANCA-associated vasculitis. Kidney Int. 60, 2025–2030 (2001).
Gan, P. Y. et al. Biologicals targeting T helper cell subset differentiating cytokines are effective in the treatment of murine anti-myeloperoxidase glomerulonephritis. Kidney Int. 96, 1121–1133 (2019).
Rousselle, A., Kettritz, R. & Schreiber, A. Monocytes promote crescent formation in anti-myeloperoxidase antibody-induced glomerulonephritis. Am. J. Pathol. 187, 1908–1915 (2017).
Terrier, B. et al. Interleukin-25: a cytokine linking eosinophils and adaptive immunity in Churg-Strauss syndrome. Blood 116, 4523–4531 (2010).
Kiene, M. et al. Elevated interleukin-4 and interleukin-13 production by T cell lines from patients with Churg-Strauss syndrome. Arthritis Rheumatol. 44, 469–473 (2001).
Jakiela, B. et al. Increased production of IL-5 and dominant Th2-type response in airways of Churg-Strauss syndrome patients. Rheumatology 51, 1887–1893 (2012).
Wechsler, M. E. et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 376, 1921–1932 (2017). A clinical trial that demonstrates that the anti-IL-5 therapy mepolizumab is an effective treatment for EGPA.
Biasci, D. et al. A blood-based prognostic biomarker in IBD. Gut 68, 1386–1395 (2019).
Jennette, J. C. et al. 2012 Revised International Chapel Hill consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 65, 1–11 (2013). A key classification paper that provides definitive definitions for each type of vasculitis.
Watts, R. A. & Robson, J. Introduction, epidemiology and classification of vasculitis. Best Pract. Res. Clin. Rheumatol. 32, 3–20 (2018).
Luqmani, R. A., Suppiah, R., Grayson, P. C., Merkel, P. A. & Watts, R. Nomenclature and classification of vasculitis - update on the ACR/EULAR Diagnosis and Classification of Vasculitis Study (DCVAS). Clin. Exp. Immunol. 164, 11–13 (2011).
Kariv, R., Sidi, Y. & Gur, H. Systemic vasculitis presenting as a tumorlike lesion. Four case reports and an analysis of 79 reported cases. Medicine 79, 349–359 (2000).
Jennette, J. C. & Falk, R. J. Small-vessel vasculitis. N. Engl. J. Med. 337, 1512–1523 (1997). This article reviews clinical and other aspects of small-vessel vasculitides, including AAVs.
Borie, R. & Crestani, B. Antineutrophil cytoplasmic antibody-associated lung fibrosis. Semin. Respir. Crit. Care Med. 39, 465–470 (2018).
Furuta, S. et al. Comparison of phenotype and outcome in microscopic polyangiitis between Europe and Japan. J. Rheumatol. 41, 325–333 (2014).
Suzuki, A. et al. Chest high-resolution CT findings of microscopic polyangiitis: a Japanese first nationwide prospective cohort study. Am. J. Roentgenol. 23, 1–11 (2019).
McAdoo, S. P. et al. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 92, 693–702 (2017).
Turner-Stokes, T. et al. Positive antineutrophil cytoplasmic antibody serology in patients with lupus nephritis is associated with distinct histopathologic features on renal biopsy. Kidney Int. 92, 1223–1231 (2017).
Anders, H. J. et al. MPO-ANCA-positive crescentic glomerulonephritis: a distinct entity of scleroderma renal disease? Am. J. Kidney Dis. 33, e3 (1999).
Quéméneur, T. et al. Systemic vasculitis during the course of systemic sclerosis: report of 12 cases and review of the literature. Medicine 92, 1–9 (2013).
Iudici, M. et al. Childhood- versus adult-onset ANCA-associated vasculitides: a nested, matched case-control study from the French Vasculitis Study Group Registry. Autoimmun. Rev. 17, 108–114 (2018).
Antonelou, M., Perea Ortega, L., Harvey, J. & Salama, A. D. Anti-myeloperoxidase antibody positivity in patients without primary systemic vasculitis. Clin. Exp. Rheumatol. 37, 86–89 (2019).
Berti, A. et al. Brief report: circulating cytokine profiles and antineutrophil cytoplasmic antibody specificity in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 70, 1114–1121 (2018).
Unizony, S. et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Ann. Rheum. Dis. 75, 1166–1169 (2016).
Cornec, D., Cornec-Le Gall, E., Fervenza, F. C. & Specks, U. ANCA-associated vasculitis - clinical utility of using ANCA specificity to classify patients. Nat. Rev. Rheumatol. 12, 570–579 (2016).
Bossuyt, X. et al. Position paper: Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat. Rev. Rheumatol. 13, 683–692 (2017). Contemporary recommendations on ANCA testing methods and procedures in suspected AAV.
Venhoff, N. et al. Reconstitution of the peripheral B lymphocyte compartment in patients with ANCA-associated vasculitides treated with rituximab for relapsing or refractory disease. Autoimmunity 47, 401–408 (2014).
von Borstel, A. et al. CD27+CD38hi B cell frequency during remission predicts relapsing disease in granulomatosis with polyangiitis patients. Front. Immunol. 10, 2221 (2019).
O’Reilly, V. P. et al. Urinary soluble CD163 in active renal vasculitis. J. Am. Soc. Nephrol. 27, 2906–2916 (2016). This study identifies soluble urinary CD163 as a potential biomarker for renal flares of AAV.
Tedesco, M., Gallieni, M., Pellegata, F., Cozzolino, M. & Alberici, F. Update on ANCA-associated vasculitis: from biomarkers to therapy. J. Nephrol. 32, 871–882 (2019).
Dekkema, G. J. et al. Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoantibody-associated vasculitis: a cohort study. Nephrol. Dial. Transpl. 34, 234–242 (2019).
Ponte, C., Agueda, A. F. & Luqmani, R. A. Clinical features and structured clinical evaluation of vasculitis. Best. Pract. Res. Clin. Rheumatol. 32, 31–51 (2018).
Mukhtyar, C. et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann. Rheum. Dis. 68, 1827–1832 (2009). An update of the BVAS, which is widely used in clinical trials to assess disease activity.
Hellmich, B. et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann. Rheum. Dis. 66, 605–617 (2007).
Guillevin, L. et al. The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 90, 19–27 (2011).
Exley, A. R. et al. Development and initial validation of the vasculitis damage index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheumatol. 40, 371–380 (1997).
Merkel, P. A. et al. The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J. Rheumatol. 38, 1480–1486 (2011).
Morgan, M. D. et al. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheumatol. 60, 3493–3500 (2009).
Robson, J. et al. Damage in the ANCA-associated vasculitides: long-term data from the European Vasculitis Study Group (EUVAS) therapeutic trials. Ann. Rheum. Dis. 74, 177–184 (2015).
Emmi, G. et al. Thrombosis in vasculitis: from pathogenesis to treatment. Thromb. J. 13, 15 (2015).
Merkel, P. A. et al. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study. Ann. Intern. Med. 142, 620–626 (2005).
Whyte, A. F., Smith, W. B., Sinkar, S. N., Kette, F. E. & Hissaria, P. Clinical and laboratory characteristics of 19 patients with Churg-Strauss syndrome from a single South Australian centre. Intern. Med. J. 43, 784–790 (2013).
Mohammad, A. J. et al. Pulmonary involvement in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis: the influence of ANCA subtype. J. Rheumatol. 44, 1458–1467 (2017).
Quinn, K. A. et al. Subglottic stenosis and endobronchial disease in granulomatosis with polyangiitis. Rheumatology 58, 2203–2211 (2019).
Churg, A. in Oxford Textbook of Vasculitis (eds Ball G. V., Fessler B. J., & Bridges S. L.) Ch. 9 101–108 (Oxford University Press, 2014).
Berden, A. E. et al. Histopathologic classification of ANCA-associated glomerulonephritis. J. Am. Soc. Nephrol. 21, 1628–1636 (2010). A classification system based on glomerular histopathology, which is associated with the outcome of renal disease in AAV.
Rahmattulla, C., Bruijn, J. A. & Bajema, I. M. Histopathological classification of antineutrophil cytoplasmic antibody-associated glomerulonephritis: an update. Curr. Opin. Nephrol. Hyperten. 23, 224–231 (2014).
Brix, S. R. et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. 94, 1177–1188 (2018).
Zhang, S., Yuan, D. & Tan, G. Neurological involvement in primary systemic vasculitis. Front. Neurol. 10, 430 (2019).
Flossmann, O. et al. Long-term patient survival in ANCA-associated vasculitis. Ann. Rheum. Dis. 70, 488–494 (2011).
Rhee, R. L. et al. Trends in long-term outcomes among patients with antineutrophil cytoplasmic antibody-associated vasculitis with renal disease. Arthritis Rheumatol. 68, 1711–1720 (2016).
Steinberg, A. W., Wechsler, M. E. & Fernandez Perez, E. R. Trends in antineutrophil cytoplasmic autoantibody-associated vasculitis-related mortality in the United States, 1999 to 2017. Ann. Intern. Med. 172, 160–163 (2020).
Scherlinger, M. et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun. Rev. 19, 102531 (2020).
Hogan, S. L. et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann. Intern. Med. 143, 621–631 (2005).
Gopaluni, S. et al. Effect of disease activity at three and six months after diagnosis on long-term outcomes in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 71, 784–791 (2019).
Walsh, M. et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N. Engl. J. Med. 382, 622–631 (2020). A clinical trial of induction therapies in severe AAV, which demonstrates that lower-dose glucocorticoids are non-inferior to standard doses and that routine use of plasma exchange as adjuvant provides no additional benefit.
Smith, R., Jayne, D. & Merkel, P. A randomized, controlled trial of rituximab versus azathioprine after induction of remission with rituximab for patients with ANCA-associated vasculitis and relapsing disease (abstract). Arthritis Rheumatol. 71 (Suppl. 10) (2019).
de Groot, K. et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann. Intern. Med. 150, 670–680 (2009).
Harper, L. et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann. Rheum. Dis. 71, 955–960 (2012).
Stone, J. H. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 363, 221–232 (2010). A clinical trial that demonstrates that rituximab is at least equivalent to cyclophosphamide in the induction of remission in AAV.
Specks, U. et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N. Engl. J. Med. 369, 417–427 (2013).
Jones, R. B. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N. Engl. J. Med. 363, 211–220 (2010).
Pepper, R. J. et al. A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology 58, 260–268 (2019).
De Groot, K. et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 52, 2461–2469 (2005).
Jones, R. B. et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann. Rheum. Dis. 78, 399–405 (2019).
Jayne, D. R. et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J. Am. Soc. Nephrol. 18, 2180–2188 (2007).
Jayne, D. R. et al. Intravenous immunoglobulin for ANCA-associated systemic vasculitis with persistent disease activity. QJM 93, 433–439 (2000).
Guillevin, L. et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N. Engl. J. Med. 371, 1771–1780 (2014). A clinical trial demonstrating that rituximab is a viable treatment and superior to azathioprine in the maintenance of remission in AAV.
Karras, A. et al. Randomised controlled trial of prolonged treatment in the remission phase of ANCA-associated vasculitis. Ann. Rheum. Dis. 76, 1662–1668 (2017).
Charles, P. et al. Long-term rituximab use to maintain remission of antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann. Intern. Med. https://doi.org/10.7326/M19-3827 (2020).
Gopaluni, S. et al. Rituximab versus azathioprine as therapy for maintenance of remission for anti-neutrophil cytoplasm antibody-associated vasculitis (RITAZAREM): study protocol for a randomized controlled trial. Trials 18, 112 (2017).
Tieu, J. et al. Rituximab for maintenance of remission in ANCA-associated vasculitis: expert consensus guidelines. Rheumatology 59, e24–e32 (2020).
Charles, P. et al. Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2). Ann. Rheum. Dis. 77, 1143–1149 (2018).
Puéchal, X. et al. Adding azathioprine to remission-induction glucocorticoids for eosinophilic granulomatosis with polyangiitis (Churg-Strauss), microscopic polyangiitis, or polyarteritis nodosa without poor prognosis factors: a randomized, controlled trial. Arthritis Rheumatol. 69, 2175–2186 (2017).
Steinfeld, J. et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immmunol. 143, 2170–2177 (2019).
Teixeira, V., Mohammad, A. J., Jones, R. B., Smith, R. & Jayne, D. Efficacy and safety of rituximab in the treatment of eosinophilic granulomatosis with polyangiitis. RMD Open 5, e000905 (2019).
Roberts, D. M. et al. Immunoglobulin G replacement for the treatment of infective complications of rituximab-associated hypogammaglobulinemia in autoimmune disease: a case series. J. Autoimmun. 57, 24–29 (2015).
De Sousa, E., Smith, R., Chaudhry, A., Willcocks, L. & Jayne, D. Venous thromboembolism with concurrent pulmonary haemorrhage in systemic vasculitis. Nephrol. Dial. Transplant. 27, 4357–4361 (2012).
Suppiah, R. et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res. 63, 588–596 (2011).
Westman, K. W., Bygren, P. G., Olsson, H., Ranstam, J. & Wieslander, J. Relapse rate, renal survival, and cancer morbidity in patients with Wegener’s granulomatosis or microscopic polyangiitis with renal involvement. J. Am. Soc. Nephrol. 9, 842–852 (1998).
Heijl, C. et al. Incidence of malignancy in patients treated for antineutrophil cytoplasm antibody-associated vasculitis: follow-up data from European Vasculitis Study Group clinical trials. Ann. Rheum. Dis. 70, 1415–1421 (2011).
van Daalen, E. E. et al. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann. Rheum. Dis. 76, 1064–1069 (2017).
Buckley, L. & Humphrey, M. B. Glucocorticoid-induced osteoporosis. N. Engl. J. Med. 379, 2547–2556 (2018).
Martinez del Pero, M. et al. Long-term outcome of airway stenosis in granulomatosis with polyangiitis (Wegener granulomatosis): an observational study. JAMA Otolaryngol. Head Neck Surg. 140, 1038–1044 (2014).
Hruskova, Z. et al. Characteristics and outcomes of granulomatosis with polyangiitis (Wegener) and microscopic polyangiitis requiring renal replacement therapy: results from the European Renal Association-European Dialysis and Transplant Association Registry. Am. J. Kidney Dis. 66, 613–620 (2015).
Herlyn, K., Hellmich, B., Seo, P., Merkel, P. A. & The Vasculitis Clinical Research Consortium. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res. 62, 1639–1645 (2010).
Robson, J. C. et al. Patient perceptions of glucocorticoids in anti-neutrophil cytoplasmic antibody-associated vasculitis. Rheumatol. Int. 38, 675–682 (2018). A study reporting the effects of glucocorticoids in AAV, both positive and negative, from the patients’ perspective.
Miloslavsky, E. M. et al. Development of a Glucocorticoid Toxicity Index (GTI) using multicriteria decision analysis. Ann. Rheum. Dis. 76, 543–546 (2017).
Robson, J. C. et al. Validation of the ANCA-associated vasculitis patient-reported outcomes (AAV-PRO) questionnaire. Ann. Rheum. Dis. 77, 1157–1164 (2018). Validation study of an AAV-specific PRO measure.
Robson, J. C. et al. OMERACT endorsement of patient-reported outcome instruments in antineutrophil cytoplasmic antibody-associated vasculitis. J. Rheumatol. 44, 1529–1535 (2017).
O’Malley, L. et al. The longitudinal course of fatigue in antineutrophil cytoplasmic antibody-associated vasculitis. J. Rheumatol. 47, 572–579 (2020). This study defines the incidence and time course of fatigue, an important symptom for patients with AAV.
Hessels, A. C. et al. Leg muscle strength is reduced and is associated with physical quality of life in Antineutrophil cytoplasmic antibody-associated vasculitis. PLoS ONE 14, e0211895 (2019).
Harper, L. et al. Treatment of fatigue with physical activity and behavioural change support in vasculitis: study protocol for an open-label randomised controlled feasibility study. BMJ Open 8, e023769 (2018).
Moran, S. M. et al. Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol. Dial. Transplant. 35, 283–291 (2020).
Pagnoux, C. et al. Treatment of systemic necrotizing vasculitides in patients aged sixty-five years or older: results of a multicenter, open-label, randomized controlled trial of corticosteroid and cyclophosphamide-based induction therapy. Arthritis Rheumatol. 67, 1117–1127 (2015).
Jones, R. B. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann. Rheum. Dis. 74, 1178–1182 (2015).
Walsh, M. et al. Long-term follow-up of patients with severe ANCA-associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclear. Kidney Int. 84, 397–402 (2013).
Merkel, P. A., Jayne, D. R., Wang, C., Hillson, J. & Bekker, P. Evaluation of the safety and efficacy of avacopan, a C5a receptor inhibitor, in patients with antineutrophil cytoplasmic antibody-associated vasculitis treated concomitantly with rituximab or cyclophosphamide/azathioprine: protocol for a randomized, double-blind, active-controlled, Phase 3 trial. JMIR Res. Protoc. 9, e16664 (2020).
Faurschou, M. et al. Brief report: long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 64, 3472–3477 (2012).
Jayne, D. et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N. Engl. J. Med. 349, 36–44 (2003).
Pagnoux, C. et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N. Engl. J. Med. 359, 2790–2803 (2008).
Puéchal, X. et al. Long-term outcomes among participants in the WEGENT trial of remission-maintenance therapy for granulomatosis with polyangiitis (Wegener’s) or microscopic polyangiitis. Arthritis Rheumatol. 68, 690–701 (2016).
Hiemstra, T. F. et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA 304, 2381–2388 (2010).
Terrier, B. et al. Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann. Rheum. Dis. 77, 1150–1156 (2018).
Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. N. Engl. J. Med. 352, 351–361 (2005).
Metzler, C. et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology 46, 1087–1091 (2007).
Jayne, D. et al. Efficacy and safety of belimumab and azathioprine for maintenance of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled study. Arthritis Rheumatol. 71, 952–963 (2019).
Stegeman, C. A., Tervaert, J. W., de Jong, P. E., Kallenberg, C. G. & Dutch Co-Trimoxazole Wegener Study Group. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. N. Engl. J. Med. 335, 16–20 (1996).
Ribi, C. et al. Treatment of Churg-Strauss syndrome without poor-prognosis factors: a multicenter, prospective, randomized, open-label study of seventy-two patients. Arthritis Rheumatol. 58, 586–594 (2008).
Puéchal, X. et al. Non-severe eosinophilic granulomatosis with polyangiitis: long-term outcomes after remission-induction trial. Rheumatology 58, 2107–2116 (2019).
Guillevin, L. et al. Lack of superiority of steroids plus plasma exchange to steroids alone in the treatment of polyarteritis nodosa and Churg-Strauss syndrome. A prospective, randomized trial in 78 patients. Arthritis Rheumatol. 35, 208–215 (1992).
Guillevin, L. et al. Corticosteroids plus pulse cyclophosphamide and plasma exchanges versus corticosteroids plus pulse cyclophosphamide alone in the treatment of polyarteritis nodosa and Churg-Strauss syndrome patients with factors predicting poor prognosis. A prospective, randomized trial in sixty-two patients. Arthritis Rheumatol. 38, 1638–1645 (1995).
Ma, T. K., McAdoo, S. P. & Tam, F. W. Targeting the tyrosine kinase signalling pathways for treatment of immune-mediated glomerulonephritis: from bench to bedside and beyond. Nephrol. Dial. Transpl. 32 (Suppl. 1), i129–i138 (2017).
Langford, C. A. et al. An open-label trial of abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener’s). Ann. Rheum. Dis. 73, 1376–1379 (2014).
Holdsworth, S. R., Gan, P. Y. & Kitching, A. R. Biologics for the treatment of autoimmune renal diseases. Nat. Rev. Nephrol. 12, 217–231 (2016).
Gan, P. Y. et al. Apoptotic cell-induced, antigen-specific immunoregulation to treat experimental antimyeloperoxidase GN. J. Am. Soc. Nephrol. 30, 1365–1374 (2019).
Bunch, D. O. et al. Gleaning relapse risk from B cell phenotype: decreased CD5+ B cells portend a shorter time to relapse after B cell depletion in patients with ANCA-associated vasculitis. Ann. Rheum. Dis. 74, 1784–1786 (2015).
Ormerod, A. S. & Cook, M. C. Epidemiology of primary systemic vasculitis in the Australian Capital Territory and south-eastern New South Wales. Intern. Med. J. 38, 816–823 (2008).
Anderson, K., Klassen, J., Stewart, S. A. & Taylor-Gjevre, R. M. Does geographic location affect incidence of ANCA-associated renal vasculitis in northern Saskatchewan, Canada? Rheumatology 52, 1840–1844 (2013).
Reinhold-Keller, E., Herlyn, K., Wagner-Bastmeyer, R. & Gross, W. L. Stable incidence of primary systemic vasculitides over five years: results from the German vasculitis register. Arthritis Rheumatol. 53, 93–99 (2005).
Panagiotakis, S. H. et al. The epidemiology of primary systemic vasculitides involving small vessels in Crete (southern Greece): a comparison of older versus younger adult patients. Clin. Exp. Rheumatol. 27, 409–415 (2009).
Fujimoto, S. et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the UK. Rheumatology 50, 1916–1920 (2011).
Dadoniene, J., Kirdaite, G., Mackiewicz, Z., Rimkevicius, A. & Haugeberg, G. Incidence of primary systemic vasculitides in Vilnius: a university hospital population based study. Ann. Rheum. Dis. 64, 335–336 (2005).
Pamuk, O., Donmez, S. & Calayir, G. B. The incidences of anti-neutrophil cytoplasmic antibody-associated vasculitis in northeastern part of Turkey. Ann. Rheum. Dis. 72, 638–638 (2013).
Sánchez Torres, A. et al. Epidemiology of primary systemic vasculitis in a Latin America population [Spanish]. Rev. Chil. Reumatol. 21, 145–150 (2005).
Gonzalez-Gay, M. A., Garcia-Porrua, C., Guerrero, J., Rodriguez-Ledo, P. & Llorca, J. The epidemiology of the primary systemic vasculitides in northwest Spain: implications of the Chapel Hill Consensus Conference definitions. Arthritis Rheumatol. 49, 388–393 (2003).
Romero-Gomez, C. et al. Epidemiological study of primary systemic vasculitides among adults in southern Spain and review of the main epidemiological studies. Clin. Exp. Rheumatol. 33 (Suppl. 89), 11–18 (2015).
Mohammad, A. J., Jacobsson, L. T. H., Westman, K. W. A., Sturfelt, G. & Segelmark, M. Incidence and survival rates in Wegener’s granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and polyarteritis nodosa. Rheumatology 48, 1560–1565 (2009).
Zeft, A. S. & Schlesinger, M. K. H. Wegener’s granulomatosis and environmental factors in Western Montana. Rheumatol. Rep. https://doi.org/10.4081/rr.2010.e8 (2010).
Nesher, G., Ben-Chetrit, E., Mazal, B. & Breuer, G. S. The incidence of primary systemic vasculitis in Jerusalem: a 20-year hospital-based retrospective study. J. Rheumatol. 43, 1072–1077 (2016).
Damoiseaux, J. et al. An international survey on anti-neutrophil cytoplasmic antibodies (ANCA) testing in daily clinical practice. Clin. Chem. Lab. Med. 56, 1759–1770 (2018).
Damoiseaux, J. et al. Detection of antineutrophil cytoplasmic antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen-specific immunoassays. Ann. Rheum. Dis. 76, 647–653 (2017).
Savige, J. et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am. J. Clin. Pathol. 111, 507–513 (1999).
Weiner, M. & Segelmark, M. The clinical presentation and therapy of diseases related to anti-neutrophil cytoplasmic antibodies (ANCA). Autoimmun. Rev. 15, 978–982 (2016).
Zhao, M. H. et al. Autoantibodies against bactericidal/permeability-increasing protein in patients with cystic fibrosis. QJM 89, 259–265 (1996).
Choi, H. K., Lamprecht, P., Niles, J. L., Gross, W. L. & Merkel, P. A. Subacute bacterial endocarditis with positive cytoplasmic antineutrophil cytoplasmic antibodies and anti-proteinase 3 antibodies. Arthritis Rheumatol. 43, 226–231 (2000).
Mahr, A. et al. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 66, 1672–1677 (2014).
Ying, C. M., Yao, D. T., Ding, H. H. & Yang, C. D. Infective endocarditis with antineutrophil cytoplasmic antibody: report of 13 cases and literature review. PLoS ONE 9, e89777 (2014).
Chen, M., Gao, Y., Guo, X. H. & Zhao, M. H. Propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis. Nat. Rev. Nephrol. 8, 476–483 (2012).
Pendergraft, W. F. & Niles, J. L. Trojan horses: drug culprits associated with antineutrophil cytoplasmic autoantibody (ANCA) vasculitis. Curr. Opin. Rheumatol. 26, 42–49 (2014).
Grau, R. G. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr. Rheumatol. Rep. 17, 71 (2015).
Lee, E. et al. Inactivation of peroxidases of rat bone marrow by repeated administration of propylthiouracil is accompanied by a change in the heme structure. Biochem. Pharmacol. 37, 2151–2153 (1988).
Nakazawa, D. et al. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 64, 3779–3787 (2012).
Lood, C. & Hughes, G. C. Neutrophil extracellular traps as a potential source of autoantigen in cocaine-associated autoimmunity. Rheumatology 56, 638–643 (2017).
Acknowledgements
H-J.A., E.B., R.K., P.A.L. and A.R.K. are principle investigators, and J.K and C.O.S.S. are scientific advisers of the European Union Horizon 20/20 RELENT (RELapses prevENTion in chronic autoimmune disease) consortium that has received funding from the European Union Horizon 2020 research and innovation programme under grant agreement 668036. A.R.K. acknowledges funding support from the Australian National Health and Medical Research Council of Australia (grant numbers 1104422, 1084869 and 1115805). H-J.A. was supported by the Deutsche Forschungsgemeinschaft (grant number AN372/24-1). P.A.L. acknowledges support from the Medical Research Council (grant number MR/L019027/1), Versus Arthritis (grant number 20593) and the British Heart Foundation (grant number PG/13/64/30435).
Author information
Authors and Affiliations
Contributions
All authors contributed to all sections of the Primer, with A.R.K. coordinating the project. The middle authors of the Primer are listed in alphabetical order.
Corresponding author
Ethics declarations
Competing interests
A.R.K. is Chair of the Board of the Australian and New Zealand Vasculitis Society and has been a consultant for CSL Limited and Visterra. N.B. has received research funding from Vifor and GSK and speaking fees from Roche and Vifor. E.B. received consultancy and speaker fees from Roche, which were paid to her employer. D.R.J. has been a consultant for ChemoCentryx, InflaRx and Insmed. P.A.L. holds founding equity in and receives consultation fees from PredictImmune Ltd. P.A.M. has been a consultant for AbbVie, Biogen, CSL Behring, Genzyme, Insmed, Janssen, Kiniska and Sparrow, received research funding and consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, Genentech/Roche, GSK and InflaRx, and grant support from Kypha. U.S. has been a consultant for AstraZeneca, Insmed, and ChemoCentryx and has received research funding from Genentech, Bristol-Myers Squibb, ChemoCentryx and GSK. The remaining authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks P. Van Paassen, B. Hellmich, M.-H. Zhao, A. Vaglio, M. Segelmark and X. Puéchal for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kitching, A.R., Anders, HJ., Basu, N. et al. ANCA-associated vasculitis. Nat Rev Dis Primers 6, 71 (2020). https://doi.org/10.1038/s41572-020-0204-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-020-0204-y
This article is cited by
-
Nailfold videocapillaroscopy in antineutrophil cytoplasmic antibody–associated vasculitis
Arthritis Research & Therapy (2024)
-
The podocytes’ inflammatory responses in experimental GN are independent of canonical MYD88-dependent toll-like receptor signaling
Scientific Reports (2024)
-
Cardiovascular Disease in Anti-neutrophil Cytoplasm Antibody-Associated Vasculitis
Current Rheumatology Reports (2024)
-
Diagnostic and prognostic role of serum interleukin-6 and carotid ultrasonography to detect subclinical atherosclerosis in patients with RA and ANCA-associated vasculitis
Rheumatology International (2024)
-
Diagnostic delays in systemic vasculitides
Rheumatology International (2024)