Abstract
Ebola virus disease (EVD) is a severe and frequently lethal disease caused by Ebola virus (EBOV). EVD outbreaks typically start from a single case of probable zoonotic transmission, followed by human-to-human transmission via direct contact or contact with infected bodily fluids or contaminated fomites. EVD has a high case–fatality rate; it is characterized by fever, gastrointestinal signs and multiple organ dysfunction syndrome. Diagnosis requires a combination of case definition and laboratory tests, typically real-time reverse transcription PCR to detect viral RNA or rapid diagnostic tests based on immunoassays to detect EBOV antigens. Recent advances in medical countermeasure research resulted in the recent approval of an EBOV-targeted vaccine by European and US regulatory agencies. The results of a randomized clinical trial of investigational therapeutics for EVD demonstrated survival benefits from two monoclonal antibody products targeting the EBOV membrane glycoprotein. New observations emerging from the unprecedented 2013–2016 Western African EVD outbreak (the largest in history) and the ongoing EVD outbreak in the Democratic Republic of the Congo have substantially improved the understanding of EVD and viral persistence in survivors of EVD, resulting in new strategies toward prevention of infection and optimization of clinical management, acute illness outcomes and attendance to the clinical care needs of patients.
Similar content being viewed by others
Introduction
To date, 12 distinct filoviruses have been described1. The seven filoviruses that have been found in humans belong either to the genus Ebolavirus (Bundibugyo virus (BDBV), Ebola virus (EBOV), Reston virus (RESTV), Sudan virus (SUDV) and Taï Forest virus (TAFV); Fig. 1) or to the genus Marburgvirus (Marburg virus (MARV) and Ravn virus (RAVV))2. The WHO International Classification of Diseases Revision 11 (ICD-11) of 2018 recognizes two major subcategories of filovirus disease (FVD): Ebola disease caused by BDBV, EBOV, SUDV or TAFV, and Marburg disease caused by MARV or RAVV. Ebola virus disease (EVD) is defined as a disease only caused by EBOV. This subcategorization of FVD is largely based on the increasing evidence of molecular differences between ebolaviruses and marburgviruses, differences that may influence virus–host reservoir tropism, pathogenesis and disease phenotype in accidental primate hosts2.
a | Taxonomy of the genus Ebolavirus. Thus far, five ebolaviruses have been associated with human infections, and four of them have been identified as pathogens. b | The natural reservoir host(s) of Ebola virus (EBOV) has (have) yet to be identified. Multiple data indicate a direct or indirect role of bats in EBOV ecology, but to date, EBOV has not been isolated from, nor has a near-complete EBOV genome been detected in any wild animal279. However, it is tempting to speculate that Ebola virus disease (EVD) is a zoonosis (that is, an infectious disease caused by an agent transmitted between animals and humans) because retrospective epidemiological investigations have often been able to track down the probable index cases of EVD outbreaks. These individuals had been in contact with wild animals or had handled the carcass of a possible accidental EBOV host7,280. c | Scanning electron microscopic (SEM) image of EBOV particles (green) budding from grivet cells. d | Transmission electron microscopic (TEM) image of EBOV particles (green) budding from grivet cells1,281. aThe kingdom name has been approved by the International Committee on Taxonomy of Viruses (ICTV) but has yet to be ratified. Parts c and d courtesy of J. Wada and J. Bernbaum, NIH/NIAID Integrated Research Facility at Fort Detrick, Frederick, MD, USA.
Since the discovery of filoviruses in 1967 (ref.3), 43 FVD outbreaks (excluding at least five laboratory-acquired infections) have been recorded in or exported from Africa4. The epidemiological definition of outbreak is one or more cases above the known endemic prevalence. For example, the single case of TAFV infection recorded in a setting in which FVD had never been reported before (Côte d’Ivoire)5 is still considered an outbreak. All FVD outbreaks, with the exception of that caused by TAFV, were characterized by extremely high case–fatality rates (CFRs, also known as lethality). Until 2013, the most extensive outbreak, caused by SUDV, involved 425 cases and 224 deaths (CFR 52.7%)6. The overall limited numbers of FVD cases (1967–2013: 2,886 cases including 1,982 deaths4), the typical remote and rural locations of outbreaks and the often delayed announcement of new outbreaks to the international community7 have prevented the systematic study of clinical FVD in humans. Thus, the commonly used description of FVD was derived either from observation of small groups of patients in care settings that were not well-equipped for diagnosis, treatment and disease characterization, or from observations of even smaller samples, such as individuals who were transferred from Equatorial Africa to Europe and the USA or who fell sick in Europe or the USA after contracting the virus elsewhere. Pathological characterization of FVD via autopsies has been rare7,8. In the absence of extensive human clinical data, FVD could only be defined further via the use of experimental animal infections9,10.
Until 2013, most EVD outbreaks originated from Middle Africa: the Democratic Republic of the Congo, Gabon and the Republic of the Congo. From late 2013 to early 2016, EBOV caused the largest outbreak to date, which spread from Guinea to other countries in Western Africa, leading to 28,652 human infections and 11,325 deaths11. The location and scale of the 2013–2016 outbreak was entirely unexpected12. Consequently, local, national and international organizations were caught unprepared for an outbreak caused by what, until then, was considered an exotic pathogen of largely negligible consequence for global public health13,14,15. After the WHO declared the outbreak a Public Health Emergency of International Concern, the global and local responses to the outbreak intensified. Ultimately, the outbreak was contained, but it devastated individuals, families, communities, health-care systems and economies16. In most affected countries, the response included the establishment of Ebola (virus disease) Treatment Units (ETUs)17,18,19, in which medical professionals and biomedical scientists managed large cohorts of patients with suspected or confirmed EVD in controlled settings. From this experience, scientists were able to better understand a virus previously best known as a potential bioweapons agent20,21. In addition to the Western African outbreak, an ongoing outbreak in the Ituri, Nord-Kivu and Sud-Kivu Provinces of the Democratic Republic of the Congo is the second largest outbreak in terms of the number of cases and deaths, with 3,418 infections and 2240 deaths (as of 28 January 2020)22 (Table 1, Fig. 2).
The map shows the location and years of all reported Ebola virus disease (EVD) outbreaks. Two cases of laboratory-acquired EVD occurred in Russia (not shown). Adapted with permission of McGraw-Hill Education, from Harrison’s principles of internal medicine, Jameson, J. L. et al, vol. 2, 20th edn, 2018 (ref.282).
Owing to observations from the 2013‒2016 Western African outbreak and, to a limited degree, from subsequent EVD outbreaks in the Democratic Republic of the Congo (Table 1)23,24,25,26, clinicians can now better describe predictable phases in the progression of EVD in humans. Typically, EVD begins with a nonspecific febrile illness followed by severe gastrointestinal symptoms and signs. In highly viraemic patients who often also have dysregulated immune responses, EVD progresses to a complex multiple organ dysfunction syndrome that can be fatal. A subset of patients, usually with lower viraemia, have less-severe disease progression and organ dysfunction27,28. Ultimately, these patients develop robust immune responses leading to clearance of viraemia and a resolution phase. However, recovery can be complicated by long-lasting clinical sequelae and/or virus persistence in immune-privileged sites that can lead to disease flares and even sexual transmission. In this Primer, we outline the current improved understanding of EVD based on the most recently published human clinical data.
Epidemiology
Classic epidemiology
Since the discovery of EBOV in 1976 in the Democratic Republic of the Congo (then Zaire)29,30,31, at least 17 EVD outbreaks have originated in Gabon, Guinea, the Republic of the Congo or Zaire/Democratic Republic of the Congo. At the time of writing, ~33,604 EBOV infections in humans, including 14,742 deaths (average CFR 43.8%) are on record4,22, although case numbers differ slightly from source to source.
Outbreaks and transmission
Most outbreaks can be traced back to a single spillover introduction of EBOV into the human population from an unknown reservoir by unknown means. Subsequently, the virus is transmitted by direct, typically non-aerosol, human-to-human contact or contact with infected tissues, bodily fluids or contaminated fomites (Fig. 1)4. Based on historical records, EBOV may have been transmitted from its natural reservoir host(s) to humans to cause disease only about 20–30 times (Table 1), although it is probable that limited EVD outbreaks may have been overlooked or not reported. The potential for infection of an index case and subsequent spread — locally and globally — has been estimated by considering reservoir species distribution, along with governance, communications, isolation, infrastructure, health care and international connectivity32. These predictions are crucial to identify regions that require increased surveillance and investments. Tracking EBOV within the human population after a zoonotic transmission event can be challenging, especially as the single natural reservoir has not been identified.
A strong risk factor linked to human-to-human EBOV propagation is contact with infected bodily fluids33,34,35. Indeed, infectious EBOV has been recovered from breast milk, saliva, urine, semen, cerebrospinal fluid, and aqueous humor, in addition to blood and blood derivatives, and detected in amniotic fluid, tears, skin swabs and stool by reverse transcription (RT)-PCR36,37,38,39,40. Although EBOV RNA has been detected in illness-related bodily fluids (such as diarrhoea and vomitus)40, infectivity is unclear. Taking care of an individual with EVD at home or in a health-care facility or following traditional funeral practices, which involve contact with the deceased’s body, substantially increases the risk of acquiring infection. This contact is one of the reasons why women, who traditionally care for the sick in certain African regions, may be at higher risk of acquiring EBOV than men41,42 (Box 1). Although rare, sexual transmission of EBOV was proven or strongly suspected during the Western African EVD outbreak. Fortunately, the risk of widespread outbreaks in middle-income and high-income countries remains relatively low, partially owing to our ability to keep the reproductive number (R0, the average number of individuals to whom an infected person will transmit the pathological agent over the course of the infectious period) of EBOV below 1 with simple infection prevention control and contact tracing measures43.
Risk factors and outcomes
Demographical risk factors for EBOV infection and subsequent development of EVD, such as age, sex and ethnicity, are not well-defined. By current (albeit incomplete) understanding, sex differences in susceptibility have not been identified, but women as care-givers may be at higher risk of being exposed to EBOV, and the incidence of EVD increases almost linearly with age to a peak at 35–44 years. Although children typically constitute a disproportionately small number of EVD cases, they have shorter incubation periods, and a more rapid disease course. Children have a higher risk of death than older populations, with children of <5 years of age at the highest risk44,45,46,47,48,49. Possible explanations for the low incidence of EVD in children include behavioural factors, such as deliberate prevention of exposure to infected individuals46, and differences in susceptibility across age groups50. Occasionally, spikes in incidence of EVD in children have been recorded in correspondence to malaria outbreaks and were probably related to nosocomial infections28.
Infected pregnant women are at high risk of miscarriage or stillbirths, and newborn babies of infected mothers rarely survive51. Indeed, EBOV can be transmitted transplacentally52 and also lead to fetal death related to placental insufficiency. Transmission of EBOV from infected pregnant women to their embryos or fetuses or from infected mothers to their children occurs frequently and is associated with elevated in utero and neonatal lethality51. The risk of fetal loss in survivors of EVD who become pregnant after recovery remains unclear; some data suggest an increased risk over baseline, especially early after recovery53, although healthy pregnancy outcomes are possible54. EBOV RNA has been detected at high concentrations in amniotic fluid, placenta, fetal tissue and breast milk39,55,56,57,58. Molecular studies of specific host factors influencing the outcome of EBOV infection in particular human populations are absent, with the exception of one study that associated the expression of killer cell immunoglobulin-like receptor (KIR) 2DS1 (KIR2DS1) and KIR2DS3 with fatal outcome59.
Case–fatality rate
Although unpublished observations of varying disease clinical signs or levels of severity depending on the specific outbreak have been described, these findings are not necessarily reflected in the published literature. On the basis of comparative statistics on CFRs, a fundamental difference in virulence between ebolaviruses that cause lethal human disease is not observed; the oft-repeated notion that EBOV is the most virulent ebolavirus (let alone filovirus) is not supported by available data4. The mean CFRs for each ebolavirus are 33.65 ± 8.38% (BDBV), 43.92 ± 0.7% (EBOV) and 53.72 ± 4.456% (SUDV)4; that is, a CFR of ~40–50% overall, with the remaining difference between the viruses compounded by the number of outbreaks recorded and the typically small number of cases in each outbreak. Accordingly, whether one ebolavirus is more dangerous than another is statistically unclear. The reasons for fluctuating CFR data are not truly understood. Possible reasons include differences in health status (nutrition, immunity and co-infection status), genetics (ethnicity-dependent haplotypes or random polymorphisms), health-seeking behaviour, case recognition and reporting capacities and the development and accessibility of health-care facilities providing supportive care in the affected African countries.
Case definitions
In response to large outbreaks of communicable diseases such as meningitis and yellow fever, in 1998, the WHO African Regional Office (WHO/AFRO) along with its Member States established an Integrated Disease Surveillance strategy (later termed Integrated Disease Surveillance and Response (IDSR)) to improve public health surveillance of and response to emerging and re-emerging diseases, including those with outbreak potential60. Revised IDSR guidelines from 2010 include guidance for developing case definitions for routine and community-based surveillance of such diseases. For EVD, the WHO has developed standard case definitions for alert, suspected, probable and confirmed cases in the context of routine and community-based surveillance (Box 2; US Centers for Disease Control and Prevention (CDC) definitions are in Box 3). These standard case definitions are utilized by public health authorities to optimize surveillance and notification of EVD, particularly before an outbreak has been identified.
As increasing numbers of patients with possible EVD present to health facilities at the beginning of an outbreak, case definitions are refined from standard public health case definitions to reflect clinical and epidemiological features associated with a particular outbreak context. A robust case definition and accurate confirmatory testing are key to ensuring that individuals with suspected EBOV infection are efficiently identified and, upon admission to an ETU, isolated for confirmation of diagnosis and treatment. Importantly, patient screening time should be minimized to limit exposure of uninfected individuals, including ETU staff, to potentially infected individuals. Within an ETU, patients with suspected EBOV infection may be further separated, based on the probability of EBOV infection or the risk of infectiousness, to avoid nosocomial infection within the ETU. The corresponding jargon for ETU wards or sections of wards to reflect levels of risk (of having EVD, and therefore infectiousness) has varied across ETUs and has included descriptions such as ‘suspect vs probable’ or ‘wet vs dry’.
Despite refinement, case definitions are rarely 100% sensitive or specific, and attempts to optimize one come at the expense of the other. A case definition with a low sensitivity will mislabel true EBOV-positive individuals as EBOV-negative, leading to an increased risk of discharge of EBOV-infected individuals back to the community, where EBOV transmission can be reinitiated. Particularly in a setting with a low community incidence of EVD, the sensitivity of the case definition should be maximized. By contrast, a case definition with a low specificity might result in misclassification of true EBOV-negative individuals as EBOV-positive. Such individuals might be admitted to an ETU with suspected EVD, placing them at increased risk of EBOV exposure and nosocomial infection, especially when the probability that other patients with suspected EVD might be EBOV-positive is high. Thus, in a community with a high incidence of EVD, increased specificity in EVD case definition may be crucial.
Given these considerations, currently no EVD case definition is globally applied. Indeed, the EVD case definition can be reiterated during the course of an outbreak; such variations in case definition were used during the 2013–2016 Western African outbreak (for example, in Sierra Leone61) as the outbreak evolved from a high incidence to a low incidence. Although novel case definitions are limited by variations in EVD prevalence during a particular outbreak and the intrinsic lack of specificity of case definitions compared with common endemic causes of acute febrile and diarrhoeal disease, their performance characteristics have been evaluated (Box 4).
Molecular epidemiology
The 2013–2016 Western African EVD outbreak was the first to be largely characterized by molecular epidemiological evidence. Deep-sequencing efforts, often performed on site and in parallel by several groups, resulted in the determination of >1,600 coding-complete (all open reading frames) or near-complete (typically coding-complete plus parts of leaders and/or trailers) EBOV genomes directly from human patient samples62,63,64,65. These samples included single genomes from single patients, multiple different genomes from the same patient and the same genome from different patients. Subsequent phylogenetic analyses traced EBOV movement through the human populations of all affected countries and pinpointed multiple back-and-forth border crossings63 (Fig. 3). The genomic data confirmed the classic epidemiological model of filovirus infections: all 28,652 human infections of this outbreak occurred via direct human-to-human contact tracing back to a single human index case (probably due to zoonotic transmission) close to Guéckédou, Nzérékoré Region, Guinea. Such molecular epidemiological investigations are now becoming routine.
Molecular evidence using hundreds of individual Ebola virus (EBOV) genomes sequenced from individual patients indicates that in the index case of the outbreak, EBOV was acquired by unknown means at the end of 2013 in or around Guéckédou in Guinea. From there, person-to-person transmission enabled EBOV to spread (coloured lines) throughout the country, to cross borders and ultimately to affect a total of 15 countries (see also Fig. 2). The direction of EBOV spread is represented by the lines and goes from the thick end to the thin end. White borders delineate the provinces (Guinea), districts (Sierra Leone) and counties (Liberia). Adapted from ref.63, Springer Nature Limited.
Molecular approaches have also enabled progress in understanding of within-outbreak and within-host viral evolution69. During the two most recent EVD outbreaks in the Democratic Republic of the Congo, deep sequencing revealed single spillover EBOV transmission events into the human populations with subsequent person-to-person transmission25,26. Molecular approaches revealed that sexual transmission of EBOV may rarely occur from apparently healthy survivors of EVD in whom EBOV may persist in the semen for extended periods of time, with the latest documented transmission event 482 days after EVD onset41,66,67,68. The importance of molecular epidemiology is not limited to an individual outbreak but provides valuable information to scientists and decision makers regarding the long-term evolution of EBOV. Current medical countermeasures (MCMs), such as vaccines and therapeutics, have often been designed to specifically match a known EBOV isolate, or designed as a consensus of multiple ones to account for genomic variation. Molecular epidemiology enables assessment of the potential efficacy of available MCMs based on the sequence of a newly circulating EBOV isolate. The analysis of 65 EBOV GP sequences from isolates collected from 1976 to 2014 demonstrated that the temporal evolution of EBOV is mostly due to neutral genetic drift, suggesting that the emergence of completely novel isolates that would not respond to current MCMs is unlikely70.
Mechanisms/pathophysiology
Many outstanding questions still surround the pathophysiology of EVD. Findings from animal studies, in vitro work and clinical data from humans are beginning to decipher the normal course of EVD in humans and to link disease progression to the molecular bases of EBOV pathogenesis. With these data, researchers may be able to identify the crucial pathways involved in effective immune responses to EBOV infection and the various candidate MCMs that may be developed to augment any host response shortcomings.
Animal models
Exposure of immunocompetent laboratory mice, Syrian hamsters (Mesocricetus auratus) and domesticated guinea pigs (Cavia porcellus) to EBOV does not yield severe (or any) disease, and EBOV must be adapted via serial passages in rodents before lethal infection is achieved71,72. Even when adapted viruses are used, these rodent models do not fully mimic human disease. Because non-human primates (NHPs) are evolutionarily much more closely related to humans than rodents, NHP models of EVD are often considered to be more useful for the study of human EBOV infection and EVD. Indeed, much of the information on viral pathogenesis has been derived from studies with wild-type EBOV predominantly in crab-eating macaques (Macaca fascicularis) and rhesus monkeys (M. mulatta)9,10. On the basis of experimental animal data, two factors that may influence development and severity of human EVD may be the EBOV exposure route and dose. Direct contact with infected biological materials or contaminated non-biological materials via cuts or scratches or via contact with mucosal membranes (oral or, theoretically, nasopharyngeal or conjunctival mucosa) is considered the most frequent mode of human-to-human EBOV transmission73. However, these transmission pathways are difficult to simulate in experimental settings. Thus, animal models of EVD have been established using injection and aerosol methods of EBOV exposure to model accidental needlestick injury and respiratory routes of exposure, respectively, despite the lack of evidence that these exposure routes have any relevant roles during natural EVD outbreaks73.
Most studies in NHPs rely on either intramuscular injection or small-particle aerosol exposure of 1,000 plaque-forming units (pfu) of EBOV, a dose that ensures that all infected animals will develop a disease that is almost always lethal74; thus, significant results can be achieved with overall low animal numbers. Interestingly, intramuscular infection of NHPs with some EBOV variants by injection of a calculated dose of 0.01 pfu (corresponding to ~90 virus particles) results in lethal disease in 100% of animals75. The lethality associated with this low virus dose suggests that very few virions may be required to initiate a lethal disease course in humans, although insufficient data preclude robust median lethal dose calculation and speculations on the effects of different variants on human disease.
During the 2013–2016 Western African EVD outbreak, molecular genomic analyses were used to observe EBOV evolution during human-to-human transmission. The comparison of the ~1,600 near-complete EBOV genome sequences obtained during that outbreak revealed several positively selected genomic mutations. A mutation leading to an amino acid residue change, A82V, in EBOV glycoprotein GP1,2 occurred in viral genomes isolated from samples collected early in the 2013–2016 Western African outbreak and remained present in genomes from all later samples76,77,78. In vitro, this mutation enhances EBOV GP1,2-mediated virus entry into human cells78, possibly by weakening the stability of the prefusion conformation of GP1,2 and hence lowering the activation barrier required for fusion of EBOV particle membranes with host cell membranes79. However, in vivo experiments have yet to unambiguously ascribe a phenotype to A82V and similar mutations in the context of pathogenesis. For instance, initial studies with Ifnar−/− immunodeficient laboratory mice and rhesus monkeys did not demonstrate an effect of A82V on disease severity or virus shedding80. This lack of an effect may be due to the true lack of effect of these mutations on pathogenesis, limitations of the in vivo studies (such as compensatory mutations for the A82V phenotype observed in vitro), or intrinsic differences among laboratory mice, NHPs and humans, such as infection cofactors or immune responses. Consequently, a convincing explanation for positive selection of certain mutations in EBOV genomes over the course of the outbreak is still lacking. Novel approaches using systems biology are used more frequently nowadays in the context of EVD and could be used to further describe the effect of such mutations on pathogenesis and transmission of EBOV81,82.
Host–pathogen determinants of outcome
Elucidation of the mechanistic determinants of the outcome of host–filovirus interaction has historically been challenging. In humans, outcome could only be correlated with very limited clinical data, providing only low-resolution associations. In proxy animal disease models, the homogeneity of highly stringent uniformly lethal models prevents the identification of any host-specific or filovirus-specific variability, much less mechanistic determinants. Ongoing analyses of samples acquired during the 2013–2016 Western African outbreak are using a systems approach to understanding the consequences to the host81,82, although these studies are at an early stage. Finally, although in vitro systems have provided valuable information regarding molecular pathogenesis, a complex variety of viruses, host cells and experimental conditions have been used. Accordingly, a single, unified picture of the host–filovirus interaction does not exist. Instead, from a ‘patchwork’ compilation of different and complex observations, key aspects of the disease in humans remain unknown.
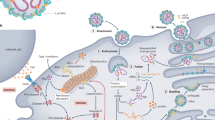
EBOV tissue and cell tropism are primarily determined by the EBOV glycoprotein GP1,2, GP1,2 attachment factors on the host cell surface and the intracellular binding of GP1,2 to the NPC intracellular cholesterol transporter 1 (NPC1, also known as Niemann–Pick C1 protein) receptor83,84 (Fig. 4). Most human cells can become infected, but mononuclear phagocytes (for example, Kupffer cells in the liver, macrophages and microglia) and dendritic cells are primary EBOV targets74,85,86,87,88,89,90,91. As the primary target cells become infected, they probably facilitate further virus dissemination86 and migrate to the regional lymph nodes and to the liver and spleen88. In vitro, infected macrophages are activated by binding to EBOV GP1,2 (ref.92) to secrete pro-inflammatory cytokines, in particular interleukins IL-1β, IL-6 and IL-8, and tumour necrosis factor (TNF). These secretions probably result in the recruitment of additional EBOV-susceptible macrophages to the site of infection and, ultimately, the breakdown of endothelial barriers. In NHP models, this breakdown frequently causes third spacing (that is, excess movement of intravascular fluid into interstitial spaces), leading to oedema and hypovolaemic shock. Although described, this manifestation is less well characterized in human patients91,93,94,95. In vitro, dendritic cells react to EBOV infection with partial suppression of major histocompatibility complex class II responses, expression of tissue factor and TNF ligand superfamily member 10 (TNFSF10), increased production of chemokines (for instance, C-C motif chemokine 2 (CCL2), CCL3, CCL4 and IL-8) and suppressed secretion of pro-inflammatory cytokines96,97,98,99,100. Together with possible abortive infection101, the aberrant cytokine responses and TNFSF10 expression are probably key to the extensive lymphocyte death. Such lymphocyte depletion possibly contributes to the susceptibility of patients with EVD to acquiring secondary infections)88,102, hypotension, disseminated intravascular coagulation, and ultimately multiple organ dysfunction syndrome that is typical of EVD8,102,103,104 (Fig. 5).
a | Ebola virus (EBOV) has a linear, non-segmented, negative-sense, single-stranded RNA genome (~19 kb) expressing seven structural proteins and several non-structural proteins from seven genes: NP encodes nucleoprotein NP, VP35 polymerase cofactor VP35, VP40 matrix protein VP40, GP glycoprotein GP1,2 and secreted glycoproteins (not shown), VP30 transcriptional activator VP30, VP24 RNA complex-associated protein VP24, and L large protein L1,281. b | The binding of EBOV particles to the attachment factors on the host cell surface is mediated by the homotrimeric structural glycoprotein GP1,2, which is formed of three heterodimers consisting of subunits GP1 and GP2 that are connected by a disulfide bond (1). Binding to the host cell membrane triggers viral particle endocytosis (2). In the late endosome, GP1,2 is sequentially cleaved by cathepsin B (CatB) and cathepsin L (CatL) (3) to expose the receptor-binding site of the GP1 subunit. A low pH induces GP1 interaction with the EBOV receptor NPC1, with subsequent GP2-mediated fusion of the particle envelope with the endosomal membrane and thereby expulsion of the ribonucleoprotein complex (predominantly RNA + NP) into the cytosol (4). There, the filovirus genome is replicated (5) and the filovirus genes are transcribed into mRNAs (6). Viral proteins are translated in the cytosol or, in the case of GP1,2, into the endoplasmic reticulum (ER) (7). Mature progeny ribonucleoprotein complexes and viral proteins are transported to the plasma membrane, where particle budding occurs (8). NPC1, NPC intracellular cholesterol transporter 1; ORF, open reading frame. Part a courtesy of J. Wada, NIH/NIAID Integrated Research Facility at Fort Detrick, Frederick, MD, USA. Part b adapted from ref.283, Springer Nature Limited.
Ebola virus particles enter the body through dermal injuries (microscopic or macroscopic wounds) or via direct contact via mucosal membranes. Primary targets of infection are macrophages and dendritic cells. Infected macrophages and dendritic cells migrate to regional lymph nodes while producing progeny virions. Through suppression of intrinsic, innate and adaptive immune responses, systemic distribution of progeny virions and infection of secondary target cells occur in almost all organs. Key organ-specific interactions occur in the gastrointestinal tract, liver and spleen, with corresponding markers of organ injury or dysfunction that correlate with human disease outcome. The question marks indicate speculated manifestations. RIG-I, antiviral innate immune response receptor RIG-I.
Immune responses
Although considerable progress has been made towards understanding the immune response to EBOV infection at the cellular level using in vitro testing, data are limited regarding the systemic immune response in humans following infection. Prior to the 2013–2016 Western African EVD outbreak, which was of such a scale and duration to enable study, opportunities were lacking to conduct thorough and simultaneous immunological analyses of the human host response to EBOV infection.
EBOV inhibits induction of intrinsic (cell-based antiviral defence mechanisms via proteins that are constitutively expressed and target specific viruses) and innate (cell-based antiviral defence mechanisms via proteins that are induced by infection and rely on pattern recognition receptors) host immune responses105,106. This inhibition permits efficient virus replication in host cells, thereby accelerating viral spread. To this end, the virus invests a substantial amount of its genome coding capacity. Perhaps the best studied inhibitor is the EBOV polymerase cofactor VP35, which is also a type I interferon (IFN) antagonist. EBOV VP35 suppresses production of type I IFN (by impairing IRF-3 phosphorylation) through its ability to bind double-stranded RNA and through direct interactions with the host proteins TBK-1, IKKε and PACT (refs107,108,109,110,111,112,113,114,115). In addition, VP35 suppresses micro-RNA silencing (an important post-translational regulatory pathway) in the host cell116, and GP1,2 antagonizes a cellular antiviral restriction factor, BST-2 (ref.117). A second EBOV-encoded protein, RNA complex-associated protein VP24, also inhibits the antiviral response by preventing the nuclear accumulation of phosphorylated signal transducer and activator of transcription 1α/β (STAT1), which is induced by type I IFN and acts as a transcription factor to increase expression of antiviral proteins118,119. Finally, EBOV VP40 is incorporated into exosomes that seem to have the potential to disrupt or kill host immune cells120,121.
Recent studies have confirmed that although EBOV has been considered immunosuppressive, EBOV-specific cellular and humoral immune responses develop but are often outpaced in the host–pathogen EVD ‘arms race’122,123, in which timing seems crucial. This host–pathogen competition might also be applied to vaccine-mediated mechanisms of protection. A vaccinated individual is assumed to be protected once outside the window for a vaccine-induced mounting of a humoral response (for example, ~10 days for the vesicular stomatitis Indiana virus-based vaccine rVSVΔG-ZEBOV-GP as defined by the Ebola ça Suffit! ring vaccination trial)124,125. Ongoing vaccine and clinical research efforts in the Democratic Republic of the Congo will help to clarify the relationship between timing of vaccine receipt with susceptibility to infection. In addition, in vaccinated individuals who then become infected, further research will clarify the relationship between previous vaccination and subsequent EVD severity or outcome. Indeed, in vaccinated individuals who then develop EVD, receipt of the rVSVΔG-ZEBOV-GP vaccine is independently associated with a decreased risk of dying126. Research on human immune responses to EBOV infection has focused largely on the detection of biomarkers of inflammation (such as CCL2, IL-8 and IL-6), endothelial dysfunction (such as selectin P), coagulation (such as d-dimer, tissue factor and von Willebrand factor) and lymphocyte function (such as CXCL3 and granzyme B)127,128; the expression of host RNA transcripts from peripheral blood mononuclear cells; and the appearance of EBOV-specific humoral and cellular immune responses.
Data from both patients with EVD treated in the USA and Western African cohorts suggest that robust adaptive immune activation, which includes antigen-specific T cell and B cell responses, occurs during acute illness123. Efforts are ongoing to define the characteristics of effective and ineffective B cell and T cell responses during acute infection and over time129,130. These limited results suggest a ‘race’ between EBOV proliferation and the ability of the human host to mount an effective and regulated anti-EBOV immune response. No study in humans has been able to measure inoculation dose and its relationship with disease severity. Interrogation of immune responses in four patients with EVD treated in the USA also revealed a second peak and persistence of T cell activation during convalescence123, which might implicate persistence of EBOV or EBOV antigen in tissue compartments (immune-privileged sites) after viral nucleic acid is no longer detected in the blood. Indeed, EBOV RNA has frequently been detected in semen from male survivors of EVD and from cerebrospinal and intraocular fluids from two convalescent patients long after blood samples tested negative for EBOV131,132,133,134.
Diagnosis, screening and prevention
Clinical manifestations of acute EVD
Given the difficulties in identifying the exposure source and route of infection in most patients, data that confidently determine the time from exposure to symptom onset are sparse. The most convincing data come from situations characterized by a single definitive exposure event. An analysis of all published human exposure data determined that the mean incubation period of EVD is 6.22 ± 1.57 days for all routes of exposure, 5.85 ± 1.42 days for percutaneous exposure and 7.34 ± 1.35 days for person-to-person contact or contact with infected animals135. Rarely, asymptomatic or pauci-symptomatic infection of individuals without known clinical manifestations of EVD has been described136,137. A study of household contacts of survivors discharged from an ETU in Sierra Leone revealed that although 47.6% of contacts (229 of 481 contacts) had high-level exposures (direct contact with a corpse, bodily fluids or a patient with bleeding, diarrhoea or vomiting), an assay detecting anti-EBOV GP IgG from oral fluid samples tested positive only in 12.0% of contacts who reported having symptoms at the same time as household members who had EVD (11 of 92 contacts) and in 2.6% of contacts who reported having no symptoms (10 of 388 contacts)138. In general, patients with EVD have a predictable clinical course (Fig. 6). During early infection (days 1–3 following disease onset), patients present with a non-specific febrile illness (symptoms may include anorexia, arthralgia, headache, malaise, myalgia and rash) that progresses in the first week to severe gastrointestinal symptoms and signs (nausea, vomiting and high-volume diarrhoea). During the 2013–2016 Western African EVD outbreak, fatigue, anorexia, abdominal pain, diarrhoea, vomiting, fever and myalgia were among the most common clinical manifestations139,140,141,142,143,144.
The time course of the clinical manifestations (top), laboratory findings (middle) and viraemia and immune responses (bottom) in patients with Ebola virus disease (EVD). The coloured lines in the top and middle panels do not have defined start and end points as these may vary. Renal dysfunction is common and not well-characterized in patients with EVD; it is probably a multifactorial combination of hypovolaemia (related to gastrointestinal fluid losses, decreased fluid input, fever, hypoalbuminaemia and sepsis pathophysiology), intrinsic renal injury (acute tubular necrosis related to myoglobin pigment injury secondary to rhabdomyolysis or direct viral infection of tubular epithelial cells) or cytokine-mediated nephrotoxicity. Whereas respiratory symptoms and signs may reflect respiratory compensation for a primary metabolic acidosis, primary causes of hypoxaemic respiratory failure include acute lung injury (related to systemic inflammatory response syndrome and/or sepsis or Ebola virus (EBOV)-related cytokinaemia), pulmonary oedema (in the setting of capillary leak or direct infection) and viral pneumonia. Respiratory muscle fatigue may also contribute to ventilatory respiratory failure. Haemorrhagic manifestations include oozing from venepuncture sites, haemoptysis (coughing up blood), haematemesis (vomiting blood), melaena (dark stools as a result of bleeding) and vaginal bleeding. Neurological manifestations include meningoencephalitis and cerebrovascular accidents (such as strokes). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CPK, creatine phosphokinase; Hb, haemoglobin; HCT, haematocrit; PLT, platelet; PMN, polymorphonuclear leukocyte; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white blood cell count. aIncubation periods of 2–21 days have been reported. Bottom panel adapted with permission from ref.284, The American Association of Immunologists, Inc.
As the EBOV load increases, typically the severity of EVD clinical manifestations increases as well. The onset of detectable viraemia and manifestations of clinical signs and symptoms in most patients occurs 6–10 days after exposure. Later in the first week of illness following disease onset, patients may have persistent fever and increased gastrointestinal fluid losses and hypotension from dehydration and, to a minor extent, vascular leakage. Rhabdomyolysis (the breakdown of muscle leading to the release of the contents of dead myofibres into the circulation) has also been observed145. Although EVD is still often referred to as a ‘viral haemorrhagic fever’, this term is discouraged2 because not all patients have overt bleeding manifestations and fever is not always present146,147. However, with observations of early consumptive coagulopathy followed by hypercoagulability in the recovery period, haematological abnormalities may be more common and complex than previously understood127,148. During the terminal phase (days 7–12 following disease onset), tissue hypoperfusion and vascular leakage, often in conjunction with dysregulated inflammation, lead to multiple organ dysfunction syndrome and/or damage, including acute kidney injury. Kidney injury is evidenced by oliguria or anuria and abnormalities in electrolytes including potassium and sodium. A subset of patients develop central nervous system manifestations and encephalopathy. Although several underlying causes could be involved, EBOV RNA has been detected in the cerebrospinal fluid of patients with EVD, suggesting that meningoencephalitis may be directly mediated by the virus149,150.
The clinical timeline and manifestations may be altered in children; although the incidence of EVD among children was lower than in adults across the three affected countries during the 2013‒2016 Western African EVD outbreak48, data regarding paediatric EVD suggest that the incubation period is shorter and the CFR is higher in younger children (<5 years of age) than in older children. Additionally, children were more likely than adults to clinically present with fever and less likely to report abdominal pain, arthritis, myalgia, dyspnoea (difficult breathing) and hiccups in the early stage of disease.
In humans, no EVD outbreak including more than a single case has ever resulted in a 100% CFR4. However, the clinical correlates of outcome following EBOV infection have been difficult to discern owing to challenges in data collection, clinical follow-up and limited laboratory services. Data from the 2013–2016 Western African EVD outbreak show that viral load or the RT quantitative PCR (RT-qPCR) cycle threshold value (which is a proxy for the viral load), age, and signs of organ dysfunction, in this order, most reliably predict outcome. The cycle threshold value refers to the number of cycles of PCR amplification required before viral RNA is detectable above a background threshold. The cycle threshold cannot be universally related to a corresponding number of viral genome copies per millilitre of blood, as it depends on the specific experimental conditions. However, a low value means that viral DNA can be detected in a short period of time, which suggests a high viral load, whereas a high value is associated with a low viral load. For instance, data from an ETU in Sierra Leone suggested a poor prognosis in patients admitted with viral loads >10 million genome copies per millilitre of blood151. The mean initial viral load at this ETU decreased over the course of the outbreak, as did the CFR, although such decreases occurred in the setting of numerous potential explanatory factors27. Other risk factors that have been sporadically linked with a fatal outcome include age ≥45 years, fever >38 °C, weakness, dizziness, diarrhoea, conjunctivitis, difficulty breathing or swallowing, confusion or disorientation, coma, haemorrhagic signs and laboratory evidence of hepatocellular damage (for example, increased concentration of blood aspartate aminotransferase AST)) and impaired kidney function (for example, increased concentrations of blood urea nitrogen and creatinine)45,152,153,154. These risk factors are an aggregate list from several distinct cohorts during the same outbreak; however, different associations were found in different cohorts, partially because the same factors were not measured across all cohorts. An increase in overall lethality has also been observed in patients co-infected with Plasmodium falciparum (the causative agent of falciparum malaria) and potentially plasmodia of other species155,156.
Diagnosis of acute EVD
In tropical areas, where numerous febrile illnesses can mimic the presentation of EVD, testing for or empirically treating parasitic (for example, Plasmodium spp.), viral (for example, Lassa virus) and bacterial (for example, Salmonella Typhi) diseases is an important consideration156,157. Given the frequency of co-infection with Plasmodium spp., the aetiological agents of malaria, all patients should receive malaria rapid diagnostic testing or be treated empirically for uncomplicated or severe malaria.
Appropriate isolation of patients with laboratory-confirmed EVD not only requires optimization of a front-line clinician’s ability to rapidly identify a patient with disease that fits the EVD case definition (see Epidemiology) but also necessitates that an EVD diagnosis is accurately confirmed with readily available laboratory tests. Until recently, field diagnosis of EVD during an outbreak has relied primarily on real-time RT-PCR assays. Although PCR assays are accurate, factors such as cost, time to processing (including sample transport time), availability and required level of operator expertise contributed to delays in provision of rapid results during the 2013–2016 Western African EVD outbreak. Improving the time to diagnosis, which includes measures to identify patients prior to the onset of symptoms, may have a major effect on transmission dynamics during an outbreak. One simulation estimated that by decreasing the average time to diagnosis with PCR and subsequent patient isolation from 5 days to 1 day in 60% of EBOV-infected patients, the virus attack rate (the proportion of people at risk of the infection who become infected) would drop from 80% to nearly 0% (ref.158). Consequently, in November 2014, the WHO issued a call for “rapid, sensitive, safe and simple EBOV diagnostic tests”159.
Since then, several diagnostic tests, which range from hand-held lateral flow assays to bench-top PCR-based technologies, have been developed, some of which have been evaluated and used in the field (Table 2). A mathematical model was used to evaluate the effect on EVD CFR and EBOV transmission dynamics of incorporating different diagnostic strategies that did or did not include the introduction of novel rapid diagnostic tests (RDTs) in various scenarios160. A strategy that coupled novel RDTs with confirmatory PCR testing was deemed superior to the use of either PCR assays or RDTs alone and would result in a reduction of the scale of an outbreak by one-third. Whether coupling RDTs with PCR influences false-positive and false-negative designations remains to be determined. Notably, in this model, the performance characteristics of RDTs were assumed to be inferior to those of PCR assays for accurately diagnosing EVD. However, with improvement in performance, RDTs alone may replace PCR assays and alter transmission dynamics during future outbreaks. Accordingly, front-line health-care workers require training on using RDTs and reading results accurately while dressed in personal protective equipment (PPE). Until RDTs for EBOV infection are advanced with appropriate validation in their intended application setting, generally they should not be used outside known outbreak settings with low pre-test probability, owing to the increased risk of false-positive tests.
Importantly, disease severity, disease acuity and sample material need to be taken into consideration before choosing a particular diagnostic test. In general, blood is the sample material of choice for live patients, whereas oropharyngeal swabs are useful for post-mortem diagnosis. The diagnostic test of choice in the acutely ill individual with suspected EVD is a PCR-based assay of a blood sample targeting one or more of the EBOV genes. In the context of the ongoing outbreak in the Democratic Republic of the Congo, diagnosis relies on the Cepheid Gene Xpert platform targeting EBOV GP and EBOV NP (Table 2). With widespread use of the same platform in multiple field laboratories in the Democratic Republic of the Congo comparison is easier across patients and outbreak samples. Regardless of the platform, a general principle is that a diagnostic strategy targeting only one EBOV gene needs to be repeated for confirmation, whereas strategies utilizing two targets do not require repeating. Asymptomatic or pauci-symptomatic EBOV-infected people may not have viraemia titres detectable by PCR assays, but typically have detectable IgG and IgM responses ~3 weeks after infection. Appropriate serological testing to confirm the presence of anti-EBOV IgG antibodies would be indicated in this setting. Of note, antibody responses may not reliably develop or may be delayed in acutely symptomatic patients with EVD. Thus, PCR-based testing is optimal in the acutely ill patient (from blood samples) and also for detection of EBOV RNA in amniotic fluid, breast milk, ocular fluid, saliva, seminal fluid, stool, sweat, tears, urine and vaginal fluid even after blood samples begin to test negative36,40,57,134,161,162.
Prevention
The overall strategy for mitigating the spread of an ongoing EVD outbreak is to interrupt community and nosocomial transmission of EBOV from patients to susceptible individuals. Effectively achieving this outcome depends upon the quality of measures in place; ideally, interruption of the chain of transmission in the community can be achieved by anthropological and sociological measures (Box 1); isolating individuals with suspected, probable or confirmed EVD for care (which includes contact tracing and following-up over 21 days); and treatment in an ETU or holding centre. The crucial importance of contact tracing is illustrated by the backdrop of the current EVD outbreak in the Democratic Republic of the Congo, where a longstanding conflict has impeded maximal tracing of contacts of patients with EVD, and violent incursions in outbreak areas are associated with increases in estimated EBOV transmission rates163. In a mathematical model estimating changes in EBOV transmission in 12 districts in Sierra Leone from June 2014 to February 2015, introduction of additional treatment beds within the area to isolate patients with suspected or confirmed EVD would have theoretically averted ~56,000 new EVD cases164. The risk of nosocomial transmission can be reduced by isolation of patients with suspected, probable or confirmed EVD, the use of appropriate PPE, strategies for donning and doffing PPE and strict adherence to infection prevention and control practices. Such practices include the provision of dedicated or disposable patient care equipment, safe injection practices, hand hygiene and attention to environmental infection control.
The provision of guidelines for discharge criteria is an important aspect of clinical care to avoid subsequent transmission events in the community. During the 2013–2016 Western African EVD outbreak, the WHO recommended that patients diagnosed with EVD can be considered for discharge from health-care facilities if ≥3 days have elapsed since resolution of clinical signs, if they show appreciable improvement in clinical condition, if they are able to perform activities of daily living and if a blood sample is negative for EBOV RNA (detected with RT-PCR tests) from the third day of the patient becoming asymptomatic. Patients with unresolved signs and symptoms should be discharged after two negative blood test results (48 h apart), and in these patients an alternative diagnosis should be sought that may explain the lack of clinical improvement165. Other health authorities (such as the US Centers for Disease Control and Prevention) have created recommendations for the discharge of patients under investigation. In most patients with EVD managed in the USA and Europe during the 2013‒2016 Western African outbreak, repeatedly negative RT-PCR tests of blood samples was the primary criterion used for discharge, along with symptomatic improvement. However, in several centres in the USA and Europe, other criteria were used, including RT-PCR tests of samples of other bodily fluids and EBOV cell culture under biosafety level 4 containment166.
The time-limited detection of infectious EBOV (by PCR and viral culture) and long-term detection of EBOV RNA in the semen of male survivors were only possible in large numbers in the 2013–2016 Western African EVD outbreak. Rare but consequential sexual transmission events have also been documented37,41,66,131. Accordingly, WHO recommendations167 (currently being updated) for the prevention of sexual transmission from survivors include routine PCR testing of semen beginning at 3 months after health-care facility discharge (as the semen should be assumed to be infectious for the first 3 months) and until two consecutive semen samples taken at least 1 week apart are negative. Abstinence or safe sexual practices should be implemented for the same period or for at least 1 year.
Candidate vaccines
Amid increasing concerns about unmitigated transmission during the 2013–2016 Western African EVD outbreak in mid-2014, a statement from a stakeholder meetings held by the WHO urged acceleration of the development and evaluation of EVD candidate vaccines. As the EBOV glycoprotein GP1,2 is the major viral immunogen, all candidate vaccines in advanced development are designed to stimulate a host immune response against this protein, among others. In the Western African outbreak, several candidate vaccines were evaluated in clinical trials168,169 (Table 3). Owing to the success of the Ebola ça Suffit! phase III ring vaccination trial in Guinea124,125, the rVSVΔG-ZEBOV-GP, a live-attenuated recombinant vesiculovirus candidate vaccine currently approved by the US Food and Drug Administration and the European Commission and is actively administered to help contain the currently ongoing EVD epidemic that started in Nord-Kivu Province of the Democratic Republic of the Congo in 2018. Using a ring vaccination strategy, whereby contacts of infected individuals (primary ring) and contacts of those contacts (secondary ring) are vaccinated, this candidate vaccine has been administered to 276,520 people in the eastern Democratic Republic of the Congo as of 26 January 2020 (ref.22). Preliminary analyses on data evaluating the first 93,965 vaccinated individuals revealed a lower estimated attack rate among individuals who were vaccinated (0.017%) than in unvaccinated individuals (0.656%)170. The WHO reported an estimated vaccine efficacy of 97.5% (95% CI 95.8–98.5%)170. However, determination of true vaccine efficacy is impossible in the absence of a placebo-controlled group. Notably, a model of the EBOV infection risk during the 2018 EVD outbreak in Équateur Province in the Democratic Republic of the Congo found that the introduction of ring vaccination with rVSVΔG-ZEBOV-GP vaccine resulted in a decrease of 70.4% of the geographical area of risk and 70.1% of the level of EBOV infection risk. However, if ring vaccination is delayed by as little as 1 week, the size of this effect is considerably diminished171. The same candidate vaccine is also used in the ongoing outbreak in the Democratic Republic of the Congo as emergency post-EBOV exposure prophylaxis in, for instance, health-care workers.
Whereas the data from the ring vaccination trials are promising, equivalent data are not yet available regarding the efficacy of a pre-exposure vaccination strategy. This strategy would be desirable particularly for informing local or international health-care workers regarding the level of protection after vaccination prior to working in settings where the risk of EBOV exposure is high. In May 2019, the WHO-convened Strategic Advisory Group of Experts (SAGE) issued recommendations regarding vaccination strategies that included the use of a second vaccine. In August 2019, a clinical trial evaluating the safety and immunogenicity of the rAd26 ZEBOV-GP–MVA-BN-Filo vaccine among health-care workers was initiated in Uganda172. In light of the risk of delayed ring vaccination with the rVSVΔG-ZEBOV-GP vaccine owing to ongoing violence, a pre-exposure vaccination strategy using the rAd26 ZEBOV-GP–MVA-BN-Filo vaccine was also introduced in Goma in the Democratic Republic of the Congo on 14 November 2019. This pre-exposure vaccine complements the ring vaccination efforts with the rVSVΔG-ZEBOV-GP vaccine currently underway. As of 10 December 2019, 1,300 people have been vaccinated with this second vaccine22.
No data are currently available to support long-term clinical protection in humans for any vaccine. In the ongoing Geneva rVSVΔG-ZEBOV-GP study, antibody titres are still present after 2 years, but whether these titres are protective is unknown173.
In October 2019, the European Medicines Agency granted the rVSVΔG-ZEBOV-GP candidate vaccine conditional marketing authorization169; soon afterwards in November and December 2019, the European Commission and US Food and Drug Administration announced approval for the same vaccine for prevention of EVD174. With full approval from the European Commission, the vaccine is cleared for use in the countries that are part of the European Union. As of December 2019, candidate vaccines have also been licensed in China and Russia168. These advances will undoubtedly facilitate production, stockpiling and wider distribution of vaccines to health-care workers and other at-risk individuals.
Management
Direct medical countermeasures
Although no national or regional regulatory bodies have yet approved the use of any medicine for the treatment of EVD, many experimental therapeutic agents have been evaluated in animal models. During the 2013–2016 Western African EVD outbreak, these agents were administered in an uncontrolled fashion to individual patients (for example, FX06, a fibrin-derived peptide for the treatment of vascular leakage175), usually through Emergency Use Authorization (a temporary authorization to use unapproved medications in public health emergencies), and, therefore, no scientifically valid conclusions could be drawn as to their efficacy. In addition to these uncoordinated efforts, several non-randomized clinical trials and one randomized controlled trial were performed during the same outbreak176.
Results from a single-arm trial conducted in Guinea evaluating the viral RNA polymerase inhibitor favipiravir did not allow efficacy conclusions to be drawn. However, a non-significant trend towards improvement in CFR was observed in patients with a low viral load (EBOV RT-qPCR threshold values ≥20 cycles) treated with favipiravir compared with historical controls177. Similarly, results from another single-arm trial conducted in Sierra Leone with TKM-130803, a formulation of small interfering RNAs that target the expression of EBOV proteins (namely, VP35 and large protein L) involved in suppression of the host’s immune system, did not demonstrate improvement in survival compared with historical controls178. Overall, the difficulty in comparing data using historical or other non-randomized control groups has been illustrated in a meta-analysis of trials conducted during the Western African outbreak, strongly emphasizing the need for randomized control groups28. A randomized controlled trial was conducted in Guinea, Liberia, Sierra Leone and the USA to evaluate the safety and efficacy of ZMapp, a cocktail of three monoclonal antibodies, added to optimized standard of care (oSOC) versus oSOC alone in the treatment of EVD. The trial was stopped early after enrolling only 71 patients because the numbers of incident cases had declined at that stage of the 2013–2016 Western African EVD outbreak. A trend towards improved survival was found in the oSOC plus ZMapp arm compared with the oSOC only arm (22.2% and 37.1%, respectively); however, a posterior probability of 91.2% failed to meet the predefined criteria for statistical significance179.
In early 2018, the WHO led a panel of experts to evaluate the latest (human and animal) efficacy data on available therapeutics to inform the Monitored Emergency Use of Unregistered Investigational Interventions (MEURI), an ethical framework to guide compassionate access to investigational therapeutics during an EVD outbreak and as a bridge to a clinical trial180. The MEURI framework was implemented during the ongoing EVD outbreak in the Democratic Republic of the Congo, whereby almost all patients admitted to ETUs since mid-August 2018 received ZMapp181,182, mAb114183,184, REGN-EB3185,186 or remdesivir187 (Table 4) either under MEURI or the Pamoja Tulinde Maisha (PALM; Swahili for “together save lives”) randomized controlled trial. The PALM study, a randomized controlled phase II/III trial evaluating the efficacy of these four candidate therapeutics, was started in eastern Democratic Republic of the Congo on 20 November 2018 (ref.180). In four trial sites in Nord-Kivu Province, patients receiving ongoing optimized supportive care were randomly assigned in a 1:1:1:1 ratio to intravenous administration of ZMapp (the control arm), the antiviral remdesivir, the monoclonal antibody mAb114 (derived from a Congolese human survivor of the 1995 Kikwit EVD outbreak) or REGN-EB3 (a cocktail of three murine-derived but fully human monoclonal antibodies) and evaluated for a primary end point of day 28 lethality. The secondary end point aimed to assess the efficacy as time to first negative test for the presence of EBOV. In August 2019, the Data Safety Monitoring Board for the PALM study recommended early halting of the trial because an interim analysis of 681 patients enrolled in the trial showed that individuals randomly assigned to either the REGN-EB3 or mAb114 arms had a higher probability of survival than individuals randomized to the ZMapp or remdesivir arms188. No difference was observed in day 28 lethality between the remdesivir group and the ZMapp group. Notably, higher cycle threshold on RT-qPCR, shorter self-reported duration of symptoms before admission and lower serum markers of renal (namely, creatinine) and hepatic (aminotransferases concentrations) function were correlated with improved survival126. Of note, the CFR of patients enrolled in the PALM trial who presented with high viral loads and late into the disease course remained at >60% even with the most effective therapeutics126. The interaction between therapeutic efficacy and EBOV viral load, viral decay kinetics and the degree of organ dysfunction is probably complex. Data from this outbreak should further inform these historically poorly understood relationships.
The transfusion of whole blood, plasma or serum from convalescent individuals (passive immunization therapy) has also been considered as a therapeutic intervention against EVD189. However, results from one study performed in Guinea in 2015 did not demonstrate a significant improvement in survival in patients who had received plasma from convalescent survivors190. In a continuation of the same study, titres of anti-EBOV IgG and other neutralizing antibodies in plasma from convalescent survivors were determined. Higher doses of anti-EBOV IgG antibodies were associated with increases in cycle threshold values following infusion compared with lower doses, but no significant difference in lethality was observed191. A case of acute respiratory distress was reported in a repatriated individual, potentially an adverse event associated with administration of plasma from convalescent individuals192. As the availability and initial effectiveness of monoclonal antibody-based therapeutic strategies in ongoing research studies in the Democratic Republic of the Congo increase, it is unlikely that polyclonal passive immunization strategies will continue to be pursued.
Supportive care for acute EVD
Aggressive supportive care includes appropriate intravenous fluid replacement with crystalloid fluids and perhaps vasopressors, to prevent patients with EVD from developing hypovolaemic shock from profound intravascular volume depletion and/or septic shock that may include vascular leak syndromes165,193. In the early stages of disease when the patient is ambulatory and able to eat and drink without nausea and excessive vomiting, oral rehydration solutions can be administered to replace gastrointestinal and insensible fluid losses (insensible water loss is attributed to evaporation from the skin and respiratory tract). Establishing early on intravenous access is crucial for administration of balanced crystalloid solutions (for example, Ringer’s lactate) as the patient’s condition worsens and nausea, vomiting, asthenia (weakness), and malaise and lassitude (lethargy) make adequate oral fluid intake impossible. In the 2013–2016 Western African EVD outbreak, central venous catheters provided better fluid optimization than peripherally inserted catheters194. However, in resource-limited settings, establishing a peripheral intravenous access is often more logistically feasible than establishing access centrally.
As illness progresses to more severe stages (peak phase), increased gastrointestinal fluid losses (secretory phase) predominate, and patients with EVD may produce large amounts of emesis and stool. Stool volumes of 5–10 l per day have been reported, leading to heavy fluid and electrolyte losses195. Managing patients’ bodily fluids is also an important infection control consideration in the health-care environment and can be accomplished with physical and pharmacological controls. Anti-emetic medications (for example, metoclopramide and ondansetron) have been used to control nausea and vomiting. Also, anti-diarrhoeal agents (for example, loperamide) have been used to reduce the frequency of diarrhoea. With potential adverse events such as intestinal ileus (that is, intestinal paralysis that can lead to obstruction), the risk–benefit ratio of using loperamide for inflammatory diarrhoea associated with EVD is uncertain196. Examples of physical controls used in the hospital include emesis bags, bedside commodes and faecal management systems (temporary containment devices composed of a rectal catheter and a collection bag) for non-ambulatory patients.
Although primary EVD-attributable respiratory disease is uncommon, patients who have respiratory signs (such as dyspnoea) and hypoxia may require conservative treatment with supplemental oxygen, particularly those with pulmonary oedema as an iatrogenic effect of aggressive fluid replacement. Haemorrhagic complications can be treated with blood products when available, but clinicians should be aware of potential hypo-coagulable and hyper-coagulable states. Severe neurological manifestations, including meningitis, encephalitis, seizures and coma, have been reported in patients with acute EVD197. Complex causes of encephalopathy include altered vital signs (hypoxemia and hypotension), metabolic dysfunction (hypoglycemia and electrolyte derangement), organ dysfunction (uraemia and hepatic encephalopathy) and also central nervous system dysfunction related directly to EBOV meningoencephalitis or to indirect micro-vascular or macrovascular infarction198. Although poorly understood, viral encephalitis or encephalopathy has been circumstantially implicated in a patient with seizures after recovery from EVD and MRI findings of encephalomalacia (softening or loss of brain tissue from injury to the brain including infarction or ischaemia) and haemorrhagic encephalitis (I.C., unpublished observations). In Guinea, EBOV RNA has been detected in the cerebrospinal fluid of four patients with clinical signs of meningoencephalitis during acute illness149,150. Delirium or agitation can be a challenging feature of EVD. Benzodiazepines or other available sedating medications may be needed to keep patients from harming themselves, other patients or health-care providers. Fever and pain may be treated by acetaminophen.
Critical care
Patients with EVD who progress to critical illness, including multiple organ dysfunction syndrome, may require advanced life support modalities. However, given the resource constraints typical of most outbreak areas, the capacity to deliver critical care is limited by gaps at the personnel, equipment and facility levels. The knowledge of modern critical care of patients with EVD stems from the care of several patients who acquired EBOV infection in Western Africa but were managed in the USA and Europe140,175,199,200,201,202, and limited experience in an ETU in Sierra Leone that was equipped with intensive care unit (ICU) capabilities203. Substantial pre-planning was necessary to provide treatment for critically ill patients, to ensure the availability of physicians with experience in airway management and dialysis, for example, and the necessary equipment and appropriate PPE for potentially aerosol-generating procedures. Invasive procedures also place health-care workers at risk of transmission of blood-borne pathogens, including EBOV.
In patients treated in the USA and Europe and those in the ICU-equipped ETU in Sierra Leone, intubation was accomplished via rapid sequence induction using neuromuscular blockade, followed by video laryngoscopy to provide direct visualization of the airway while reducing the likelihood of aerosol or bodily fluid exposure via coughing or vomiting175,201. Validation of correct endotracheal tube placement was often difficult, as some centres did not have the ability to auscultate the lungs or monitor end-tidal CO2 concentrations175. In patients with EVD who developed acute kidney injury, continuous renal replacement therapy (CRRT) was performed199,204. CRRT was chosen over intermittent haemodialysis to decrease the frequency of exposure to blood and bodily fluids. Frequent laboratory monitoring, including electrolytes, was performed while patients remained on CRRT, and regional citrate anticoagulation204. Regional citrate anticoagulation is achieved by administering citrate at the proximal portion of the RRT circuit. Citrate reduces the blood concentration of calcium, a cofactor required to activate the coagulation cascade, and, therefore, prolongs the life of the RRT circuit. The safe and effective provision of CRRT in an isolation setting posed many challenges, including the need to minimize contact with blood and bodily fluids, the generation of effluent waste, the training of health-care workers to operate equipment and terminal cleaning of devices. Effluent waste was found to be negative for EBOV by RT-PCR on three separate occasions at one centre, probably owing to the inability of EBOV particles to cross the dialyzer membrane. However, as the effluent waste was found to be positive for EBOV by RT-PCR in one of three samples at another centre, the CDC and some clinicians recommend that effluent waste be generally handled as potentially contaminated175,205.
Conflicting ethical arguments exist regarding the utility of advanced cardiac life support measures and cardiopulmonary resuscitation in patients with EVD206,207,208. Ultimately, cardiopulmonary resuscitation and other interventions, including defibrillation and cardioversion, should be assessed on an individual case-by-case basis. A careful risk–benefit assessment should be performed prior to all critical care interventions to provide optimal care for the patient while reducing the risk of health-care worker exposure. Of note, the CFR of 27 patients with EVD managed with aggressive supportive care measures in Europe and the US was only 18.5% compared with the overall mean CFR of 39.5% in Western Africa, suggesting that availability of aggressive interventions may have a substantial effect on CFR during an EVD outbreak199. However, substantial heterogeneity in reported CFRs was noted in the 2013–2016 Western African EVD outbreak due to a number of variables209. In the ongoing outbreak in the Democratic Republic of the Congo, substantial efforts have been made to improve and optimize the supportive care provided to patients with EVD in an African setting. These efforts have often occurred in innovatively designed ETU spaces that enable a higher degree of monitoring and care, and have been catalysed by newly developed protocols to support care delivery210.
Monitoring
Profound gastrointestinal fluid losses and concomitant kidney injury require timely monitoring, replacement of electrolytes and restoration of the acid–base balance to prevent potentially lethal arrhythmias and fluid shifts152,211,212. Life-threatening hypoglycaemia has been observed. Also, liver injury is very common during EVD and is associated with a disproportionate increase in AST concentrations over alanine aminotransferase (ALT) concentrations. Although mild aberrations in liver function tests (including bilirubin and international normalized ratio) have been reported, these alterations are less common211. The performance of clinical laboratory testing varies according to the resources available, and a risk assessment should be performed to ensure the safety of the laboratory staff who process the samples213. Point-of-care testing214 can be useful if the temperature and humidity conditions do not affect performance. In the current outbreak in the Democratic Republic of the Congo, access to point-of-care testing has become routine. The clinical parameters that can be monitored closely include: blood chemistry (sodium, potassium chloride, ionized calcium, glucose and creatinine concentrations), markers of liver injury (AST and ALT), creatine phosphokinase, C-reactive protein, haematological parameters (white blood cells, haematocrit and platelets count) and urine analyses (glucose, ketones, ascorbic acid, protein, blood, leukocytes, nitrite, pH, bilirubin and urobilinogen), and therapies can be determined on the basis of the daily values. In the setting of multiple organ dysfunction syndrome and critical illness, frequent laboratory monitoring becomes a cornerstone to guide supportive therapy. In addition, viral load monitoring (or the proxy of cycle threshold value) is also helpful as a surrogate of viral replication, immune containment and (in retrospective studies) CFR, and it may assist in risk stratification of patients with EVD.
Complications
In general, patients with EVD should either receive reliable testing for malaria or be treated empirically with artemether–lumefantrine or other artemisinin-based therapy. Regardless of testing results, all ill patients who meet criteria for severe malaria should receive intravenous artesunate empirically. Secondary infectious complications in patients with EVD, including sepsis induced by Gram-negative bacteria, have been observed38. Patients with EVD may be at high risk of bacterial translocation of the commensal gut microbiota into the bloodstream, owing to substantial inflammation in the gastrointestinal tract157. Patients with EVD treated in Western Africa received empirical antibiotic therapy to prevent and treat bacterial infection and sepsis but also, particularly in children, to treat other potentially life-threatening infections that can mimic the clinical signs of EVD (for example, Salmonella Typhi bacteraemia). Initiation of broad-spectrum antibiotics is recommended in patients with EVD who are critically ill193,199. Antimalarial treatments were administered to patients from the Western African outbreak with suspected EVD, since malaria co-infection was common in patients presenting to ETUs155,215. Since patients with EVD may remain hospitalized for prolonged periods and may undergo invasive procedures, they should be monitored closely for the development of nosocomial infections such as central line-associated bloodstream infections, ventilator-associated pneumonia and urinary tract infections. As performing blood cultures for full identification of the causative bacterium and its antimicrobial susceptibility pattern can be logistically challenging in ETUs, broad-spectrum PCR-based methods may prove useful in some settings for rapid identification of secondary or nosocomial infections216.
Special populations
Over 3,000 patients with EVD were of <15 years of age during the 2013–2016 Western African outbreak. The clinical characteristics of paediatric patients with EVD have been described, and CFRs of 42–76% in this population were reported in the 2013–2016 Western African outbreak44,217,218,219. The clinical management of children with EVD introduces unique challenges, including the need for health-care workers trained in paediatrics and the issue of parental presence and its associated benefits and risks220. Similarly, the care of obstetrical patients also presents remarkable challenges, especially regarding infection control during the provision of surgical procedures such as caesarean section221. Survival of neonates born to EBOV-infected mothers is rare, and the frequency of miscarriage and the maternal CFR are considerable222,223,224,225. Whether the management of these patients in better-resourced settings or with experimental therapeutic agents would have an effect on survival remains unclear226. To reduce health-care worker risk, creation of protocols is of high importance to maintain the standards of infection control and to ensure the availability of staff who are trained to provide safe and effective care to these special patient populations227.
Quality of life
Owing to the high CFR and overall low case numbers of EVD outbreaks prior to 2014, survivors of EVD were rare and not followed-up systematically with modern clinical research methodology. Limited case-controlled data from an EVD outbreak in 1995 first suggested that convalescence could be complicated by substantial morbidity that might limit a survivor’s ability to resume a pre-EVD quality of life. Reported sequelae included arthralgias, myalgias, visual and auditory changes and extreme fatigue228,229. Almost two-thirds of survivors continued to experience one or more of these clinical signs for 2 years following disease onset, and many survivors reported that their capacity to work was decreased compared with their pre-EVD state229.
By contrast, during the 2013–2016 Western African outbreak the number of survivors exceeded the number of fatalities, and the majority of survivors who participated in observational cohort studies reported symptoms similar to those described in the 1995 outbreak230. In the largest controlled observational study of survivors of EVD to date, certain symptoms (headache, joint and muscle pain, memory loss, fatigue and increased urinary frequency) and signs (as revealed by abnormal abdominal, chest, neurological, musculoskeletal and ocular examinations) were statistically significantly more common in survivors than in controls (antibody-negative close contacts). In general, these conditions improved over time, with the exception of uveitis (intra-ocular inflammation), for which the prevalence increased slightly over the follow-up period of the study230 (Fig. 7).
Clinical sequelae in survivors of Ebola virus disease (EVD) that are supported by evidence that includes physical examination of the individuals. Studies reporting patient-reported symptoms are not included in this summary figure. EBOV, Ebola virus; PTSD, post-traumatic stress disorder. aIn the PREVAIL III clinical trial, a prospective, controlled study assessing symptoms in survivors that had a >10% increase in prevalence compared with control close contacts, this symptom had an increased odds ratio (P < 0.0001) compared with close contact controls. bIn the PREVAIL III clinical trial, in which symptoms in survivors were compared with symptoms in control close contacts (regardless of any increase in their prevalence in survivors), this symptom had an increased odds ratio (P < 0.01) compared with control close contacts. cData from uncontrolled cohorts, case series or case reports. dMost common abnormalities in neurological examinations are abnormal oculomotor examination, abnormal reflexes, tremor and abnormal sensory examination. eMost common abnormalities include irregular heart rate, cardiac murmur, decreased breath sounds, rales (crackling lung sounds) and wheezes. fMost common abnormalities include abdominal tenderness, mass or distension. gMost common abnormalities include muscle tenderness and decreased range of motion. Based on refs230,234,239,243,253,254,285,286,287.
Clinical sequelae in survivors of EVD
WHO treatment guidelines for the clinical management of survivors of EVD were rapidly developed and published during the Western African outbreak. These guidelines, by necessity, were based on consensus expert opinion on the best management of a clinical disease that was still being characterized; clinical evaluation of therapeutic interventions had not yet been performed231. With the exception of EVD-associated uveitis, for which specific treatment includes topical and/or oral anti-inflammatory steroids and cycloplegics (to paralyse the ciliary muscle)232, symptom alleviation focusing on pain management is a primary focus, with analgesics and NSAIDs recommended. Also, improving diagnosis and appropriate management of ophthalmological and mental health conditions will require increasing the overall low number of well-trained and equipped eye care and mental health providers and limited supplies of ophthalmological and psychotropic medications in Africa.
Physical sequelae
Up to 87% of survivors of EVD report arthralgias — with symmetric polyarticular involvement affecting (in order of decreasing frequency) the knees, back, hips, fingers, wrists, neck, shoulders, ankles and elbows229. The presence and severity of arthralgias has been reported to directly impede recovery of functional status233. Although rarely actually characterized, physical findings are usually unremarkable without overt erythema or swelling229,233,234. Imaging of a limited number of joints has thus far been unrevealing. In the only report of joint arthrocentesis (aspiration of synovial fluid from within a joint capsule), EBOV RNA could not be detected in the synovial fluid235.
Ocular symptoms and signs, including retro-orbital pain, blurry vision, eye pain, sensitivity to light, and conjunctival injection also seem to complicate EVD recovery in a substantial proportion of adult (14–60%) and paediatric (32%) survivors228,229,233,234,236,237,238,239,240,241,242. These symptoms and signs are most frequently due to uveitis, which has been reported most frequently within the first 12 weeks (but sometimes even after a year) following hospital or ETU discharge232. However, the true incidence and prevalence of ocular complications are uncertain, as diagnosis requires advanced ocular equipment, including a slit lamp, and ophthalmological expertise, which are less commonly available in resource-limited settings. Careful characterization of the clinical phenotype and natural history of uveitis in survivors of EVD is ongoing, but emerging reports suggest involvement of all anatomical locations including anterior uveitis (affecting the anterior chamber, iris or ciliary body) in 46–62%, posterior uveitis (affecting the choroid or posterior retina) in 26%, and pan-uveitis in 21–25% of examined populations with uveitis234,239. Patients may also develop structural ocular complications, most commonly cataracts, which require surgical intervention. In one study of 57 patients with uveitis after EVD, seven (12%) were also diagnosed with cataracts concurrently with uveitis, and at least three others developed cataract(s) following the onset of uveitis234. These findings raise the concern for long-term visual disability if complications of uveitis are not diagnosed and treated early234. Timely diagnosis enabling early appropriate cycloplegic and anti-inflammatory treatment (topical or systemic steroids depending on severity) for uveitis and recognition and management of complications are crucial to avoid long-term visual disability. Recurrent uveitis has been described243.
Neurological issues (headache, memory loss, mental status changes, seizures and insomnia), psychiatric conditions (anxiety and depressive disorders and post-traumatic stress disorder; see next section), dermatological disorders (alopecia and rashes), gastrointestinal issues (poorly defined abdominal pain syndromes), auditory issues (hearing loss and tinnitus), and generalized symptoms, including severe and persistent fatigue, have also been reported in a substantial number of survivors of EVD229,233,234,236,237,240,241. Many of these conditions are associated with important functional limitations; more than one-third of survivors in a single study reported health problems lasting >1 year, and 29% indicated that their health problems limited their ability to walk or run230. Additionally, gender-specific complications (for example, orchitis and amenorrhoea) and sexual dysfunction in both women and men have been reported. Association of these complications with EVD and the consequences for future fertility remain unclear228,233,234,240.
Mental health and psychosocial sequelae
In addition to the physical complications of EVD, reports from the current epidemic indicate that psychological sequelae have a substantial effect on the lives of survivors. Survivors not only experienced a life-threatening event but often also the loss of immediate family members to EVD. In one study of 24 survivors, all reported losing at least one family member, and 67% reported witnessing the death244. The loss of a family member was significantly associated with signs of depression (odds ratio 5.7, 95% CI 1.2–28.0) and an inability to concentrate (adjusted odds ratio 10.1, 95% CI 1.7–60.7)245. Difficulty coping with such loss was probably compounded by an inability to observe traditional burial practices owing to infection control procedures designed to mitigate EBOV transmission244,246. Furthermore, the 2013‒2016 Western African EVD outbreak resulted in >16,000 orphans, further complicating the recovery of these children in particular and affected communities in general247,248.
Survival can be complicated by symptoms of post-traumatic stress and post-traumatic stress disorder and stigma. Within the first month of discharge, 71% of survivors experienced arousal reactions, such as racing heart, abdominal discomfort and dizziness, when reminded of their experiences. In addition, 21% of survivors reported distressing thoughts about their experiences and difficulty sleeping244,245. Furthermore, survivors have only limited access to psychiatrists or counsellors who are trained to evaluate and manage post-traumatic stress disorders. Additionally, almost one-third of survivors reported experiencing stigma from their community upon return from an ETU, as manifested by social distancing by community members and even family members and by verbal abuse244. Questions regarding viral persistence and possible shedding also contribute to social isolation and stigmatization.
Compounding these emotional sequelae is the loss of employment opportunities. A substantial proportion of survivors of EVD lose their livelihood249. Many health-care workers who were occupationally exposed and infected were not welcomed back to their previous positions. The loss of employment exacerbates social isolation, feelings of worthlessness and poverty. Full recovery from the trauma of the EVD experience is not possible without full reintegration into society, including its workforce.
Mechanisms of EVD sequelae
Putative inflammatory and immune activation pathways that may or may not be associated with EBOV or EBOV antigen persistence may underly the pathogenesis of clinical sequelae in survivors of EVD, but these pathways remain speculative and data to support specific therapeutic approaches are sparse. In a few survivors, an inflammatory component to joint and muscle pain is suggested by stiffness associated with inactivity and response to steroid therapy, although the classic signs of infectious or inflammatory arthritis (heat, pain, redness and swelling, or ‘calor, dolor, rubor and tumour’) are typically not present230. Significantly increased anti-EBOV IgG antibody titres in survivors with arthralgias compared with survivors without arthralgias support a role for sustained immune activation and inflammation in the pathogenesis of arthralgias after EVD229. This hypothesis is consistent with findings of polyarthropathies associated with other viral infections250,251,252. However, this finding has yet to be confirmed in survivors of the Western African outbreak.
Longitudinal characterization of sustained immune activation several months after clearance of viraemia has been described in some survivors of EVD. The presence of persistent peripheral EBOV antigen-specific activated CD8+ and CD4+ T cells123 suggests ongoing viral antigen stimulation in these survivors. Intra-ocular inflammation suggests ongoing antigen presentation with intense immune responses leading to local inflammation in many survivors with uveitis. In one survivor, severe sight-threatening uveitis was associated with persistent infectious EBOV in the aqueous humor. In one study, high viral load in patients with acute EVD at presentation (as measured by the proxy low RT-PCR cycle threshold) was associated with uveitis after recovery239. These findings suggest that disease severity that may include direct viral injury and/or the immunopathology at the time of acute EVD may contribute to complications after EVD, and that markers of severe disease, in particular the viral load, may predict sequelae230,239.
Viral persistence
Although EBOV RNA can be found in virtually any bodily fluid from patients with acute EVD40, historically little is known about viral kinetics in bodily fluids other than blood. EBOV can persist in immune-privileged sites (including the central nervous system, eye, urogenital system, placenta and potentially breast milk) and viral persistence may be associated with recrudescent organ-specific inflammatory disease (uveitis and meningoencephalitis). Two case reports have documented the development of encephalopathy and meningoencephalitis 13 days and 9 months after clearance of viraemia, respectively, and viable EBOV was detected in the cerebrospinal fluid in one patient235,253. Additionally, in a patient with severe unilateral uveitis, replication-competent EBOV was detected in the aqueous humor at high levels 9 weeks after clearance of viraemia254.
Despite clearance of viraemia, some male survivors continue to shed live EBOV in the semen, thereby posing a public health risk of sexual transmission and reignition of outbreaks40,41,68,123,229,254,255,256,257. Studies have investigated clusters of EVD cases that occurred sporadically after the peak of the 2013–2016 Western African EVD outbreak, each cluster thought to be initiated by a transmission event involving viral persistence in survivors of EVD, typically in semen68,258,259.
The last flare of the 2013‒2016 Western African outbreak in 2016 was attributed via molecular epidemiology to a sexual transmission event from a survivor at 482 days after disease onset. EBOV RNA was detected in his semen ~500 days after EVD onset, although isolation of infectious virus was not possible66. These findings led to the implementation of new recommendations regarding the practice of safe sex for survivors of EVD167. Despite the detection of EBOV RNA in a few vaginal fluid swabs from female survivors of EVD (up to 37 days after disease onset)260, no studies have thoroughly evaluated the persistence of EBOV in cervical or vaginal fluid261. Though rarely documented, viral persistence has been associated with transplacental transmission. EBOV was detected in a stillborn infant from a mother whose blood was negative for EBOV RNA detected by PCR tests, but who had detectable levels of anti-EBOV IgM and anti-EBOV IgG antibodies262. EBOV RNA was also recovered from breast milk following the death of an infant, suggesting transmission via breastfeeding263. In many of these studies, unvalidated diagnostic tests for the detection of EBOV RNA were used, and, therefore, the limits of detection and the repeatability of the assays are unknown. EBOV persistence in immune-privileged sites has been reported in NHPs who survive infection; these and future studies will provide crucial insights into the mechanisms of viral persistence264.
Outlook
The 2013–2016 Western African EVD outbreak considerably increased the evidence base and the attention and approach of the global community to this disease. During the outbreak, the rapid evaluation and implementation of effective MCMs, appropriate supportive clinical care and preventive measures lacked coordination. However, important lessons learned have been subsequently applied during more-recent EVD outbreaks, including the ongoing second-largest outbreak in history in the Democratic Republic of the Congo, where the will and capacity of response teams to understand and apply new strategies have been remarkably accelerated. Hopefully, multi-pronged approaches including novel (for example, ring vaccination, new EBOV-specific therapeutics and advanced supportive care) and well-established pillars of outbreak response (for example, contact tracing and infection prevention and control) will not only interrupt transmission and prevent new cases but also decrease the CFR inside the ETUs to reduce overall morbidity and fatality.
Although the success of the aforementioned PALM trial has been a remarkable demonstration of the possibility of conducting timely and rigorous clinical research in a complex outbreak setting, many questions remain. For example, the role for combination EBOV-specific therapeutics in further reducing CFR, clinical sequelae or viral persistence in survivors is unclear. The role for host-directed ‘agnostic’ approaches to reduce immunopathology remains to be determined. In addition, the role for specific therapeutics to prevent or treat viral persistence or clinical sequelae that are directly or indirectly associated with viral persistence remains unclear. The optimal supportive care regimen that can be safely and effectively delivered in the field requires further evaluation. Additionally, whether there is a role for specific therapeutics in targeted high-risk contacts for prophylaxis after exposure remains to be studied.
Lessons from the 2013–2016 Western African outbreak suggest that advanced supportive care may have contributed to improved outcomes, particularly in patients medically evacuated to the USA or Europe. Improved ETU design combined with the will and capacity to train and adequately equip staff has advanced provision of supportive treatment on a widespread basis in African settings199,265. However, despite encouraging progress, the principles of supportive treatment of EVD are largely borrowed from other disease syndromes (for example, septic shock) and from observations of very small numbers of patients. Data to support or refute current supportive care approaches are scant and difficult to obtain given the difficulties in ethically exploring these approaches in the research setting in humans with EVD. Nonetheless, the authors’ opinion is that newly identified EBOV-specific therapeutics cannot be considered in isolation as ‘magic bullets’. These therapeutics must not be uncoupled from the supportive care required to care for patients with EVD, a disease that often presents late in illness with ominous multiple organ dysfunction syndrome. Similarly, the influence of pre-existing or coexisting infection or superinfection with endemic pathogens (for example, Plasmodium spp., HIV-1, measles virus or mycobacteria) on the course and outcome of EVD remains to be determined. In addition, the influence of genetic or other host factors in ethnically different populations and different regions is also unclear.
The medical follow-up of survivors of EVD is also not trivial. For those with physical or psychological sequelae, even the most basic medications are scarce, and simple diagnostic testing is non-existent or prohibitively expensive. Ultimately, longitudinal research to inform an evidence base for the care of survivors of EVD is crucial to understanding better the mechanisms underlying complications after EVD to implement appropriate treatment. Further study is needed to define the outer time limit of viral shedding to inform public health recommendations, and support the unprecedented number of survivors who continue to experience social isolation and stigma.
The typical setting of EVD outbreaks in under-developed areas (with limited access to medical infrastructure) and in multiethnic populations (characterized by vastly different mobility and health-seeking behaviours), the presence of armed conflicts and the unpredictability and typically limited size of EVD outbreaks in general pose immense challenges to conducting clinical trials. Clinical trials are also not necessarily welcomed by local populations, who may be understandably suspicious of outsiders’ intentions in post-colonial settings. In the very challenging setting of the current EVD outbreak in the Democratic Republic of the Congo, the clinical research response represents an important step forward but also emphasizes these challenges.
On a positive note, EBOV diagnostics have greatly improved over recent years and are now relatively broadly available in countries with a history of EVD outbreaks. With local or international support, crucial infrastructures (for example, laboratories, field stations and treatment centres) have been built in several countries. National and international health agencies (including the WHO) and non-governmental organizations have streamlined their interactions and communication strategies to be able to work together to curtail future outbreaks. Whereas many problems remain to be tackled, we are hopeful that the response to future outbreaks will be even more rapid and more coordinated than before, and that future outbreaks will involve an increasing number of EVD-experienced health-care staff using clinically tested MCMs to decrease CFR and prevent or ameliorate sequelae.
Zooming out from the outbreak setting, ecological and epidemiological uncertainties remain to be clarified, including the identity of the natural EBOV reservoir host and the determinants and dynamics of EBOV spillover and subsequent human-to-human transmission. Understanding the animal–human interface may prevent the introduction of EBOV into the human population. Finally, EBOV is not the only filovirus that causes severe human disease; indeed, MARV seems statistically to be even more lethal than EBOV4. By extrapolation, MARV could cause an outbreak of the scale of the 2013–2016 EVD outbreak. Our understanding of MARV disease, let alone the diseases caused by other ebolaviruses and marburgviruses, is limited with large knowledge gaps in the entire spectrum from basic science to human clinical disease. Progress in the prevention and treatment of FVD, including nascent MCMs and vaccine development endeavours, lags far behind the recent progress made specifically in EVD.
References
Kuhn, J. H. et al. ICTV virus taxonomy profile: Filoviridae. J. Gen. Virol. 100, 911–912 (2019).
Kuhn, J. H. et al. New filovirus disease classification and nomenclature. Nat. Rev. Microbiol. 17, 261–263 (2019). Outlines the current official WHO International Classification of Diseases version 11 (ICD-11) subdivisions of filovirus disease (FVD), including Ebola virus disease (EVD).
Siegert, R., Shu, H.-L., Slenczka, W., Peters, D. & Müller, G. On the etiology of an unknown human infection originating from monkeys [German]. Dtsch. Med. Wochenschr. 92, 2341–2343 (1967).
Kuhn, J. H., Amarasinghe, G. & Perry, D. L. in Fields Virology: Emerging Viruses 7th edn Ch. 12 (eds Sean P. J. Whelan, Peter M. Howley, & David M. Knipe) in the press (Wolters Kluwer, 2020).
Formenty, P. et al. Human infection due to Ebola virus, subtype Côte d’Ivoire: clinical and biologic presentation. J. Infect. Dis. 179, S48–S53 (1999).
Okware, S. I. et al. An outbreak of Ebola in Uganda. Trop. Med. Int. Health 7, 1068–1075 (2002).
Kuhn, J. H. Filoviruses. A compendium of 40 years of epidemiological, clinical, and laboratory studies. Arch.Virol. Suppl. 20, 13–360 (2008).
Martines, R. B., Ng, D. L., Greer, P. W., Rollin, P. E. & Zaki, S. R. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J. Pathol. 235, 153–174 (2015). An updated and comprehensive summary of filovirus disease autopsy data.
Siragam, V., Wong, G. & Qiu, X.-G. Animal models for filovirus infections. Zool. Res. 39, 15–24 (2018).
Nakayama, E. & Saijo, M. Animal models for Ebola and Marburg virus infections. Front. Microbiol. 4, 267 (2013).
World Health Organization. Ebola Outbreak 2014–2016. http://www.who.int/csr/disease/ebola/en/ (2017).
Chippaux, J.-P. Outbreaks of Ebola virus disease in Africa: the beginnings of a tragic saga. J. Venom. Anim. Toxins Incl. Trop. Dis. 20, 44 (2014).
Check Hayden, E. Ebola failures prompt WHO rethink. Nature 521, 137 (2015).
Levett, J. Disastrous events and political failures. Prehosp. Disaster Med. 30, 227–228 (2015).
[No authors listed] Ebola: a failure of international collective action. Lancet 384, 637 (2014).
Ippolito, G., Di Caro, A. & Capobianchi, M. R. The chronology of the international response to Ebola in Western Africa: lights and shadows in a frame of conflicting position and figures. Infect. Dis. Rep. 7, 5957 (2015).
Kiiza, P., Adhikari, N. K. J., Mullin, S., Teo, K. & Fowler, R. A. Principles and practices of establishing a hospital-based Ebola treatment unit. Crit. Care Clin. 35, 697–710 (2019). Informative discussion of the process and resources necessary to initiate an Ebola (virus) Treatment Unit (ETU).
Janke, C. et al. Beyond Ebola treatment units: severe infection temporary treatment units as an essential element of Ebola case management during an outbreak. BMC Infect. Dis. 17, 124 (2017).
Lamb, L. E., Cox, A. T., Fletcher, T. & McCourt, A. L. Formulating and improving care while mitigating risk in a military Ebola virus disease treatment unit. J. R. Army Med. Corps 163, 2–6 (2017).
Leitenberg, M., Zilinskas, R. A. & Kuhn, J. H. The Soviet Biological Weapons Program — a History (Harvard Univ. Press, 2012).
Radoshitzky, S. R., Bavari, S., Jahrling, P. B. & Kuhn, J. H. in Medical Aspects of Biological Warfare (Textbooks of Military Medicine) Ch. 23 (eds Bozue, J., Cote, C. K & Glass, P. J.) 569–614 (Borden Institute, US Army Medical Department Center and School, Health Readiness Center of Excellence, 2018).
World Health Organization. Ebola Virus Disease Democratic Republic of Congo: External Situation Report 77/2020. https://reliefweb.int/sites/reliefweb.int/files/resources/SITREP_EVD_DRC_20200128-eng.pdf (2020).
Maganga, G. D. et al. Ebola virus disease in the Democratic Republic of Congo. N. Engl. J. Med. 371, 2083–2091 (2014).
Ebola Outbreak Epidemiology Team. Outbreak of Ebola virus disease in the Democratic Republic of the Congo, April-May, 2018: an epidemiological study. Lancet 392, 213–221 (2018).
Mbala-Kingebeni, P. et al. Medical countermeasures during the 2018 Ebola virus disease outbreak in the North Kivu and Ituri provinces of the Democratic Republic of the Congo: a rapid genomic assessment. Lancet Infect. Dis. 19, 648–657 (2019).
Mbala-Kingebeni, P. et al. 2018 Ebola virus disease outbreak in Équateur province, Democratic Republic of the Congo: a retrospective genomic characterisation. Lancet Infect. Dis. 19, 641–647 (2019).
de La Vega, M.-A. et al. Ebola viral load at diagnosis associates with patient outcome and outbreak evolution. J. Clin. Invest. 125, 4421–4428 (2015).
Dodd, L. E. et al. A meta-analysis of clinical studies conducted during the West Africa Ebola virus disease outbreak confirms the need for randomized control groups. Sci. Transl Med. 11, eaaw1049 (2019). Meta-analysis of data from individual patients with EVD highlights the difficulty in making meaningful comparisons between studies in the absence of randomization.
Bowen, E. T. W. et al. Viral hæmorrhagic fever in southern Sudan and northern Zaire. Preliminary studies on the aetiological agent. Lancet 309, 571–573 (1977).
Johnson, K. M., Lange, J. V., Webb, P. A. & Murphy, F. A. Isolation and partial characterisation of a new virus causing acute hæmorrhagic fever in Zaire. Lancet 309, 569–571 (1977).
Pattyn, S., van der Groen, G., Jacob, W., Piot, P. & Courteille, G. Isolation of Marburg-like virus from a case of hæmorrhagic fever in Zaire. Lancet 309, 573–574 (1977).
Pigott, D. M. et al. Local, national, and regional viral haemorrhagic fever pandemic potential in Africa: a multistage analysis. Lancet 390, 2662–2672 (2017). A multi-factorial picture of subnational African risk for viral haemorrhagic fever, toward preparation efforts around improving surveillance systems, diagnostics and health systems in parallel at national and international levels.
Roels, T. H. et al. Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure. J. Infect. Dis. 179, S92–S97 (1999).
Dowell, S. F. et al. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J. Infect. Dis. 179, S87–S91 (1999).
Galas, A. The determinants of spread of Ebola virus disease - an evidence from the past outbreak experiences. Folia Med. Cracov. 54, 17–25 (2014).
Bausch, D. G. et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J. Infect. Dis. 196, S142–S147 (2007). Describes stability of the virus in clinical fluids and the associated risk of transmission.
Deen, G. F. et al. Ebola RNA persistence in semen of Ebola virus disease survivors - final report. N. Engl. J. Med. 377, 1428–1437 (2017). First large study to evaluate the long-term presence of EBOV RNA in the semen of male survivors of EVD in Sierra Leone.
Kreuels, B., Addo, M. M. & Schmiedel, S. Severe Ebola virus infection complicated by gram-negative septicemia. N. Engl. J. Med. 372, 1377 (2015).
Moreau, M. et al. Lactating mothers infected with Ebola virus: EBOV RT-PCR of blood only may be insufficient. Euro Surveill. 20, 21017 (2015).
Vetter, P. et al. Ebola virus shedding and transmission: review of current evidence. J. Infect. Dis. 214, S177–S184 (2016).
Mate, S. E. et al. Molecular evidence of sexual transmission of Ebola virus. N. Engl. J. Med. 373, 2448–2454 (2015). First molecular and epidemiological evidence that EBOV can be transmitted from the semen of a male survivor by sexual transmission.
Dean, N. E., Halloran, M. E., Yang, Y. & Longini, I. M. Transmissibility and pathogenicity of Ebola virus: a systematic review and meta-analysis of household secondary attack rate and asymptomatic infection. Clin. Infect. Dis. 62, 1277–1286 (2016).
Chowell, G., Hengartner, N. W., Castillo-Chavez, C., Fenimore, P. W. & Hyman, J. M. The basic reproductive number of Ebola and the effects of public health measures: the cases of Congo and Uganda. J. Theor. Biol. 229, 119–126 (2004).
Smit, M. A., Michelow, I. C., Glavis-Bloom, J., Wolfman, V. & Levine, A. C. Characteristics and outcomes of pediatric patients with Ebola virus disease admitted to treatment units in Liberia and Sierra Leone: a retrospective cohort study. Clin. Infect. Dis. 64, 243–249 (2017).
Aylward, B. et al. Ebola virus disease in West Africa - the first 9 months of the epidemic and forward projections. N. Engl. J. Med. 371, 1481–1495 (2014).
Dowell, S. F. Ebola hemorrhagic fever: why were children spared? Pediatr. Infect. Dis. J. 15, 189–191 (1996).
Glynn, J. R. Age-specific incidence of Ebola virus disease. Lancet 386, 432 (2015).
Agua-Agum, J. et al. Ebola virus disease among children in West Africa. N. Engl. J. Med. 372, 1274–1277 (2015).
Chérif, M. S. et al. Ebola virus disease in children during the 2014-2015 epidemic in Guinea: a nationwide cohort study. Eur. J. Pediatr. 176, 791–796 (2017).
Bower, H. et al. Exposure-specific and age-specific attack rates for Ebola virus disease in Ebola-affected households, Sierra Leone. Emerg. Infect. Dis. 22, 1403–1411 (2016).
Schwartz, D. A., Anoko, J. N. & Abramowitz, S. A. (eds) Pregnant in the Time of Ebola: Women and Their Children in the 2013–2015 West African Epidemic (Springer, 2019).
Okoror, L., Kamara, A., Kargbo, B., Bangura, J. & Lebby, M. Transplacental transmission: a rare case of Ebola virus transmission. Infect. Dis. Rep. 10, 7725 (2018).
Fallah, M. P. et al. Pregnancy outcomes in Liberian women who conceived after recovery from Ebola virus disease. Lancet. Glob. Health 4, e678–e679 (2016).
Haddad, L. B., Horton, J., Ribner, B. S. & Jamieson, D. J. Ebola infection in pregnancy: a global perspective and lessons learned. Clin. Obstet. Gynecol. 61, 186–196 (2018).
Baggi, F. M. et al. Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Euro Surveill. 19, 20983 (2014).
Oduyebo, T. et al. A pregnant patient with Ebola virus disease. Obstet. Gynecol. 126, 1273–1275 (2015).
Nordenstedt, H. et al. Ebola virus in breast milk in an Ebola virus-positive mother with twin babies, Guinea, 2015. Emerg. Infect. Dis. 22, 759–760 (2016).
Arias, A. et al. Rapid outbreak sequencing of Ebola virus in Sierra Leone identifies transmission chains linked to sporadic cases. Virus Evol. 2, vew016 (2016).
Wauquier, N., Padilla, C., Becquart, P., Leroy, E. & Vieillard, V. Association of KIR2DS1 and KIR2DS3 with fatal outcome in Ebola virus infection. Immunogenetics 62, 767–771 (2010).
World Health Organization. Technical Guidelines for Integrated Disease Surveillance and Response in the African Region. https://www.afro.who.int/publications/technical-guidelines-integrated-disease-surveillance-and-response-african-region-0 (2010).
Huizenga, E. et al. A modified case definition to facilitate essential hospital care during Ebola outbreaks. Clin. Infect. Dis. 68, 1763–1768 (2019).
Gire, S. K. et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 345, 1369–1372 (2014). First description of mass genomic data for EBOV during the Western African outbreak.
Dudas, G. et al. Virus genomes reveal factors that spread and sustained the Ebola epidemic. Nature 544, 309–315 (2017). Describes the spread of EBOV during the Western African EVD outbreak.
Carroll, M. W. et al. Temporal and spatial analysis of the 2014–2015 Ebola virus outbreak in West Africa. Nature 524, 97–101 (2015).
Tong, Y.-G. et al. Genetic diversity and evolutionary dynamics of Ebola virus in Sierra Leone. Nature 524, 93–96 (2015).
Diallo, B. et al. Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin. Infect. Dis. 63, 1353–1356 (2016).
Schindell, B. G., Webb, A. L. & Kindrachuk, J. Persistence and sexual transmission of filoviruses. Viruses 10, 683 (2018).
Den Boon, S. et al. Ebola virus infection associated with transmission from survivors. Emerg. Infect. Dis. 25, 249–255 (2019).
Whitmer, S. L. M. et al. Active Ebola virus replication and heterogeneous evolutionary rates in EVD survivors. Cell Rep. 22, 1159–1168 (2018). First-in-kind examination of intra-host EBOV genome evolution in survivors of EVD, highlighting in general the slow-down of the molecular clock in immune-privileged fluids, although also identifying individuals and samples with unusual hyper-mutation.
Azarian, T. et al. Impact of spatial dispersion, evolution, and selection on Ebola Zaire virus epidemic waves. Sci. Rep. 5, 10170 (2015).
Bray, M., Davis, K., Geisbert, T., Schmaljohn, C. & Huggins, J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis. 179, S248–S258 (1999).
Bente, D., Gren, J., Strong, J. E. & Feldmann, H. Disease modeling for Ebola and Marburg viruses. Dis. Model. Mech. 2, 12–17 (2009).
Osterholm, M. T. et al. Transmission of Ebola viruses: what we know and what we do not know. MBio 6, e00137 (2015).
Geisbert, T. W., Strong, J. E. & Feldmann, H. Considerations in the use of nonhuman primate models of Ebola virus and Marburg virus infection. J. Infect. Dis. 212, S91–S97 (2015).
Alfson, K. J. et al. Particle-to-PFU ratio of Ebola virus influences disease course and survival in cynomolgus macaques. J. Virol. 89, 6773–6781 (2015).
Urbanowicz, R. A. et al. Human adaptation of Ebola virus during the West African outbreak. Cell 167, 1079–1087.e5 (2016). Highlights the early emergence of EBOV GP 1,2 (for example, A82V) and other mutations that became dominant in the EVD-affected population in Western Africa.
Dietzel, E., Schudt, G., Krähling, V., Matrosovich, M. & Becker, S. Functional characterization of adaptive mutations during the West African Ebola virus outbreak. J. Virol. 91, e01913–e01916 (2017).
Diehl, W. E. et al. Ebola virus glycoprotein with increased infectivity dominated the 2013–2016 epidemic. Cell 167, 1088–1098.e6 (2016).
Wang, M. K., Lim, S.-Y., Lee, S. M. & Cunningham, J. M. Biochemical basis for increased activity of Ebola glycoprotein in the 2013–16 epidemic. Cell Host Microbe 21, 367–375 (2017).
Marzi, A. et al. Recently identified mutations in the Ebola virus-Makona genome do not alter pathogenicity in animal models. Cell Rep. 23, 1806–1816 (2018).
Kyle, J. E. et al. Plasma lipidome reveals critical illness and recovery from human Ebola virus disease. Proc. Natl Acad. Sci. USA 116, 3919–3928 (2019).
Eisfeld, A. J. et al. Multi-platform’omics analysis of human Ebola virus disease pathogenesis. Cell Host Microbe 22, 817–829.e8 (2017). A first-in-kind comparison of host signatures between those who did and did not survive using multi-platfom “omics” in patients with EVD in Sierra Leone, implicating sepsis-like pathophysiology, pancreatic damage and neutrophil dysfunction, and describing an integrated approach to identifying a discriminatory biomarker for survival or death.
Carette, J. E. et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477, 340–343 (2011).
Côté, M. et al. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 477, 344–348 (2011).
Takada, A. et al. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl Acad. Sci. USA 94, 14764–14769 (1997).
Schnittler, H.-J. & Feldmann, H. Marburg and Ebola hemorrhagic fevers: does the primary course of infection depend on the accessibility of organ-specific macrophages? Clin. Infect. Dis. 27, 404–406 (1998).
Ryabchikova, E. I. & Price, B. B. S. Ebola and Marburg Viruses: a View of Infection Using Electron Microscopy. (Battelle, 2004).
Geisbert, T. W. et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 163, 2347–2370 (2003).
Zaki, S. R. & Peters, C. J. in Pathology of infectious diseases (ed. Connor D. H.) 347–364 (Appleton & Lange, 1997).
Geisbert, T. W., Jahrling, P. B., Hanes, M. A. & Zack, P. M. Association of Ebola-related Reston virus particles and antigen with tissue lesions of monkeys imported to the United States. J. Comp. Pathol. 106, 137–152 (1992).
Bray, M. & Geisbert, T. W. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int. J. Biochem. Cell Biol. 37, 1560–1566 (2005).
Wahl-Jensen, V. et al. Role of Ebola virus secreted glycoproteins and virus-like particles in activation of human macrophages. J. Virol. 79, 2413–2419 (2005).
Wahl-Jensen, V. et al. Ebola virion attachment and entry into human macrophages profoundly effects early cellular gene expression. PLoS Negl. Trop. Dis. 5, e1359 (2011).
Gupta, M., Mahanty, S., Ahmed, R. & Rollin, P. E. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with Ebola virus secrete MIP-1α and TNF-α and inhibit poly-IC-induced IFN-α in vitro. Virology 284, 20–25 (2001).
Ströher, U. et al. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 75, 11025–11033 (2001).
Bosio, C. M. et al. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis. 188, 1630–1638 (2003).
Geisbert, T. W. et al. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 188, 1618–1629 (2003).
Mahanty, S. et al. Impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J. Immunol. 170, 2797–2801 (2003).
Bosio, C. M. et al. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology 326, 280–287 (2004).
Hensley, L. E., Young, H. A., Jahrling, P. B. & Geisbert, T. W. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol. Lett. 80, 169–179 (2002).
Younan, P. et al. Ebola virus-mediated T-lymphocyte depletion is the result of an abortive infection. PLoS Pathog. 15, e1008068 (2019).
Geisbert, T. W. et al. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab. Invest. 80, 171–186 (2000).
Baize, S. et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5, 423–426 (1999). First comprehensive description of ineffective humoral immunity and lymphocyte death in humans with EVD.
Ryabchikova, E. I., Kolesnikova, L. V. & Luchko, S. V. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J. Infect. Dis. 179, S199–S202 (1999).
Harcourt, B. H., Sanchez, A. & Offermann, M. K. Ebola virus inhibits induction of genes by double-stranded RNA in endothelial cells. Virology 252, 179–188 (1998).
Harcourt, B. H., Sanchez, A. & Offermann, M. K. Ebola virus selectively inhibits responses to interferons, but not to interleukin-1β, in endothelial cells. J. Virol. 73, 3491–3496 (1999).
Basler, C. F. et al. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 77, 7945–7956 (2003).
Basler, C. F. et al. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl Acad. Sci. USA 97, 12289–12294 (2000).
Cárdenas, W. B. et al. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 80, 5168–5178 (2006).
Feng, Z., Cerveny, M., Yan, Z. & He, B. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J. Virol. 81, 182–192 (2007).
Leung, D. W. et al. Structure of the Ebola VP35 interferon inhibitory domain. Proc. Natl Acad. Sci. USA 106, 411–416 (2009).
Leung, D. W. et al. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat. Struct. Mol. Biol. 17, 165–172 (2010).
Luthra, P. et al. Mutual antagonism between the Ebola virus VP35 protein and the RIG-I activator PACT determines infection outcome. Cell Host Microbe 14, 74–84 (2013).
Prins, K. C., Cárdenas, W. B. & Basler, C. F. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKε and TBK-1. J. Virol. 83, 3069–3077 (2009).
Woolsey, C. et al. A VP35 mutant Ebola virus lacks virulence but can elicit protective immunity to wild-type virus challenge. Cell Rep. 28, 3032–3046.e6 (2019).
Zhu, Y. et al. Characterization of the RNA silencing suppression activity of the Ebola virus VP35 protein in plants and mammalian cells. J. Virol. 86, 3038–3049 (2012).
Kaletsky, R. L., Francica, J. R., Agrawal-Gamse, C. & Bates, P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl Acad. Sci. USA 106, 2886–2891 (2009).
Reid, S. P. et al. Ebola virus VP24 binds karyopherin α1 and blocks STAT1 nuclear accumulation. J. Virol. 80, 5156–5167 (2006).
Reid, S. P., Valmas, C., Martinez, O., Sanchez, F. M. & Basler, C. F. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin α proteins with activated STAT1. J. Virol. 81, 13469–13477 (2007).
Pleet, M. L., DeMarino, C., Lepene, B., Aman, M. J. & Kashanchi, F. The role of exosomal VP40 in Ebola virus disease. DNA Cell Biol. 36, 243–248 (2017).
Pleet, M. L. et al. Ebola VP40 in exosomes can cause immune cell dysfunction. Front. Microbiol. 7, 1765 (2016).
Mandl, J. N. & Feinberg, M. B. Robust and sustained immune activation in human Ebola virus infection. Proc. Natl Acad. Sci. USA 112, 4518–4519 (2015).
McElroy, A. K. et al. Human Ebola virus infection results in substantial immune activation. Proc. Natl Acad. Sci. USA 112, 4719–4724 (2015). Landmark description of the longitudinal kinetics of B cell and T cell adaptive immune responses in four acutely ill patients with EVD cared for in a US setting. Highlights robust antigen-specific immune activation (as opposed to historical presumption of mainly immune suppressive effects) and sustained immune activation potentially associated with viral persistence.
Henao-Restrepo, A. M. et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 389, 505–518 (2017). Final results from a first cluster randomized trials of a ring vaccination strategy using a vesiculovirus platform in Western Africa. Sets the stage for similar use in subsequent EVD outbreaks in the Democratic Republic of the Congo.
Henao-Restrepo, A. M. et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 386, 857–866 (2015).
Mulangu, S. et al. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 381, 2293–2303 (2019). Landmark first randomized clinical trial of four EBOV-specific therapeutics conducted during the current EVD outbreak in the Democratic Republic of the Congo, identifying REGN-EB3 and mAb114 monoclonal antibody-based therapies as more effective than the ZMapp control arm and the antiviral remdesivir.
McElroy, A. K. et al. Kinetic analysis of biomarkers in a cohort of US patients with Ebola virus disease. Clin. Infect. Dis. 63, 460–467 (2016).
Muñoz-Fontela, C. & McElroy, A. K. Ebola virus disease in humans: pathophysiology and immunity. Curr. Top. Microbiol. Immunol. 411, 141–169 (2017).
Ruibal, P. et al. Unique human immune signature of Ebola virus disease in Guinea. Nature 533, 100–104 (2016). One of the first highly detailed descriptions of T cell responses in Western African patients with EVD, identifying a PD-1/CTLA-4 T cell signature of fatal outcome.
Davis, C. W. et al. Longitudinal analysis of the human B cell response to Ebola virus infection. Cell 177, 1566–1582.e7 (2019). Long-term kinetics, quality, and quantity of B cell responses in four human survivors over a 3-year period, including isotypes, hypermutation and convergent evolution, and identification of monoclonal antibodies.
Sow, M. S. et al. New evidence of long-lasting persistence of Ebola virus genetic material in semen of survivors. J. Infect. Dis. 214, 1475–1476 (2016).
Abbate, J. L., Murall, C. L., Richner, H. & Althaus, C. L. Potential impact of sexual transmission on Ebola virus epidemiology: Sierra Leone as a case study. PLoS Negl. Trop. Dis. 10, e0004676 (2016).
Chancellor, J. R. et al. Uveitis and systemic inflammatory markers in convalescent phase of Ebola virus disease. Emerg. Infect. Dis. 22, 295–297 (2016).
Chughtai, A. A., Barnes, M. & Macintyre, C. R. Persistence of Ebola virus in various body fluids during convalescence: evidence and implications for disease transmission and control. Epidemiol. Infect. 144, 1652–1660 (2016).
Velásquez, G. E. et al. Time from infection to disease and infectiousness for Ebola virus disease, a systematic review. Clin. Infect. Dis. 61, 1135–1140 (2015).
Richardson, E. T. et al. Minimally symptomatic infection in an Ebola ‘hotspot’: a cross-sectional serosurvey. PLoS Negl. Trop. Dis. 10, e0005087 (2016).
Timothy, J. W. S. et al. Early transmission and case fatality of Ebola virus at the index site of the 2013–16 west African Ebola outbreak: a cross-sectional seroprevalence survey. Lancet. Infect. Dis. 19, 429–438 (2019).
Glynn, J. R. et al. Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: a cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet. Infect. Dis. 17, 645–653 (2017).
Xu, Z. et al. Epidemiologic characteristics, clinical manifestations, and risk factors of 139 patients with Ebola virus disease in western Sierra Leone. Am. J. Infect. Control. 44, 1285–1290 (2016).
Fowler, R. A. et al. Caring for critically ill patients with Ebola virus disease. Perspectives from West Africa. Am. J. Respir. Crit. Care Med. 190, 733–737 (2014). Provides a broad perspective on the approach to the care of critically ill patients with EVD.
Barry, M. et al. Clinical predictors of mortality in patients with Ebola virus disease. Clin. Infect. Dis. 60, 1821–1824 (2015).
Qin, E. et al. Clinical features of patients with Ebola virus disease in Sierra Leone. Clin. Infect. Dis. 61, 491–495 (2015).
Chertow, D. S. et al. Ebola virus disease in West Africa - clinical manifestations and management. N. Engl. J. Med. 371, 2054–2057 (2014).
Dietz, P. M., Jambai, A., Paweska, J. T., Yoti, Z. & Ksiazek, T. G. Epidemiology and risk factors for Ebola virus disease in Sierra Leone-23 May 2014 to 31 January 2015. Clin. Infect. Dis. 61, 1648–1654 (2015).
Cournac, J. M. et al. Rhabdomyolysis in Ebola virus disease. Results of an observational study in a treatment center in Guinea. Clin. Infect. Dis. 62, 19–23 (2016).
Lado, M. et al. Clinical features of patients isolated for suspected Ebola virus disease at Connaught Hospital, Freetown, Sierra Leone: a retrospective cohort study. Lancet Infect. Dis. 15, 1024–1033 (2015).
Rojek, A., Horby, P. & Dunning, J. Insights from clinical research completed during the west Africa Ebola virus disease epidemic. Lancet Infect. Dis. 17, e280–e292 (2017). Excellent broad review of clinical care-focused EVD research during the Western African EVD outbreak, including priority focus areas for future research.
Wilson, A. J. et al. Thromboelastography in the management of coagulopathy associated with Ebola virus disease. Clin. Infect. Dis. 62, 610–612 (2016).
Sagui, E. et al. Severe Ebola virus infection with encephalopathy: evidence for direct virus involvement. Clin. Infect. Dis. 61, 1627–1628 (2015).
de Greslan, T. et al. Ebola virus-related encephalitis. Clin. Infect. Dis. 63, 1076–1078 (2016).
Fitzpatrick, G. et al. The contribution of Ebola viral load at admission and other patient characteristics to mortality in a Médecins Sans Frontières Ebola case management centre, Kailahun, Sierra Leone, June–October 2014. J. Infect. Dis. 212, 1752–1758 (2015).
Schieffelin, J. S. et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N. Engl. J. Med. 371, 2092–2100 (2014).
Rollin, P. E., Bausch, D. G. & Sanchez, A. Blood chemistry measurements and D-dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J. Infect. Dis. 196, S364–S371 (2007).
Bah, E. I. et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N. Engl. J. Med. 372, 40–47 (2015). Describes important aspects of the clinical presentation of EVD.
Waxman, M., Aluisio, A. R., Rege, S. & Levine, A. C. Characteristics and survival of patients with Ebola virus infection, malaria, or both in Sierra Leone: a retrospective cohort study. Lancet Infect. Dis. 17, 654–660 (2017).
Vernet, M.-A. et al. Clinical, virological, and biological parameters associated with outcomes of Ebola virus infection in Macenta, Guinea. JCI Insight 2, e88864 (2017).
Carroll, M. W. et al. Deep sequencing of RNA from blood and oral swab samples reveals the presence of nucleic acid from a number of pathogens in patients with acute Ebola virus disease and is consistent with bacterial translocation across the gut. mSphere 2, e00325-17 (2017). RNA sequencing detects the molecular footprints of an array of pathogens (bacterial, viral, fungal and parasitic) in diagnostic blood samples from patients with EVD.
Dhillon, R. S., Srikrishna, D., Garry, R. F. & Chowell, G. Ebola control: rapid diagnostic testing. Lancet Infect. Dis. 15, 147–148 (2015).
World Health Organization. Urgently Needed: Rapid, Sensitive, Safe and Simple Ebola Diagnostic Tests. http://www.who.int/mediacentre/news/ebola/18-november-2014-diagnostics/en (2014).
Nouvellet, P. et al. The role of rapid diagnostics in managing Ebola epidemics. Nature 528, S109–S116 (2015).
Diallo, M. S. K. et al. Prevalence of infection among asymptomatic and paucisymptomatic contact persons exposed to Ebola virus in Guinea: a retrospective, cross-sectional observational study. Lancet Infect. Dis. 19, 308–316 (2019).
Erickson, B. R. et al. Ebola virus disease diagnostics, Sierra Leone: analysis of real-time reverse transcription-polymerase chain reaction values for clinical blood and oral swab specimens. J. Infect. Dis. 214, S258–S262 (2016).
Wannier, S. R. et al. Estimating the impact of violent events on transmission in Ebola virus disease outbreak, Democratic Republic of the Congo, 2018–2019. Epidemics 28, 100353 (2019).
Kucharski, A. J. et al. Measuring the impact of Ebola control measures in Sierra Leone. Proc. Natl Acad. Sci. USA 112, 14366–14371 (2015).
World Health Organization. Clinical management of patients with viral haemorrhagic fever: a pocket guide for front-line health workers. http://apps.who.int/iris/bitstream/10665/205570/1/9789241549608_eng.pdf?ua=1 (2016).
Bevilacqua, N. et al. Criteria for discharge of patients with Ebola virus diseases in high-income countries. Lancet Glob. Health 3, e739–e740 (2015).
World Health Organization. Interim advice on the sexual transmission of the Ebola virus disease. http://www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/ (2016).
Henao Restrepo, A. M. Update on candidate Ebola vaccines: available data on immunogenicity, efficacy and safety. http://www.who.int/immunization/sage/meetings/2018/october/SAGE_october_2018_ebola_Henaorestrepo.pdf (WHO, 2018).
European Commission. Vaccine against Ebola: Commission grants first-ever market authorisation. https://ec.europa.eu/commission/presscorner/detail/en/IP_19_6246 (2019). Market authorization in Europe for Ebola virus vaccine.
World Health Organization. Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response. https://www.who.int/csr/resources/publications/ebola/ebola-ring-vaccination-results-12-april-2019.pdf?ua=1 (2019).
Wells, C. R. et al. Ebola vaccination in the Democratic Republic of the Congo. Proc. Natl Acad. Sci. USA 116, 10178–10183 (2019).
London School of Hygiene & Tropical Medicine. Uganda starts Ebola vaccine trial among healthcare and frontline workers. https://www.lshtm.ac.uk/newsevents/news/2019/uganda-starts-ebola-vaccine-trial-among-healthcare-and-frontline-workers (2019).
Huttner, A. et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 15, 1156–1166 (2015).
US Food and Drug Administration. First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response. https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health (2019).
Wolf, T. et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet 385, 1428–1435 (2015).
World Health Organization. Essential medicines and health products. Table of drug clinical trials. https://www.who.int/medicines/ebola-treatment/ebola_drug_clinicaltrials/en/ (2019).
Sissoko, D. et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 13, e1001967 (2016).
Dunning, J. et al. Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med. 13, e1001997 (2016).
The PREVAIL II Writing Group, et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N. Engl. J. Med. 375, 1448–1456 (2016).
World Health Organization. Notes for the record: consultation on Monitored Emergency Use of Unregistered and Investigational Interventions (MEURI) for Ebola virus disease (EVD). http://www.who.int/ebola/drc-2018/notes-for-the-record-meuri-ebola.pdf (2018).
Qiu, X. et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514, 47–53 (2014).
Davey, R. T. Jr., Nordwall, J. & Proschan, M. A. Trial of ZMapp for Ebola virus infection. N. Engl. J. Med. 376, 700–701 (2017).
Gaudinski, M. R. et al. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Lancet 393, 889–898 (2019).
Corti, D. et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 351, 1339–1342 (2016).
Sivapalasingam, S. et al. Safety, pharmacokinetics, and immunogenicity of a co-formulated cocktail of three human monoclonal antibodies targeting Ebola virus glycoprotein in healthy adults: a randomised, first-in-human phase 1 study. Lancet Infect. Dis. 18, 884–893 (2018).
Pascal, K. E. et al. Development of clinical-stage human monoclonal antibodies that treat advanced Ebola virus disease in nonhuman primates. J. Infect. Dis. 218, S612–S626 (2018).
Warren, T. K. et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531, 381–385 (2016).
National Instite of Allergy and Infectious Diseases. Independent Monitoring Board recommends early termination of Ebola therapeutics trial in DRC because of favorable results with two of four candidates. https://www.niaid.nih.gov/news-events/independent-monitoring-board-recommends-early-termination-ebola-therapeutics-trial-drc (2019).
van Griensven, J. et al. The use of Ebola convalescent plasma to treat Ebola virus disease in resource-constrained settings: a perspective from the field. Clin. Infect. Dis. 62, 69–74 (2016).
van Griensven, J. et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N. Engl. J. Med. 374, 33–42 (2016).
van Griensven, J., Edwards, T. & Baize, S. Efficacy of convalescent plasma in relation to dose of Ebola virus antibodies. N. Engl. J. Med. 375, 2307–2309 (2016).
Mora-Rillo, M. et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir. Med. 3, 554–562 (2015).
Lamontagne, F. et al. Evidence-based guidelines for supportive care of patients with Ebola virus disease. Lancet 391, 700–708 (2018). Supportive care guidelines for patients with EVD.
Cotte, J. et al. Fluid resuscitation in Ebola virus disease: a comparison of peripheral and central venous accesses. Anaesth. Crit. Care Pain. Med. 34, 317–320 (2015).
Kraft, C. S. et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin. Infect. Dis. 61, 496–502 (2015).
Chertow, D. S., Uyeki, T. M. & DuPont, H. L. Loperamide therapy for voluminous diarrhea in Ebola virus disease. J. Infect. Dis. 211, 1036–1037 (2015).
Billioux, B. J., Smith, B. & Nath, A. Neurological complications of Ebola virus infection. Neurotherapeutics 13, 461–470 (2016).
Chertow, D. S. et al. Severe meningoencephalitis in a case of ebola virus disease: a case report. Ann. Intern. Med. 165, 301–304 (2016).
Uyeki, T. M. et al. Clinical management of Ebola virus disease in the United States and Europe. N. Engl. J. Med. 374, 636–646 (2016). Summary of 27 patients with EVD medically evacuated to or infected in and cared for in the USA and Europe highlighting the complexity of disease, delivery of advanced supportive care and therapeutics and improved outcomes.
Sueblinvong, V. et al. Critical care for multiple organ failure secondary to Ebola virus disease in the United States. Crit. Care Med. 43, 2066–2075 (2015). Describes critical care modalities utilized in the care of patients with EVD.
Johnson, D. W. et al. Lessons learned: critical care management of patients with Ebola in the United States. Crit. Care Med. 43, 1157–1164 (2015).
Auffermann, W. F., Kraft, C. S., Vanairsdale, S., Lyon, G. M. III & Tridandapani, S. Radiographic imaging for patients with contagious infectious diseases: how to acquire chest radiographs of patients infected with the Ebola virus. AJR Am. J. Roentgenol. 204, 44–48 (2015).
Langer, M. et al. Intensive care support and clinical outcomes of patients with Ebola virus disease (EVD) in West Africa. Intensive Care Med. 44, 1266–1275 (2018).
Connor, M. J. Jr. et al. Successful delivery of RRT in Ebola virus disease. J. Am. Soc. Nephrol. 26, 31–37 (2015). Details the use of RRT in patients with EVD and renal failure.
Centers for Disease Control and Prevention. Recommendations for safety performing acute hemodialysis in patients with Ebola virus disease (EVD) in U.S. hospitals. http://www.cdc.gov/vhf/ebola/healthcare-us/hospitals/acute-hemodialysis.html (2015).
Torabi-Parizi, P., Davey, R. T. Jr., Suffredini, A. F. & Chertow, D. S. Ethical and practical considerations in providing critical care to patients with Ebola virus disease. Chest 147, 1460–1466 (2015).
Murthy, S. Ebola and provision of critical care. Lancet 385, 1392–1393 (2015).
Halpern, S. D. & Emanuel, E. J. Use of life-sustaining therapies for patients with Ebola virus disease. Ann. Intern. Med. 163, 70 (2015).
Garske, T. et al. Heterogeneities in the case fatality ratio in the West African Ebola outbreak 2013–2016. Philos. Trans. R Soc. Lond. B Biol. Sci. 372, 20160308 (2017).
World Health Organization. Optimized supportive care for Ebola virus disease. Clinical management standard operating procedures. https://www.who.int/csr/resources/publications/optimized-supportive-care/en/ (2019). Field manual focusing on delivery of optimized supportive care in the 2018-present EVD outbreak in the Democratic Republic of the Congo.
Lyon, G. M. et al. Clinical care of two patients with Ebola virus disease in the United States. N. Engl. J. Med. 371, 2402–2409 (2014).
Hunt, L. et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect. Dis. 15, 1292–1299 (2015). Descriptive paper details laboratory findings in patients with EVD.
Iwen, P. C. et al. Safety considerations in the laboratory testing of specimens suspected or known to contain Ebola virus. Am. J. Clin. Pathol. 143, 4–5 (2015).
de La Vega, M.-A., Bello, A., Chaillet, P. & Kobinger, G. P. Diagnosis and management of Ebola samples in the laboratory. Expert. Rev. Anti Infect. Ther. 14, 557–567 (2016).
Gignoux, E. et al. Effect of artesunate-amodiaquine on mortality related to Ebola virus disease. N. Engl. J. Med. 374, 23–32 (2016).
O’Shea, M. K. et al. Diagnosis of febrile illnesses other than Ebola virus disease at an Ebola Treatment Unit in Sierra Leone. Clin. Infect. Dis. 61, 795–798 (2015).
Kangbai, J. B., Heumann, C., Hoelscher, M., Sahr, F. & Froeschl, G. Epidemiological characteristics, clinical manifestations, and treatment outcome of 139 paediatric Ebola patients treated at a Sierra Leone Ebola treatment center. BMC Infect. Dis. 19, 81 (2019).
Damkjær, M., Rudolf, F., Mishra, S., Young, A. & Storgaard, M. Clinical features and outcome of Ebola virus disease in pediatric patients: a retrospective case series. J. Pediatr. 182, 378–381.e1 (2017).
Shah, T. et al. Inpatient signs and symptoms and factors associated with death in children aged 5 years and younger admitted to two Ebola management centres in Sierra Leone, 2014: a retrospective cohort study. Lancet Glob. Health 4, e495–e501 (2016).
Trehan, I., Kelly, T., Marsh, R. H., George, P. M. & Callahan, C. W. Moving towards a more aggressive and comprehensive model of care for children with Ebola. J. Pediat. 170, 28–33.e1-7 (2016).
Caluwaerts, S. et al. Dilemmas in managing pregnant women with Ebola: 2 case reports. Clin. Infect. Dis. 62, 903–905 (2016).
Nelson, J. M., Griese, S. E., Goodman, A. B. & Peacock, G. Live neonates born to mothers with Ebola virus disease: a review of the literature. J. Perinatol. 36, 411–414 (2016).
Bebell, L. M., Oduyebo, T. & Riley, L. E. Ebola virus disease and pregnancy: a review of the current knowledge of Ebola virus pathogenesis, maternal, and neonatal outcomes. Birth Defects Res. 109, 353–362 (2017).
Haddad, L. B., Jamieson, D. J. & Rasmussen, S. A. Pregnant women and the Ebola crisis. N. Engl. J. Med. 379, 2492–2493 (2018).
Mupapa, K. et al. Ebola hemorrhagic fever and pregnancy. J. Infect. Dis. 179, S11–S12 (1999).
Dörnemann, J. et al. First newborn baby to receive experimental therapies survives Ebola virus disease. J. Infect. Dis. 215, 171–174 (2017). Case report of the clinical course and care of the first neonate to survive EVD, including the use of EBOV-specific mAb-based and antiviral therapeutics.
Centers for Disease Control and Prevention. Care of a neonate born to a mother who is confirmed to have Ebola, is a person under investigation, or has been exposed to Ebola. Interim Guidance for U.S. Hospitals on the Care of a Neonate Born to a Mother who is Confirmed to have Ebola, is a Person under Investigation (PUI), or has been Exposed to Ebola. http://www.cdc.gov/vhf/ebola/healthcare-us/hospitals/neonatal-care.html (2016).
Bwaka, M. A. et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J. Infect. Dis. 179, S1–S7 (1999).
Rowe, A. K. et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. J. Infect. Dis. 179, S28–S35 (1999).
Sneller, M. C. et al. A longitudinal study of Ebola sequelae in Liberia. N. Engl. J. Med. 380, 924–934 (2019). A large, prospective, controlled observational study of Liberian survivors of EVD detailing symptoms and signs of clinical sequelae and EBOV semen persistence.
World Health Organization. Caring for Ebola survivors: Supporting survivors to recover their lives and livelihoods. http://www.who.int/csr/disease/ebola/survivors/caring-for-survivors/en/ (2016).
Yeh, S., Shantha, J. G., Hayek, B., Crozier, I. & Smith, J. R. Clinical manifestations and pathogenesis of uveitis in Ebola virus disease survivors. Ocul. Immunol. Inflamm. 26, 1128–1134 (2018).
Qureshi, A. I. et al. Study of Ebola virus disease survivors in Guinea. Clin. Infect. Dis. 61, 1035–1042 (2015).
Tiffany, A. et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin. Infect. Dis. 62, 1360–1366 (2016).
Howlett, P. et al. Ebola virus disease complicated by late-onset encephalitis and polyarthritis, Sierra Leone. Emerg. Infect. Dis. 22, 150–152 (2016).
Clark, D. V. et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect. Dis. 15, 905–912 (2015).
Epstein, L., Wong, K. K., Kallen, A. J. & Uyeki, T. M. Post-Ebola signs and symptoms in U.S. survivors. N. Engl. J. Med. 373, 2484–2486 (2015).
Kibadi, K. et al. Late ophthalmologic manifestations in survivors of the 1995 Ebola virus epidemic in Kikwit, Democratic Republic of the Congo. J. Infect. Dis. 179, S13–S14 (1999).
Mattia, J. G. et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect. Dis. 16, 331–338 (2016).
Nanyonga, M., Saidu, J., Ramsay, A., Shindo, N. & Bausch, D. G. Sequelae of Ebola virus disease, Kenema District, Sierra Leone. Clin. Infect. Dis. 62, 125–126 (2016).
Scott, J. T. et al. Post-Ebola syndrome, Sierra Leone. Emerg. Infect. Dis. 22, 641–646 (2016).
Shantha, J. G., Crozier, I. & Yeh, S. An update on ocular complications of Ebola virus disease. Curr. Opin. Ophthalmol. 28, 600–606 (2017).
Shantha, J. G. et al. Long-term management of panuveitis and iris heterochromia in an Ebola survivor. Ophthalmology 123, 2626–2628.e2 (2016).
Hugo, M. et al. Post-traumatic stress reactions in Ebola virus disease survivors in Sierra Leone. Emerg. Med. 5, 285 (2015).
Mohammed, A. et al. An evaluation of psychological distress and social support of survivors and contacts of Ebola virus disease infection and their relatives in Lagos, Nigeria: a cross sectional study–2014. BMC Public Health 15, 824 (2015).
Reardon, S. Ebola’s mental-health wounds linger in Africa. Nature 519, 13–14 (2015).
Evans, D. K. & Popova, A. West African Ebola crisis and orphans. Lancet 385, 945–946 (2015).
Save the Children Foundation. Ebola crisis: facts, FAQs, and how to help. http://www.savethechildren.org/site/c.8rKLIXMGIpI4E/b.9208421/k.244F/Ebola_Response_in_West_Africa.htm?msource=weklpebo1014 (2015).
Gatiso, T. T. et al. The impact of the Ebola virus disease (EVD) epidemic on agricultural production and livelihoods in Liberia. PLoS Negl. Trop. Dis. 12, e0006580 (2018).
Chow, A. et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis. 203, 149–157 (2011).
Hoarau, J.-J. et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 184, 5914–5927 (2010).
Roques, P. & Gras, G. Chikungunya fever: focus on peripheral markers of pathogenesis. J. Infect. Dis. 203, 141–143 (2011).
Jacobs, M. et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 388, 498–503 (2016). Detailed case report of severe meningoencephalitis associated with infectious EBOV persistence in cerebrospinal fluid of a survivor of EVD more than 10 months after acute disease onset.
Varkey, J. B. et al. Persistence of Ebola virus in ocular fluid during convalescence. N. Engl. J. Med. 372, 2423–2427 (2015). Detailed case report of severe pan-uveitis associated with persistent infectious EBOV in the aqueous humor more than 3 months after acute disease onset.
Christie, A. et al. Possible sexual transmission of Ebola virus - Liberia, 2015. Morb. Mortal. Wkly. Rep. 64, 479–481 (2015).
Fischer, W. A. II & Wohl, D. A. Confronting Ebola as a sexually transmitted infection. Clin. Infect. 62, 1272–1276 (2016).
Martini, G. A. & Schmidt, H. A. Spermatogenic transmission of the “Marburg virus”. (Causes of “Marburg simian disease”) [German]. Klin. Wochenschr. 46, 398–400 (1968).
Dokubo, E. K. et al. Persistence of Ebola virus after the end of widespread transmission in Liberia: an outbreak report. Lancet Infect. Dis. 18, 1015–1024 (2018).
Subissi, L. et al. Ebola virus transmission caused by persistently infected survivors of the 2014–2016 outbreak in West Africa. J. Infect. Dis. 218, S287–S291 (2018).
Liu, W. J. et al. Comprehensive clinical and laboratory follow-up of a female patient with Ebola virus disease: Sierra Leone Ebola virus persistence study. Open Forum Infect. Dis. 6, ofz068 (2019).
Rodriguez, L. L. et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J. Infect. Dis. 179, S170–S176 (1999).
Bower, H. et al. Delivery of an Ebola virus-positive stillborn infant in a rural community health center, Sierra Leone, 2015. Am. J. Trop. Med. Hyg. 94, 417–419 (2016).
Sissoko, D. et al. Ebola virus persistence in breast milk after no reported illness: a likely source of virus transmission from mother to child. Clin. Infect. Dis. 64, 513–516 (2017).
Zeng, X. et al. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat. Microbiol. 2, 17113 (2017).
Fischer, W. A. II et al. Shifting the paradigm - applying universal standards of care to Ebola virus disease. N. Engl. J. Med. 380, 1389–1391 (2019).
Epelboin, A. & Formenty, P. in Les Maladies Infectieuses Émergentes: État de la Situation et Perspectives (eds Leport, C. & Guégan, J. F.) 111–113 (Haut Conseil de la Santé Publique, 2011).
Hewlett, B. S. & Hewlett, B. L. Ebola, Culture and Politics: the Anthropology of an Emerging Disease (Thomson Wadsworth, 2008).
Prinz, A. Contributions to visual anthropology - ethnomedical background of the Ebola epidemic 2004 in Yambio, South Sudan. Vienn. Ethnomed. Newsl. 7, 16–19 (2005).
Chandler, C. et al. Ebola: limitations of correcting misinformation. Lancet 385, 1275–1277 (2015).
Richards, P. et al. Social pathways for Ebola virus disease in rural Sierra Leone, and some implications for containment. PLoS Negl. Trop. Dis. 9, e0003567 (2015).
Sams, K., Desclaux, A. & Sow, S. ‘They’ll inject you and you’ll die’: from medication non-compliance to acceptance in Guinea’s Ebola treatment units. Anthropol. Med. https://doi.org/10.1080/13648470.2019.1615749 (2019).
Thys, S. & Boelaert, M. The origin of Ebola: biomedical approach versus popular interpretations in Macenta, Guinea [French]. Santé Publique 29, 497–507 (2017).
Desclaux, A. & Anoko, J. Anthropology engaged against Ebola (2014–2016): approaches, contributions and new questions [French]. Santé Publique 29, 477–485 (2017).
Kasereka, M. C. & Hawkes, M. T. ‘The cat that kills people:’ community beliefs about Ebola origins and implications for disease control in Eastern Democratic Republic of the Congo. Pathog. Glob. Health 113, 149–157 (2019).
World Health Organization. Case definition recommendations for Ebola or Marburg virus diseases. https://www.who.int/csr/resources/publications/ebola/case-definition/en/ (2014).
Centers for Disease Control and Prevention. Case definition for Ebola virus disease (EVD). https://www.cdc.gov/vhf/ebola/clinicians/evaluating-patients/case-definition.html (2014).
Centers for Disease Control and Prevention. Ebola (Ebola Virus Disease). Transmission. https://www.cdc.gov/vhf/ebola/transmission/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvhf%2Febola%2Fexposure%2Frisk-factors-when-evaluating-person-for-exposure.html (2019).
Levine, A. C. et al. Derivation and internal validation of the Ebola prediction score for risk stratification of patients with suspected Ebola virus disease. Ann. Emerg. Med. 66, 285–293.e1 (2015).
Wahl-Jensen, V. et al. in Viral Hemorrhagic Fevers Ch. 7 (eds Singh, S. K. & Ruzek, D.) 99–127 (CRC Press, 2013).
Judson, S. D., Fischer, R., Judson, A. & Munster, V. J. Ecological contexts of index cases and spillover events of different ebolaviruses. PLoS Pathog. 12, e1005780 (2016).
Emanuel, J., Marzi, A. & Feldmann, H. Filoviruses: ecology, molecular biology, and evolution. Adv. Virus Res. 100, 189–221 (2018).
Kuhn, J. H. in Harrison’s principles of internal medicine Vol. 2 Ch. 205 (eds J. Larry Jameson et al.) (McGraw-Hill Education, 2018).
White, J. M. & Schornberg, K. L. A new player in the puzzle of filovirus entry. Nat. Rev. Microbiol. 10, 317–322 (2012).
Ploquin, A., Zhou, Y. & Sullivan, N. J. Ebola immunity: gaining a winning position in lightning chess. J. Immunol. 201, 833–842 (2018). Detailed review of the “timing is everything” host–pathogen arms race after EBOV infections in humans and non-human primates.
Etard, J.-F. et al. Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect. Dis. 17, 545–552 (2017).
Shantha, J. G. et al. Ophthalmic manifestations and causes of vision impairment in Ebola virus disease survivors in Monrovia, Liberia. Ophthalmology 124, 170–177 (2017).
Hereth-Hebert, E. et al. Ocular complications in survivors of the Ebola outbreak in Guinea. Am. J. Ophthalmol. 175, 114–121 (2017).
Cnops, L. et al. Where are the Ebola diagnostics from last time? Nature 565, 419–421 (2019).
Dahlke, C. et al. Dose-dependent T-cell dynamics and cytokine cascade following rVSV-ZEBOV immunization. EBioMedicine 19, 107–118 (2017).
Milligan, I. D. et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA 315, 1610–1623 (2016).
Huttner, A. et al. Determinants of antibody persistence across doses and continents after single-dose rVSV-ZEBOV vaccination for Ebola virus disease: an observational cohort study. Lancet Infect. Dis. 18, 738–748 (2018).
Kennedy, S. B. et al. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N. Engl. J. Med. 377, 1438–1447 (2017).
Samai, M. et al. The Sierra Leone trial to introduce a vaccine against Ebola: an evaluation of rVSVG-ZEBOV-GP vaccine tolerability and safety during the West Africa Ebola outbreak. J. Infect. Dis. 217, S6–S15 (2018).
McWilliams, I. L. et al. Pseudovirus rVSVΔG-ZEBOV-GP infects neurons in retina and CNS, causing apoptosis and neurodegeneration in neonatal mice. Cell Rep. 26, 1718–1726.e4 (2019).
van den Pol, A. N., Mao, G., Chattopadhyay, A., Rose, J. K. & Davis, J. N. Chikungunya, influenza, Nipah, and Semliki Forest chimeric viruses with vesicular stomatitis virus: actions in the brain. J. Virol. 91, e02154-16 (2017).
Halperin, S. A. et al. Immunogenicity, lot consistency, and extended safety of rVSVDeltaG-ZEBOV-GP vaccine: a phase 3 randomized, double-blind, placebo-controlled study in healthy adults. J. Infect. Dis. 220, 1127–1135 (2019).
Winslow, R. L. et al. Immune responses to novel adenovirus type 26 and modified vaccinia virus Ankara-vectored Ebola vaccines at 1 year. JAMA 317, 1075–1077 (2017).
Acknowledgements
The authors thank J. Wada and J. Bernbaum (NIH/NIAID Integrated Research Facility at Fort Detrick, Frederick, MD, USA) for critically editing the manuscript and helping with creating Figs 1c, 1d, 2 and 4a. This work was supported in part through Battelle Memorial Institute’s prime contract with the US National Institute of Allergy and Infectious Diseases (NIAID) under contract no. HHSN272200700016I (to J.H.K.) and with federal funds from the National Cancer Institute (NCI), National Institutes of Health (NIH), under contract no. HHSN261200800001E to I.C., who was supported by the Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research, sponsored by NCI. This work was also funded in part under contract no. HSHQDC-15-C-00064 awarded by Department of Homeland Security Science and Technology Directorate for the management and operation of the National Biodefense Analysis and Countermeasures Center (NBACC), a federally funded research and development centre (to V.W.).
Author information
Authors and Affiliations
Contributions
Introduction (J.H.K.); Epidemiology (S.T.J., M.-A.d.L.V. and J.H.K.); Mechanisms/pathophysiology (M.-A.d.L.V., V.W., A.G. and J.H.K.); Diagnosis, screening and prevention (S.T.J., I.C., A.G., L.B. and J.H.K.); Management (S.T.J., I.C., W.A.F.II, A.H., C.S.K., M.J.S., L.B. and J.H.K.); Quality of life (S.T.J., I.C., W.A.F.II, A.H., C.S.K. and M.J.S., J.H.K.); Outlook (all authors); Overview of Primer (all authors).
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests.
Additional information
Disclaimer
The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the US Department of Health and Human Services, the Department of Homeland Security Science and Technology Directorate, or of the institutions and companies affiliated with the authors. In no event shall any of these entities have any responsibility or liability for any use, misuse, inability to use, or reliance upon the information contained herein. The US departments do not endorse any products or commercial services mentioned in this publication.
Peer review information
Nature Reviews Disease Primers thanks T. Brooks, P. Horby, G. Ippolito and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov
Rights and permissions
About this article
Cite this article
Jacob, S.T., Crozier, I., Fischer, W.A. et al. Ebola virus disease. Nat Rev Dis Primers 6, 13 (2020). https://doi.org/10.1038/s41572-020-0147-3
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-020-0147-3
This article is cited by
-
Monoclonal antibody applications in travel medicine
Tropical Diseases, Travel Medicine and Vaccines (2024)
-
Evaluating the risk of conflict on recent Ebola outbreaks in Guinea and the Democratic Republic of the Congo
BMC Public Health (2024)
-
Addressing biodiversity conservation, disease surveillance, and public health interventions through One Health approach in Hainan’s tropical rainforest
One Health Advances (2024)
-
Asymptomatic viruses detectable in saliva in the first year of life: a narrative review
Pediatric Research (2024)
-
Emerging and re-emerging pediatric viral diseases: a continuing global challenge
Pediatric Research (2024)