Abstract
Systemic immunoglobulin light chain amyloidosis is a protein misfolding disease caused by the conversion of immunoglobulin light chains from their soluble functional states into highly organized amyloid fibrillar aggregates that lead to organ dysfunction. The disease is progressive and, accordingly, early diagnosis is vital to prevent irreversible organ damage, of which cardiac damage and renal damage predominate. The development of novel sensitive biomarkers and imaging technologies for the detection and quantification of organ involvement and damage is facilitating earlier diagnosis and improved evaluation of the efficacy of new and existing therapies. Treatment is guided by risk assessment, which is based on levels of cardiac biomarkers; close monitoring of clonal and organ responses guides duration of therapy and changes in regimen. Several new classes of drugs, such as proteasome inhibitors and immunomodulatory drugs, along with high-dose chemotherapy and autologous haematopoietic stem cell transplantation, have led to rapid and deep suppression of amyloid light chain production in the majority of patients. However, effective therapies for patients with advanced cardiac involvement are an unmet need. Passive immunotherapies targeting clonal plasma cells and directly accelerating removal of amyloid deposits promise to further improve the overall outlook of this increasingly treatable disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Merlini, G. & Bellotti, V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 349, 583–596 (2003).
Pepys, M. B. Amyloidosis. Annu. Rev. Med. 57, 223–241 (2006).
Sipe, J. D. et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 23, 209–213 (2016).
Kourelis, T. V. et al. Presentation and outcomes of localized immunoglobulin light chain amyloidosis: the Mayo Clinic experience. Mayo Clin. Proc. 92, 908–917 (2017).
Obici, L. & Merlini, G. Amyloidosis in autoinflammatory syndromes. Autoimmun. Rev. 12, 14–17 (2012).
Nasr, S. H., Dogan, A. & Larsen, C. P. Leukocyte cell-derived chemotaxin 2-associated amyloidosis: a recently recognized disease with distinct clinicopathologic characteristics. Clin. J. Am. Soc. Nephrol. 10, 2084–2093 (2015).
Merlini, G. AL amyloidosis: from molecular mechanisms to targeted therapies. Hematology Am. Soc. Hematol. Educ. Program 2017, 1–12 (2017).
Muchtar, E. et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood 129, 2111–2119 (2017).
Quock, T. P., Yan, T., Chang, E., Guthrie, S. & Broder, M. S. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2, 1046–1053 (2018).
Kyle, R. A. et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 378, 241–249 (2018).
Desikan, K. R. et al. Incidence and impact of light chain associated (AL) amyloidosis on the prognosis of patients with multiple myeloma treated with autologous transplantation. Leuk. Lymphoma 27, 315–319 (1997).
Madan, S. et al. Clinical features and treatment response of light chain (AL) amyloidosis diagnosed in patients with previous diagnosis of multiple myeloma. Mayo Clin. Proc. 85, 232–238 (2010).
da Silva Filho, M. I. et al. Genome-wide association study of immunoglobulin light chain amyloidosis in three patient cohorts: comparison with myeloma. Leukemia 31, 1735–1742 (2017).
Kyle, R. A. et al. Incidence and natural history of primary systemic amyloidosis in Olmsted county, Minnesota, 1950 through 1989. Blood 79, 1817–1822 (1992).
Pinney, J. H. et al. Systemic amyloidosis in England: an epidemiological study. Br. J. Haematol. 161, 525–532 (2013).
Hemminki, K., Li, X., Forsti, A., Sundquist, J. & Sundquist, K. Incidence and survival in non-hereditary amyloidosis in Sweden. BMC Public Health 12, 974 (2012).
Quock, T. P., Yan, T., Chang, E., Guthrie, S. & Broder, M. S. Healthcare resource utilization and costs in amyloid light-chain amyloidosis: a real-world study using US claims data. J. Comp. Eff. Res. 7, 549–559 (2018).
Duhamel, S. et al. Incidence and prevalence of light chain amyloidosis: a population-based study. Blood 130, 5577 (2017).
Aguirre, M. A. et al. Incidence rate of amyloidosis in patients from a medical care program in Buenos Aires, Argentina: a prospective cohort. Amyloid 23, 184–187 (2016).
Hipp, M. S., Park, S. H. & Hartl, F. U. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 24, 506–514 (2014).
Yerbury, J. J. et al. Walking the tightrope: proteostasis and neurodegenerative disease. J. Neurochem. 137, 489–505 (2016).
Labbadia, J. & Morimoto, R. I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 84, 435–464 (2015).
Eanes, E. D. & Glenner, G. G. X-ray diffraction studies on amyloid filaments. J. Histochem. Cytochem. 16, 673–677 (1968).
Shirahama, T. & Cohen, A. S. High-resolution electron microscopic analysis of the amyloid fibril. J. Cell Biol. 33, 679–708 (1967).
Merlini, G. & Stone, M. J. Dangerous small B cell clones. Blood 108, 2520–2530 (2006). This paper presents pioneering work that introduces the concept that small, indolent clones can produce systemic damage through the production of immunoglobulin or fragments thereof.
Bochtler, T. et al. Hyperdiploidy is less frequent in AL amyloidosis compared with monoclonal gammopathy of undetermined significance and inversely associated with translocation t(11;14). Blood 117, 3809–3815 (2011).
Morgan, G. J. & Kelly, J. W. The kinetic stability of a full-length antibody light chain dimer determines whether endoproteolysis can release amyloidogenic variable domains. J. Mol. Biol. 428, 4280–4297 (2016).
Blancas-Mejia, L. M. et al. Thermodynamic and fibril formation studies of full length immunoglobulin light chain AL-09 and its germline protein using scan rate dependent thermal unfolding. Biophys. Chem. 207, 13–20 (2015).
Oberti, L. et al. Concurrent structural and biophysical traits link with immunoglobulin light chains amyloid propensity. Sci. Rep. 7, 16809 (2017).
Wyatt, A. R., Yerbury, J. J., Dabbs, R. A. & Wilson, M. R. Roles of extracellular chaperones in amyloidosis. J. Mol. Biol. 421, 499–516 (2012).
Ami, D. et al. In situ characterization of protein aggregates in human tissues affected by light chain amyloidosis: a FTIR microspectroscopy study. Sci. Rep. 6, 29096 (2016).
Diomede, L. et al. Cardiac light chain amyloidosis: the role of metal ions in oxidative stress and mitochondrial damage. Antioxid. Redox Signal. 27, 567–582 (2017).
Marin-Argany, M. et al. Cell damage in light chain amyloidosis: fibril internalization, toxicity and cell-mediated seeding. J. Biol. Chem. 291, 19813–19825 (2016).
Pepys, M. B., Dyck, R. F., de Beer, F. C., Skinner, M. & Cohen, A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin. Exp. Immunol. 38, 284–293 (1979).
Tennent, G. A., Lovat, L. B. & Pepys, M. B. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proc. Natl Acad. Sci. USA 92, 4299–4303 (1995). This study supports the role of SAP in preventing amyloid resorption, and the findings from this study led to anti-SAP therapies.
Shi, J. et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc. Natl Acad. Sci. USA 107, 4188–4193 (2010).
Brenner, D. A. et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ. Res. 94, 1008–1010 (2004). This is a pioneering paper on the cardiotoxicity of amyloidogenic light chains.
Imperlini, E. et al. Proteotoxicity in cardiac amyloidosis: amyloidogenic light chains affect the levels of intracellular proteins in human heart cells. Sci. Rep. 7, 15661 (2017).
Westermark, G. T., Fandrich, M., Lundmark, K. & Westermark, P. Noncerebral amyloidoses: aspects on seeding, cross-seeding, and transmission. Cold Spring Harb. Perspect. Med. 8, a024323 (2018).
Nystrom, S. N. & Westermark, G. T. AA-amyloid is cleared by endogenous immunological mechanisms. Amyloid 19, 138–145 (2012).
Comenzo, R. L., Zhang, Y., Martinez, C., Osman, K. & Herrera, G. A. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V-L germ line gene use and clonal plasma cell burden. Blood 98, 714–720 (2001).
Perfetti, V. et al. The repertoire of lambda light chains causing predominant amyloid heart involvement and identification of a preferentially involved germline gene, IGLV1-44. Blood 119, 144–150 (2012).
Kourelis, T. V. et al. Clarifying immunoglobulin gene usage in systemic and localized immunoglobulin light-chain amyloidosis by mass spectrometry. Blood 129, 299–306 (2017).
Ma, K. K., Ogawa, T. & de Bold, A. J. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J. Mol. Cell. Cardiol. 36, 505–513 (2004).
Merlini, G. et al. Rationale, application and clinical qualification for NT-proBNP as a surrogate end point in pivotal clinical trials in patients with AL amyloidosis. Leukemia 30, 1979–1986 (2016).
Wechalekar, A. D., Gillmore, J. D. & Hawkins, P. N. Systemic amyloidosis. Lancet 387, 2641–2654 (2016).
Lovat, L. B., Persey, M. R., Madhoo, S., Pepys, M. B. & Hawkins, P. N. The liver in systemic amyloidosis: insights from 123I serum amyloid P component scintigraphy in 484 patients. Gut 42, 727–734 (1998).
Quarta, C. C. et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur. Heart J. 38, 1905–1908 (2017).
Muchtar, E. et al. Overuse of organ biopsies in immunoglobulin light chain amyloidosis (AL): the consequence of failure of early recognition. Ann. Med. 49, 545–551 (2017). This study shows that, if the diagnosis of amyloidosis is considered in the differential diagnosis, invasive procedures can be avoided.
Schonland, S. O. et al. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Blood 119, 488–493 (2012).
Linke, R. P. On typing amyloidosis using immunohistochemistry. Detailled illustrations, review and a note on mass spectrometry. Prog. Histochem. Cytochem. 47, 61–132 (2012).
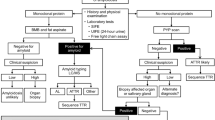
Fernandez de Larrea, C. et al. A practical approach to the diagnosis of systemic amyloidoses. Blood 125, 2239–2244 (2015).
Vrana, J. A. et al. Clinical diagnosis and typing of systemic amyloidosis in subcutaneous fat aspirates by mass spectrometry-based proteomics. Haematologica 99, 1239–1247 (2014).
Pont, L. et al. A chemometric approach for characterization of serum transthyretin in familial amyloidotic polyneuropathy type I (FAP-I) by electrospray ionization-ion mobility mass spectrometry. Talanta 181, 87–94 (2018).
Geller, H. I. et al. Prevalence of monoclonal gammopathy in wild-type transthyretin amyloidosis. Mayo Clin. Proc. 92, 1800–1805 (2017).
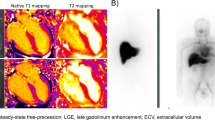
Aljaroudi, W. A. et al. Role of imaging in the diagnosis and management of patients with cardiac amyloidosis: state of the art review and focus on emerging nuclear techniques. J. Nucl. Cardiol. 21, 271–283 (2014).
Gillmore, J. D. et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 133, 2404–2412 (2016). This is a consensus paper that facilitates a differential diagnosis with wild-type ATTR amyloidosis.
de Miguel, C. et al. Myocardial uptake of 99mTc-DPD in patients with AL amyloidosis. Amyloid. 24, 48–49 (2017).
Palladini, G. et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 124, 2325–2332 (2014). This study presents a validated staging system for renal outcomes and criteria for renal response.
Lousada, I., Comenzo, R. L., Landau, H., Guthrie, S. & Merlini, G. Light chain amyloidosis: patient experience survey from the Amyloidosis Research Consortium. Adv. Ther. 32, 920–928 (2015).
Kourelis, T. V. et al. Immunoglobulin light chain amyloidosis is diagnosed late in patients with preexisting plasma cell dyscrasias. Am. J. Hematol. 89, 1051–1054 (2014).
Weiss, B. M. et al. Increased serum free light chains precede the presentation of immunoglobulin light chain amyloidosis. J. Clin. Oncol. 32, 2699–2704 (2014). Among the 20 patients with AL amyloidosis in this study, a monoclonal protein is shown to be present by free light chain assay and/or immunofixation in 100%, 80% and 42% of patients at <4 years, 4–11 years and >11 years before diagnosis, respectively.
Palladini, G. et al. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation 107, 2440–2445 (2003). This study introduces NT-proBNP as a sensitive marker for the diagnosis and follow-up after therapy of amyloid cardiac dysfunction.
Wechalekar, A. D. et al. Abnormal N-terminal fragment of brain natriuretic peptide in patients with light chain amyloidosis without cardiac involvement at presentation is a risk factor for development of cardiac amyloidosis. Haematologica 96, 1079–1080 (2011).
Merlini, G. & Palladini, G. Differential diagnosis of monoclonal gammopathy of undetermined significance. Hematology Am. Soc. Hematol. Educ. Program 2012, 595–603 (2012).
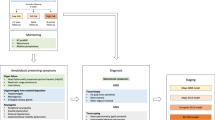
Merlini, G., Wechalekar, A. D. & Palladini, G. Systemic light chain amyloidosis: an update for treating physicians. Blood 121, 5124–5130 (2013).
Palladini, G. et al. Biomarker-based screening of organ dysfunction in patients with MGUS allows early diagnosis of AL amyloidosis. Blood 130, 1760 (2017).
Wechalekar, A. D. et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 121, 3420–3427 (2013).
Dispenzieri, A. et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J. Clin. Oncol. 22, 3751–3757 (2004). This paper presents a widely used staging system, which is based on cardiac biomarkers, that is essential in the management of patients with AL amyloidosis.
Kristen, A. V. et al. Assessment of disease severity and outcome in patients with systemic light-chain amyloidosis by the high-sensitivity troponin T assay. Blood 116, 2455–2461 (2010).
Palladini, G. et al. The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood 116, 3426–3430 (2010).
Kourelis, T. V. et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J. Clin. Oncol. 31, 4319–4324 (2013).
Dittrich, T. et al. AL amyloidosis patients with low amyloidogenic free light chain levels at first diagnosis have an excellent prognosis. Blood 130, 632–642 (2017).
Milani, P. et al. Patients with light-chain amyloidosis and low free light-chain burden have distinct clinical features and outcome. Blood 130, 625–631 (2017).
Sidana, S. et al. Clinical presentation and outcomes in light chain amyloidosis patients with non-evaluable serum free light chains. Leukemia 32, 729–735 (2017).
Kumar, S. et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 30, 989–995 (2012).
Palladini, G. et al. Oral melphalan and dexamethasone grants extended survival with minimal toxicity in AL amyloidosis: long-term results of a risk-adapted approach. Haematologica 99, 743–750 (2014).
Kastritis, E. et al. Renal outcomes in patients with AL amyloidosis: prognostic factors, renal response and the impact of therapy. Am. J. Hematol. 92, 632–639 (2017).
Kastritis, E. et al. Clinical and prognostic significance of serum levels of von Willebrand factor and ADAMTS-13 antigens in AL amyloidosis. Blood 128, 405–409 (2016).
Kastritis, E. et al. Growth differentiation factor-15 is a new biomarker for survival and renal outcomes in light chain amyloidosis. Blood 131, 1568–1575 (2018).
Palladini, G. et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J. Clin. Oncol. 30, 4541–4549 (2012). This paper provides new consensus criteria for haematological and cardiac response, which are now universally used.
Tandon, N. et al. Impact of involved free light chain (FLC) levels in patients achieving normal FLC ratio after initial therapy in light chain amyloidosis (AL). Am. J. Hematol. 93, 17–22 (2018).
Kastritis, E. et al. Evaluation of minimal residual disease using next-generation flow cytometry in patients with AL amyloidosis. Blood Cancer J. 8, 46 (2018).
Palladini, G. et al. Persistence of minimal residual disease by multiparameter flow cytometry can hinder recovery of organ damage in patients with AL amyloidosis otherwise in complete response. Blood 128, 3261–3261 (2016).
Comenzo, R. L. et al. Dose-intensive melphalan with blood stem cell support for the treatment of AL amyloidosis: one-year follow-up in five patients. Blood 88, 2801–2806 (1996). This report introduces autologous stem cell transplantation as an effective treatment for AL amyloidosis.
Skinner, M. et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann. Intern. Med. 140, 85–93 (2004).
Tsai, S. B. et al. High-dose melphalan and stem cell transplantation for patients with AL amyloidosis: trends in treatment-related mortality over the past 17 years at a single referral center. Blood 120, 4445–4446 (2012).
Batalini, F. et al. High-dose melphalan and stem cell transplantation in patients on dialysis due to immunoglobulin light-chain amyloidosis and monoclonal immunoglobulin deposition disease. Biol. Blood Marrow Transplant. 24, 127–132 (2018).
Jaccard, A. et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N. Engl. J. Med. 357, 1083–1093 (2007).
D’Souza, A. et al. Improved outcomes after autologous hematopoietic cell transplantation for light chain amyloidosis: a Center for International Blood and Marrow Transplant Research Study. J. Clin. Oncol. 33, 3741–3749 (2015).
Nguyen, V. P. et al. Modified high-dose melphalan and autologous stem cell transplantation for immunoglobulin light chain amyloidosis. Biol. Blood Marrow Transplant. 24, 1823–1827 (2018).
Comenzo, R. L. & Gertz, M. A. Autologous stem cell transplantation for primary systemic amyloidosis. Blood 99, 4276–4282 (2002).
Tandon, N. et al. Revisiting conditioning dose in newly diagnosed light chain amyloidosis undergoing frontline autologous stem cell transplant: impact on response and survival. Bone Marrow Transplant. 52, 1126–1132 (2017).
Cibeira, M. T. et al. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood 118, 4346–4352 (2011).
Browning, S. et al. Hematologic relapse in AL amyloidosis after high-dose melphalan and stem cell transplantation. Blood 130, 1383–1386 (2017).
Gertz, M. A. et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 48, 557–561 (2013).
Dember, L. M. et al. Effect of dose-intensive intravenous melphalan and autologous blood stem-cell transplantation on AL amyloidosis-associated renal disease. Ann. Intern. Med. 134, 746–753 (2001).
Seldin, D. C. et al. Improvement in quality of life of patients with AL amyloidosis treated with high-dose melphalan and autologous stem cell transplantation. Blood 104, 1888–1893 (2004).
Girnius, S. et al. Hepatic response after high-dose melphalan and stem cell transplantation in patients with AL amyloidosis associated liver disease. Haematologica 94, 1029–1032 (2009).
Meier-Ewert, H. K. et al. Regression of cardiac wall thickness following chemotherapy and stem cell transplantation for light chain (AL) amyloidosis. Amyloid 18, S130–S131 (2011).
Salinaro, F. et al. Longitudinal systolic strain, cardiac function improvement, and survival following treatment of light-chain (AL) cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging 18, 1057–1064 (2017).
Sanchorawala, V. et al. Induction therapy with bortezomib followed by bortezomib-high dose melphalan and stem cell transplantation for light chain amyloidosis: results of a prospective clinical trial. Biol. Blood Marrow Transplant. 21, 1445–1451 (2015).
Sanchorawala, V. et al. High-dose intravenous melphalan and autologous stem cell transplantation as initial therapy or following two cycles of oral chemotherapy for the treatment of AL amyloidosis: results of a prospective randomized trial. Bone Marrow Transplant. 33, 381–388 (2004).
Sanchorawala, V., Quillen, K., Sloan, J. M., Andrea, N. T. & Seldin, D. C. Bortezomib and high-dose melphalan conditioning for stem cell transplantation for AL amyloidosis: a pilot study. Haematologica 96, 1890–1892 (2011).
Landau, H. et al. Bortezomib and dexamethasone consolidation following risk-adapted melphalan and stem cell transplantation for patients with newly diagnosed light-chain amyloidosis. Leukemia 27, 823–828 (2013).
Dittus, C., Uwumugambi, N., Sun, F., Sloan, J. M. & Sanchorawala, V. The effect of bone marrow plasma cell burden on survival in patients with light chain amyloidosis undergoing high-dose melphalan and autologous stem cell transplantation. Biol. Blood Marrow Transplant. 22, 1729–1732 (2016).
Landau, H. et al. Long-term event-free and overall survival after risk-adapted melphalan and SCT for systemic light chain amyloidosis. Leukemia 31, 136–142 (2017).
Palladini, G. et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 126, 612–615 (2015).
Palladini, G. et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood 103, 2936–2938 (2004). This paper details how the addition of dexamethasone to melphalan rather than prednisone revolutionized the treatment of intermediate-risk and high-risk patients: both haematological and organ response rates doubled with this innovation.
Palladini, G. et al. Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: a matched case-control study on 174 patients. Leukemia 28, 2311–2316 (2014).
Venner, C. P. et al. A matched comparison of cyclophosphamide, bortezomib and dexamethasone (CVD) versus risk-adapted cyclophosphamide, thalidomide and dexamethasone (CTD) in AL amyloidosis. Leukemia 28, 2304–2310 (2014).
Kastritis, E. et al. A randomized phase III trial of melphalan and dexamethasone (MDex) versus bortezomib, melphalan and dexamethasone (BMDex) for untreated patients with AL amyloidosis. Blood 128, 646 (2016).
Bochtler, T. et al. Gain of chromosome 1q21 is an independent adverse prognostic factor in light chain amyloidosis patients treated with melphalan/dexamethasone. Amyloid 21, 9–17 (2014).
Bochtler, T. et al. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J. Clin. Oncol. 33, 1371–1378 (2015).
Bochtler, T. et al. Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study. Blood 128, 594–602 (2016).
Muchtar, E. et al. Interphase fluorescence in situ hybridization in untreated AL amyloidosis has an independent prognostic impact by abnormality type and treatment category. Leukemia 31, 1562–1569 (2017).
Kastritis, E. et al. Addition of cyclophosphamide and higher doses of dexamethasone do not improve outcomes of patients with AL amyloidosis treated with bortezomib. Blood Cancer J. 7, e570 (2017).
Wechalekar, A. et al. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood 109, 457–464 (2007).
Dispenzieri, A. et al. Poor tolerance to high doses of thalidomide in patients with primary systemic amyloidosis. Amyloid 10, 257–261 (2003).
Palladini, G. et al. The combination of thalidomide and intermediate-dose dexamethasone is an effective but toxic treatment for patients with primary amyloidosis (AL). Blood 105, 2949–2951 (2005).
Moreau, P. et al. Lenalidomide in combination with melphalan and dexamethasone in patients with newly diagnosed AL amyloidosis: a multicenter phase 1/2 dose-escalation study. Blood 116, 4777–4782 (2010).
Sanchorawala, V. et al. Melphalan, lenalidomide and dexamethasone for the treatment of immunoglobulin light chain amyloidosis: results of a phase II trial. Haematologica 98, 789–792 (2013).
Hegenbart, U. et al. Lenalidomide/melphalan/dexamethasone in newly diagnosed patients with immunoglobulin light chain amyloidosis: results of a prospective phase 2 study with long-term follow up. Haematologica 102, 1424–1431 (2017).
Kumar, S. K. et al. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) for light-chain amyloidosis: long-term results from a phase 2 trial. Blood 119, 4860–4867 (2012).
Kastritis, E. et al. A phase 1/2 study of lenalidomide with low-dose oral cyclophosphamide and low-dose dexamethasone (RdC) in AL amyloidosis. Blood 119, 5384–5390 (2012).
Cibeira, M. T. et al. A phase II trial of lenalidomide, dexamethasone and cyclophosphamide for newly diagnosed patients with systemic immunoglobulin light chain amyloidosis. Br. J. Haematol. 170, 804–813 (2015).
Palladini, G., Milani, P. & Merlini, G. Novel strategies for the diagnosis and treatment of cardiac amyloidosis. Expert Rev. Cardiovasc. Ther. 13, 1195–1211 (2015).
Manwani, R. et al. Rapid hematological responses improve outcomes in patients with very advanced (stage IIIb) cardiac immunoglobulin light chain amyloidosis. Haematologica 103, e165–e168 (2018).
Palladini, G. & Merlini, G. What is new in diagnosis and management of light chain amyloidosis? Blood 128, 159–168 (2016).
Warsame, R. et al. Outcomes and treatments of patients with immunoglobulin light chain amyloidosis who progress or relapse postautologous stem cell transplant. Eur. J. Haematol. 92, 485–490 (2014).
Palladini, G. et al. Presentation and outcome with second-line treatment in AL amyloidosis previously sensitive to nontransplant therapies. Blood 131, 525–532 (2018).
Milani, P., Gertz, M. A., Merlini, G. & Dispenzieri, A. Attitudes about when and how to treat patients with AL amyloidosis: an international survey. Amyloid 24, 213–216 (2017).
Tandon, N. et al. Treatment patterns and outcome following initial relapse or refractory disease in patients with systemic light chain amyloidosis. Am. J. Hematol. 92, 549–554 (2017).
Dispenzieri, A. et al. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood 109, 465–470 (2007).
Sanchorawala, V. et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood 109, 492–496 (2007).
Mahmood, S. et al. Lenalidomide and dexamethasone for systemic AL amyloidosis following prior treatment with thalidomide or bortezomib regimens. Br. J. Haematol. 166, 842–848 (2014).
Palladini, G. et al. Salvage therapy with lenalidomide and dexamethasone in patients with advanced AL amyloidosis refractory to melphalan, bortezomib, and thalidomide. Ann. Hematol. 91, 89–92 (2012).
Specter, R. et al. Kidney dysfunction during lenalidomide treatment for AL amyloidosis. Nephrol. Dial. Transplant. 26, 881–886 (2011).
Dispenzieri, A. et al. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood 119, 5397–5404 (2012).
Sanchorawala, V. et al. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 1 and 2 trial. Blood 128, 1059–1062 (2016).
Palladini, G. et al. A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood 129, 2120–2123 (2017).
Sanchorawala, V. et al. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood 130, 597–605 (2017).
Roussel, M. et al. A prospective phase II of daratumumab in previously-treated systemic light-chain (AL) amyloidosis. Blood 130, 508 (2017).
Sanchorawala, V. et al. Safety and tolerability of daratumumab in patients with relapsed light chain (AL) amyloidosis: preliminary results of a phase II study. Blood 130, 507 (2017).
Kaufman, G. P. et al. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood 130, 900–902 (2017). This paper shows that daratumumab is rapidly effective in relapsed and/or refractory patients.
Gertz, M. A. et al. First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J. Clin. Oncol. 34, 1097–1103 (2016).
Edwards, C. V. et al. Interim analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1F4 in patients with AL amyloidosis. Amyloid 24, 58–59 (2017). This paper shows that the monoclonal antibody 11–11F4 is well tolerated and safe when administered as a single infusion or as a weekly treatment for 4 weeks.
Solomon, A., Weiss, D. T. & Wall, J. S. Immunotherapy in systemic primary (AL) amyloidosis using amyloid-reactive monoclonal antibodies. Cancer Biother. Radiopharm. 18, 853–860 (2003).
Edwards, C. V. et al. Final analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1F4 in patients with relapsed or refractory AL amyloidosis. Blood 130, 509 (2017).
Richards, D. B. et al. Repeat doses of antibody to serum amyloid P component clear amyloid deposits in patients with systemic amyloidosis. Sci. Transl Med. 10, eaan3128 (2018).
Richards, D. B. et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N. Engl. J. Med. 373, 1106–1114 (2015). This paper shows that anti-SAP therapy, combining miridesap followed by dezamizumab, safely triggers clearance of amyloid deposits from the liver and some other tissues.
Tan, N. Y. et al. Catheter ablation for atrial arrhythmias in patients with cardiac amyloidosis. J. Cardiovasc. Electrophysiol. 27, 1167–1173 (2016).
Muchtar, E. et al. Digoxin use in systemic light-chain (AL) amyloidosis: contra-indicated or cautious use? Amyloid. 25, 86–92 (2018).
Palladini, G. et al. Holter monitoring in AL amyloidosis: prognostic implications. Pacing Clin. Electrophysiol 24, 1228–1233 (2001).
Itoh, M. et al. Implantable cardioverter defibrillator therapy in a patient with cardiac amyloidosis. Am. J. Hematol. 81, 560–561 (2006).
Lin, G., Dispenzieri, A., Kyle, R., Grogan, M. & Brady, P. A. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J. Cardiovasc. Electrophysiol. 24, 793–798 (2013).
Wright, B. L., Grace, A. A. & Goodman, H. J. Implantation of a cardioverter-defibrillator in a patient with cardiac amyloidosis. Nat. Clin. Pract. Cardiovasc. Med. 3, 110–114 (2006).
Rezk, T. et al. Role of implantable intracardiac defibrillators in patients with cardiac immunoglobulin light chain amyloidosis. Br. J. Haematol. 182, 145–148 (2018).
Sayed, R. H. et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur. Heart J. 36, 1098–1105 (2015).
Ward, J. E. et al. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood 118, 6610–6617 (2011).
Diomede, L. et al. A Caenorhabditis elegans-based assay recognizes immunoglobulin light chains causing heart amyloidosis. Blood 123, 3543–3552 (2014).
Wechalekar, A. D. & Whelan, C. Encouraging impact of doxycycline on early mortality in cardiac light chain (AL) amyloidosis. Blood Cancer J. 7, e546 (2017).
Dispenzieri, A., Gertz, M. A. & Buadi, F. What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Rev. 26, 137–154 (2012).
Kristen, A. V. et al. Improved outcomes after heart transplantation for cardiac amyloidosis in the modern era. J. Heart Lung Transplant. 37, 611–618 (2018).
Gray Gilstrap, L. et al. Predictors of survival to orthotopic heart transplant in patients with light chain amyloidosis. J. Heart Lung Transplant. 33, 149–156 (2014).
Davis, M. K. et al. Outcomes after heart transplantation for amyloid cardiomyopathy in the modern era. Am. J. Transplant. 15, 650–658 (2015).
Grogan, M. et al. Long term outcomes of cardiac transplant for immunoglobulin light chain amyloidosis: the Mayo Clinic experience. World J. Transplant. 6, 380–388 (2016).
Gertz, M. A. et al. Clinical outcome of immunoglobulin light chain amyloidosis affecting the kidney. Nephrol. Dial Transplant. 24, 3132–3137 (2009).
Sattianayagam, P. T. et al. Solid organ transplantation in AL amyloidosis. Am. J. Transplant. 10, 2124–2131 (2010).
Herrmann, S. M. et al. Long-term outcomes of patients with light chain amyloidosis (AL) after renal transplantation with or without stem cell transplantation. Nephrol. Dial Transplant. 26, 2032–2036 (2011).
White, M. K., McCausland, K. L., Sanchorawala, V., Guthrie, S. D. & Bayliss, M. S. Psychometric validation of the SF-36 Health Survey in light chain amyloidosis: results from community-based and clinic-based samples. Patient Relat. Outcome Meas. 8, 157–167 (2017).
Warsame, R. et al. Hematology patient reported symptom screen to assess quality of life for AL amyloidosis. Am. J. Hematol. 92, 435–440 (2017).
Sanchorawala, V. et al. A longitudinal evaluation of health-related quality of life in patients with AL amyloidosis: associations with health outcomes over time. Br. J. Haematol. 179, 461–470 (2017).
Oliva, L. et al. The amyloidogenic light chain is a stressor that sensitizes plasma cells to proteasome inhibitor toxicity. Blood 129, 2132–2142 (2017). This paper shows that amyloidogenic light chain production is an intrinsic cellular stressor that sensitizes to proteasome inhibitor toxicity.
Song, Y. et al. Blockade of deubiquitylating enzyme Rpn11 triggers apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Oncogene 36, 5631–5638 (2017).
Kumar, S. K. et al. Multiple myeloma. Nat. Rev. Dis. Primers 3, 17046 (2017).
Mills, J. R., Barnidge, D. R. & Murray, D. L. Detecting monoclonal immunoglobulins in human serum using mass spectrometry. Methods 81, 56–65 (2015).
Thoren, K. L. Mass spectrometry methods for detecting monoclonal immunoglobulins in multiple myeloma minimal residual disease. Semin. Hematol. 55, 41–43 (2018).
Bai, Y., Orfao, A. & Chim, C. S. Molecular detection of minimal residual disease in multiple myeloma. Br. J. Haematol. 181, 11–26 (2018).
Flores-Montero, J. et al. Next generation flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 31, 2094–2103 (2017).
Lavatelli, F. et al. Novel mitochondrial protein interactors of immunoglobulin light chains causing heart amyloidosis. FASEB J. 29, 4614–4628 (2015).
Mishra, S. et al. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am. J. Physiol. Heart Circ. Physiol. 305, H95–H103 (2013).
Palladini, G. et al. Treatment of al amyloidosis guided by biomarkers [abstract PO-910]. Haematologica 92, (Suppl. 2), 199 (2007).
Fontana, M., Chung, R., Hawkins, P. N. & Moon, J. C. Cardiovascular magnetic resonance for amyloidosis. Heart Fail. Rev. 20, 133–144 (2015).
Benson, M. D., Liepnieks, J. J. & Kluve-Beckerman, B. Hereditary systemic immunoglobulin light-chain amyloidosis. Blood 125, 3281–3286 (2015).
Acknowledgements
G.M. and G.P. are supported in part by grants from ‘Associazione Italiana per la Ricerca sul Cancro–Special Program Molecular Clinical Oncology 5 per mille n. 9965’, from CARIPLO ‘Structure-function relation of amyloid: understanding the molecular bases of protein misfolding diseases to design new treatments n. 2013–0964’ and from CARIPLO ‘Molecular mechanisms of immunoglobulin toxicity in age-related plasma cell dyscrasias n. 2015–0591’. G.P. is supported in part by the Bart Barlogie Young Investigator Award from the International Myeloma Society.
Reviewer information
Nature Reviews Disease Primers thanks J. Blade, R. Comenzo, B. Hazenberg, S. Ikeda, A. Jaccard, R. Linke and the other, anonymous referee(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (G.M.); Epidemiology (A.D. and M.A.G.); Mechanisms/pathophysiology (G.M.); Diagnosis, screening and prevention (A.D., G.P., P.N.H. and M.A.G.); Management (G.M., A.D., V.S., S.O.S., G.P., P.N.H. and M.A.G.); Quality of life (V.S.); Outlook (G.M. and P.N.H.); Overview of the Primer (G.M.).
Corresponding author
Ethics declarations
Competing interests
G.M. is on the advisory board for Caelum, Janssen and Pfizer and has received travel support from Janssen and Prothena. A.D. receives research support from Alnylam, Celgene, GlaxoSmithKline, Pfizer and Takeda and has received research support from Prothena. V.S. sits on the advisory boards of Caelum, Janssen, receives research support from Celgene, Janssen and Takeda and has received research support from Prothena. S.O.S. has received honoraria and research support from Prothena and receives honoraria from Janssen, travel support from Jazz and Takeda and research support from Janssen and Sanofi. G.P. sits on the advisory board of Janssen, has received honoraria from Prothena and receives honoraria from Sebia and travel support from Celgene. P.N.H. receives honoraria from Alnylam and GlaxoSmithKline and is a director and stockholder of Pentraxin Therapeutics. M.A.G. receives honoraria from Abbvie, Alnylam, Amgen, Annexon, Appellis, Celgene, Ionis, Janssen, Johnson and Johnson, Medscape, Physicians Education Resource, Prothena, Research to Practice, Spectrum and Teva and receives research support from Spectrum.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Merlini, G., Dispenzieri, A., Sanchorawala, V. et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers 4, 38 (2018). https://doi.org/10.1038/s41572-018-0034-3
Published:
DOI: https://doi.org/10.1038/s41572-018-0034-3
This article is cited by
-
Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases
Molecular Neurodegeneration (2024)
-
Minimal residual disease in systemic light chain amyloidosis: a systematic review and meta-analysis
Journal of Cancer Research and Clinical Oncology (2024)
-
First third-generation CAR T cell application targeting CD19 for the treatment of systemic IgM AL amyloidosis with underlying marginal zone lymphoma
Biomarker Research (2023)
-
The exploration of B cell maturation antigen expression in plasma cell dyscrasias beyond multiple myeloma
BMC Cancer (2023)
-
A constant domain mutation in a patient-derived antibody light chain reveals principles of AL amyloidosis
Communications Biology (2023)