Abstract
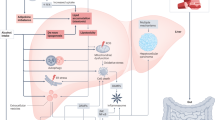
Alcoholic liver disease (ALD) is the most prevalent type of chronic liver disease worldwide. ALD can progress from alcoholic fatty liver (AFL) to alcoholic steatohepatitis (ASH), which is characterized by hepatic inflammation. Chronic ASH can eventually lead to fibrosis and cirrhosis and in some cases hepatocellular cancer (HCC). In addition, severe ASH (with or without cirrhosis) can lead to alcoholic hepatitis, which is an acute clinical presentation of ALD that is associated with liver failure and high mortality. Most individuals consuming >40 g of alcohol per day develop AFL; however, only a subset of individuals will develop more advanced disease. Genetic, epigenetic and non-genetic factors might explain the considerable interindividual variation in ALD phenotype. The pathogenesis of ALD includes hepatic steatosis, oxidative stress, acetaldehyde-mediated toxicity and cytokine and chemokine-induced inflammation. Diagnosis of ALD involves assessing patients for alcohol use disorder and signs of advanced liver disease. The degree of AFL and liver fibrosis can be determined by ultrasonography, transient elastography, MRI, measurement of serum biomarkers and liver biopsy histology. Alcohol abstinence achieved by psychosomatic intervention is the best treatment for all stages of ALD. In the case of advanced disease such as cirrhosis or HCC, liver transplantation may be required. Thus, new therapies are urgently needed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Change history
28 August 2018
A Correction to this paper has been published: https://doi.org/10.1038/s41572-018-0021-8
References
Blachier, M., Leleu, H., Peck-Radosavljevic, M., Valla, D. C. & Roudot-Thoraval, F. The burden of liver disease in Europe: A review of available epidemiological data. J. Hepatol. 58, 593–608 (2013).
Pimpin, L. et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. https://doi.org/10.1016/j.jhep.2018.05.011 (2018).
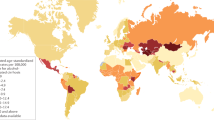
Rehm, J., Samokhvalov, A. V. & Shield, K. D. Global burden of alcoholic liver diseases. J. Hepatol. 59, 160–168 (2013).
Rehm, J. et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 29, 437–445 (2010).
Bellentani, S. & Tiribelli, C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J. Hepatol. 35, 531–537 (2001).
O’Shea, R. S., Dasarathy, S. & McCullough, A. J. Alcoholic liver disease. Hepatology 51, 307–328 (2010).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of alcohol-related liver disease. J. Hepatol. 69, 154–181 (2018). This paper provides a complete overview of the latest developments in the management of ALD.
Mathurin, P. et al. EASL clinical practical guidelines: Management of alcoholic liver disease. J. Hepatol. 57, 399–420 (2012).
WHO. Global Status Report on Noncommunicable Diseases 2014 (World Health Organization, 2014).
Shield, K. D., Rylett, M., Rehm, J. Public health successes and missed opportunities. trends in alcohol consumption and attributable mortality in the WHO European region, 1990–2014. (World Health Organization, 2016).
EASL. HEPAHEALTH Project Report. Risk Factors and the Burden of Liver Disease in Europe and Selected Central Asian Countries. (EASL, 2018).
Naghavi, M. et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1151–1210 (2017).
Akinyemiju, T. et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level. JAMA Oncol. 3, 1683 (2017).
Sheron, N. Alcohol and liver disease in Europe - simple measures have the potential to prevent tens of thousands of premature deaths. J. Hepatol. 64, 957–967 (2016).
Sandahl, T. D., Jepsen, P., Thomsen, K. L. & Vilstrup, H. Incidence and mortality of alcoholic hepatitis in Denmark 1999-2008: a nationwide population based cohort study. J. Hepatol. 54, 760–764 (2011).
Liangpunsakul, S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J. Clin. Gastroenterol. 45, 714–719 (2011).
Mathurin, P. et al. Fibrosis progression occurs in a subgroup of heavy drinkers with typical histological features. Aliment. Pharmacol. Ther. 25, 1047–1054 (2007).
Goldberg, D. et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 152, 1090–1099 (2017).
Cholankeril, G. et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig. Dis. Sci. 62, 2915–2922 (2017).
European Liver and Intestine Transplant Association. European Liver Transplant Registry. ELITA http://www.eltr.org/ (2018).
O’Grady, J. G. Liver transplantation alcohol related liver disease: (deliberately) stirring a hornet’s nest! Gut 55, 1529–1531 (2006).
Lelbach, W. K. Cirrhosis in the alcoholic and its relation to the volume of alcohol abuse. Ann. NY Acad. Sci. 252, 85–105 (1975).
Becker, U. et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 23, 1025–1029 (1996).
Shimizu, I., Kamochi, M., Yoshikawa, H. & Nakayama, Y. in Trends in Alcoholic Liver Disease Research. Clinical and Scientific Aspects (ed. Shimizu, I.) 23–40 (InTech, 2012).
Strnad, P. et al. Heterozygous carriage of the alpha1-antitrypsin Z variant rs28929474 predisposes to the development of cirrhosis in the presence of alcohol misuse and non-alcohol-related fatty liver disease. J. Hepatol. 66, S177 (2017).
Chen, C.-J. et al. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology 13, 398–406 (1991).
Mueller, S., Millonig, G. & Seitz, H. K. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J. Gastroenterol. 15, 3462–3471 (2009).
Fletcher, L. M. & Powell, L. W. Hemochromatosis and alcoholic liver disease. Alcohol 30, 131–136 (2003).
Seitz, H. K., Mueller, S., Hellerbrand, C. & Liangpunsakul, S. Effect of chronic alcohol consumption on the development and progression of non-alcoholic fatty liver disease (NAFLD). Hepatobiliary Surg. Nutr. 4, 147–151 (2015).
Boyle, M., Masson, S. & Anstee, Q. M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: cofactors for progressive fatty liver disease. J. Hepatol. 68, 251–267 (2017). This paper updates all current knowledge of an important issue — namely, the interaction between alcohol, obesity and metabolic syndrome.
Morgan, T. R., Mandayam, S. & Jamal, M. M. Alcohol and hepatocellular carcinoma. Gastroenterology 127, S87–S96 (2004).
Ascha, M. S. et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 51, 1972–1978 (2010).
Naveau, S. et al. Excess weight is a risk factor for alcoholic liver disease. Hepatology 25, 108–111 (1997).
Seitz, H. K. & Mueller, S. Metabolism of alcohol and its consequences. Metabolism Drugs Other Xenobiot. 493–516 (2012).
Hagström, H. Alcohol, smoking and the liver disease patient. Best Pract. Res. Clin. Gastroenterol. 31, 537–543 (2017).
Stickel, F., Moreno, C., Hampe, J. & Morgan, M. Y. The genetics of alcohol dependence and alcohol-related liver disease. J. Hepatol. 66, 195–211 (2017).
Hrubec, Z. & Omenn, G. S. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcohol. Clin. Exp. Res. 5, 207–215 (1981).
Edenberg, H. J. & Foroud, T. Genetics of alcoholism. Handb. Clin. Neurol. 125, 561–571 (2014).
Salameh, H. et al. PNPLA3 gene polymorphism is associated with predisposition to and severity of alcoholic liver disease. Am. J. Gastroenterol. 110, 846–856 (2015).
Stickel, F. et al. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology 53, 86–95 (2011).
Buch, S. et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 47, 1443–1448 (2015). This paper presents probably the best genome-wide association study with respect to ALD, demonstrating three important gene loci for risk.
BasuRay, S., Smagris, E., Cohen, J. C. & Hobbs, H. H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology 66, 1111–1124 (2017).
Bataller, R., North, K. E. & Brenner, D. A. Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology 37, 493–503 (2003).
Lieber, C. S., Rubin, E. & DeCarli, L. M. Hepatic microsomal ethanol oxidizing system (MEOS): differentiation from alcohol dehydrogenase and NADPH oxidase. Biochem. Biophys. Res. Commun. 40, 858–865 (1970).
Seitz, H. K. & Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 7, 599–612 (2007). This article is an excellent overview on the various molecular mechanisms on alcohol and cancer including the liver.
Seitz, H. K. & Mueller, S. Alcoholic liver disease. Clin. Hepatol. 2, 1111–1151 (2010).
Albano, E. et al. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology 23, 155–163 (1996).
Linhart, K., Bartsch, H. & Seitz, H. K. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox Biol. 3, 56–62 (2014).
Wang, Y. et al. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology 50, 453–461 (2009).
Mueller, S. et al. Carcinogenic etheno DNA adducts in alcoholic liver disease: correlation with cytochrome P-4502E1 and fibrosis. Alcohol. Clin. Exp. Res. 42, 252–259 (2018). This article shows for the first time a correlation between CYP2E1 and fibrosis in a large cohort of alcoholic patients with various severities of ALD.
Albano, E. Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 65, 278–290 (2006).
Leung, T. M. & Nieto, N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 58, 395–398 (2013).
Bautista, A. P. Neutrophilic infiltration in alcoholic hepatitis. Alcohol 27, 17–21 (2002).
Bailey, S. M. & Cunningham, C. C. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic. Biol. Med. 32, 11–16 (2002).
García-Ruiz, C., Colell, a, París, R. & Fernández-Checa, J. C. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 14, 847–858 (2000).
Chamulitrat, W. & Spitzer, J. J. Nitric oxide and liver injury in alcohol-fed rats after lipopolysaccharide administration. Alcohol. Clin. Exp. Res. 20, 1065–1070 (1996).
Oneta, C. M. et al. Dynamics of cytochrome P4502E1 activity in man: Induction by ethanol and disappearance during withdrawal phase. J. Hepatol. 36, 47–52 (2002).
Lieber, C. S. et al. Role of medium-chain triglycerides in the alcohol-mediated cytochrome P450 2E1 induction of mitochondria. Alcohol. Clin. Exp. Res. 31, 1660–1668 (2007).
Harrison-Findik, D. D. et al. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J. Biol. Chem. 281, 22974–22982 (2006).
Butura, A. et al. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J. Hepatol. 50, 572–583 (2009).
Morgan, K., French, S. W. & Morgan, T. R. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology 36, 122–134 (2002).
Lu, Y., Wu, D., Wang, X., Ward, S. C. & Cederbaum, A. I. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic. Biol. Med. 49, 1406–1416 (2010).
Gouillon, Z. et al. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc. Soc. Exp. Biol. Med. 224, 302–308 (2000).
Ye, Q. et al. Cytochrome P450 2E1 inhibition prevents hepatic carcinogenesis induced by diethylnitrosamine in alcohol-fed rats. Hepatobiliary Surg. Nutr. 1, 5–18 (2012).
Seitz, H. K. & Stickel, F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 387, 349–360 (2006).
Cederbaum, A. I. Nrf2 and antioxidant defense against CYP2E1 toxicity. Subcell. Biochem. 67, 105–130 (2013).
Szabo, G. & Satishchandran, A. MicroRNAs in alcoholic liver disease. Semin. Liver Dis. 35, 36–42 (2015).
Park, P.-H. Involvement of histone acetyltransferase (HAT) in ethanol-induced acetylation of histone H3 in hepatocytes: potential mechanism for gene expression. AJP Gastrointest. Liver Physiol. 289, G1124–G1136 (2005).
You, M., Liang, X., Ajmo, J. M. & Ness, G. C. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. AJP Gastrointest. Liver Physiol. 294, G892–G898 (2008).
Lu, S. C. et al. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G178–G185 (2000).
Lippai, D., Bala, S., Catalano, D., Kodys, K. & Szabo, G. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol. Clin. Exp. Res. 38, 2217–2224 (2014).
Purohit, V., Gao, B. & Song, B.-J. Molecular mechanisms of alcoholic fatty liver. Alcohol. Clin. Exp. Res. 33, 191–205 (2009).
Baraona, E. & Lieber, C. S. Effects of ethanol on lipid metabolism. J. Lipid Res. 20, 289–315 (1979).
You, M., Fischer, M., Deeg, M. A. & Crabb, D. W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 277, 29342–29347 (2002).
Galli, A., Pinaire, J., Fischer, M., Dorris, R. & Crabb, D. W. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor α is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J. Biol. Chem. 276, 68–75 (2001).
You, M., Matsumoto, M., Pacold, C. M., Cho, W. K. & Crabb, D. W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 127, 1798–1808 (2004).
Zhong, W. et al. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am. J. Pathol. 180, 998–1007 (2012).
Sebastian, B. M. et al. Identification of a cytochrome P4502E1/Bid/C1q-dependent axis mediating inflammation in adipose tissue after chronic ethanol feeding to mice. J. Biol. Chem. 286, 35989–35997 (2011).
Parker, R., Kim, S. J. & Gao, B. Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations. Nat. Rev. Gastroenterol. Hepatol. 15, 50–59 (2018).
Dolganiuc, A., Thomes, P. G., Ding, W. X., Lemasters, J. J. & Donohue, T. M. Autophagy in alcohol-induced liver diseases. Alcohol. Clin. Exp. Res. 36, 1301–1308 (2012).
Ding, W. et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 139, 1740–1752 (2010).
Pone, E. J. Toll-like receptors. Signal. Pathways Liver Diseases 1390, 149–159 (2016).
Iracheta-Vellve, A. et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J. Hepatol. 63, 1147–1155 (2015).
Petrasek, J. et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J. Leukoc. Biol. 98, 249–256 (2015).
Iracheta-Vellve, A. et al. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int. 37, 968–973 (2017).
Negrin, K. A. et al. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLOS One 9, e107265 (2014).
Petrasek, J. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 122, 3476–3489 (2012).
Bukong, T. N. et al. Therapeutic benefits of spleen tyrosine kinase inhibitor administration on binge drinking-induced alcoholic liver injury, steatosis, and inflammation in mice. Alcohol. Clin. Exp. Res. 40, 1524–1530 (2016).
Mandrekar, P. Epigenetic regulation in alcoholic liver disease. World J. Gastroenterol. 17, 2456–2464 (2011).
Bala, S. et al. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J. Hepatol. 64, 1378–1387 (2016).
Bala, S. et al. Alcohol-induced miR-155 and HDAC11 inhibit negative regulators of the TLR4 pathway and lead to increased LPS responsiveness of Kupffer cells in alcoholic liver disease. J. Leukoc. Biol. 102, 487–498 (2017).
Lippai, D., Bala, S., Csak, T., Kurt-Jones, E. A. & Szabo, G. Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice. PLOS One 8, e70945 (2013).
Bala, S. et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 56, 1946–1957 (2012).
Satishchandran, A. et al. MicroRNA 122, regulated by GRLH2, protects livers of mice and patients from ethanol-induced liver disease. Gastroenterology 154, 238–252 (2018).
Juskeviciute, E. et al. Inhibition of miR-21 rescues liver regeneration after partial hepatectomy in ethanol-fed rats. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G794–G806 (2016).
Dippold, R. P., Vadigepalli, R., Gonye, G. E., Patra, B. & Hoek, J. B. Chronic ethanol feeding alters miRNA expression dynamics during liver regeneration. Alcohol. Clin. Exp. Res. 37, E59–E69 (2013).
French, S. W. & Bardag-Gorce, F. in Signaling Pathways in Liver Diseases (eds Dufour, J.-F. et al.) 377–389 (Springer, 2005).
Wang, S., Pacher, P., De Lisle, R. C., Huang, H. & Ding, W. X. A. Mechanistic review of cell death in alcohol-induced liver injury. Alcohol. Clin. Exp. Res. 40, 1215–1223 (2016).
Petrasek, J. et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl Acad. Sci. USA 110, 16544–16549 (2013).
Iracheta-Vellve, A. et al. Endoplasmic reticulum stress-induced hepatocellular death pathways mediate liver injury and fibrosis via stimulator of interferon genes. J. Biol. Chem. 291, 26794–26805 (2016).
Nagy, L. E., Ding, W. X., Cresci, G., Saikia, P. & Shah, V. H. Linking pathogenic mechanisms of alcoholic liver disease with clinical phenotypes. Gastroenterology 150, 1756–1768 (2016).
Lackner, C. et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J. Hepatol. 66, 610–618 (2017).
Tsuchida, T. & Friedman, S. L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14, 397–411 (2017). This manuscript describes in detail the mechanisms of stellate cell activation leading to hepatic fibrosis.
Ge, X. High-mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). Alcohol 48, 729 (2014).
Kwon, H. J. et al. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology 60, 146–157 (2014).
Enomoto, N. et al. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol. Clin. Exp. Res. 25, 51S–54S (2001).
Paik, Y. H. et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 37, 1043–1055 (2003).
Radaeva, S. et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 130, 435–452 (2006).
Jeong, W. Il, Park, O. & Gao, B. Abrogation of the antifibrotic effects of natural killer cells/interferon-γ contributes to alcohol acceleration of liver fibrosis. Gastroenterology 134, 248–258 (2008).
Baan, R. et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 8, 292–293 (2007).
Yokoyama, A. et al. Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics. Carcinogenesis 19, 1383–1387 (1998).
Jin, S. et al. ALDH2(E487K) mutation increases protein turnover and promotes murine hepatocarcinogenesis. Proc. Natl Acad. Sci. USA 112, 9088–9093 (2015).
Theruvathu, J. A., Jaruga, P., Nath, R. G., Dizdaroglu, M. & Brooks, P. J. Polyamines stimulate the formation of mutagenic 1,N2/-propanodeoxyguanosine adducts from acetaldehyde. Nucleic Acids Res. 33, 3513–3520 (2005).
Mechilli, M., Schinoppi, A., Kobos, K., Natarajan, A. T. & Palitti, F. DNA repair deficiency and acetaldehyde-induced chromosomal alterations in CHO cells. Mutagenesis 23, 51–56 (2008).
Wilson, D. M., Tentler, J. J., Carney, J. P., Wilson, T. M. & Kelley, M. R. Acute ethanol exposure suppresses the repair of O6-methylguanine DNA lesions in castrated adult male rats. Alcohol. Clin. Exp. Res. 18, 1267–1271 (1994).
Tuma, D. J., Thiele, G. M., Xu, D., Klassen, L. W. & Sorrell, M. F. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology 23, 872–880 (1996).
Garro, A. J., Seitz, H. K. & Lieber, C. S. Enhancement of dimethylnitrosamine metabolism and activation to a mutagen following chronic ethanol consumption. Cancer Res. 41, 120–124 (1981).
Varela-Rey, M., Woodhoo, A., Martinez-Chantar, M.-L., Mato, J. M. & Lu, S. C. Alcohol, DNA methylation, and cancer. Alcohol Res. 35, 25–35 (2013).
Wilson, C. L. et al. NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat. Commun. 6, 6618 (2015).
Machida, K. et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc. Natl Acad. Sci. USA 106, 1548–1553 (2009).
Chen, C. L. et al. Reciprocal regulation by TLR4 and TGF-β in tumor-initiating stem-like cells. J. Clin. Invest. 123, 2832–2849 (2013).
Coulouarn, C. & Clément, B. Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J. Hepatol. 60, 1306–1309 (2014).
Lai, K. K. Y. et al. Stearoyl-CoA desaturase promotes liver fibrosis and tumor development in mice via a Wnt Positive-signaling loop by stabilization of low-density lipoprotein-receptor-related proteins 5 and 6. Gastroenterology 152, 1477–1491 (2017).
Nieto, N., Friedman, S. L. & Cederbaum, A. I. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J. Biol. Chem. 277, 9853–9864 (2002).
Seki, E. et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Mercer, K. E. et al. Alcohol consumption promotes diethylnitrosamine-induced hepatocarcinogenesis in male mice through activation of the Wnt/β-catenin signaling pathway. Cancer Prev. Res. 7, 675–685 (2014).
Groll, N. et al. Coordinate regulation of Cyp2e1 by β-catenin- and hepatocyte nuclear factor 1α-dependent signaling. Toxicology 350–352, 40–48 (2016).
Yan, G. et al. Chronic alcohol consumption promotes diethylnitrosamine-induced hepatocarcinogenesis via immune disturbances. Sci. Rep. 7, 2567 (2017).
Ambade, A., Satishchandran, A. & Szabo, G. Alcoholic hepatitis accelerates early hepatobiliary cancer by increasing stemness and MIR-122-mediated HIF-1α activation. Sci. Rep. 6, 21340 (2016).
Mitchell, A. J., Meader, N., Bird, V. & Rizzo, M. Clinical recognition and recording of alcohol disorders by clinicians in primary and secondary care: meta-analysis. Br. J. Psychiatry 201, 93–100 (2012).
Tavakoli, H. R., Hull, M. & Michael Okasinski, L. Review of current clinical biomarkers for the detection of alcohol dependence. Innov. Clin. Neurosci. 8, 26–33 (2011).
American Psychiatric Association in Diagnostic and Statistical Manual of Mental Disorders 863–877 (American Psychiatric Association, 2014).
Thiele, M. et al. Controlled attenuation parameter for the assessment of alcoholic hepatic steatosis: biopsy-controlled diagnostic accuracy and role of detoxification. J. Hepat 68, 1025–1032 (2018).
Thiele, M. et al. Controlled attenuation parameter and alcoholic hepatic steatosis: diagnostic accuracy and role of alcohol detoxification. J. Hepatol. 68, 1025–1032 (2018).
Imajo, K. et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 150, 626–637 (2016).
Dulai, P. S., Sirlin, C. B. & Loomba, R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J. Hepatol. 65, 1006–1016 (2016).
Mueller, S., Seitz, H. K. & Rausch, V. Non-invasive diagnosis of alcoholic liver disease. World J. Gastroenterol. 20, 14626–14641 (2014).
Anton, R. F., Lieber, C. & Tabakoff, B. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol. Clin. Exp. Res. 26, 1215–1222 (2002).
Bell, H. et al. Serum carbohydrate-deficient transferrin as a marker of alcohol consumption in patients with chronic liver diseases. Alcohol. Clin. Exp. Res. 17, 246–252 (1993).
Mukai, M., Ozasa, K., Hayashi, K. & Kawai, K. Various S-GOT/S-GPT ratios in nonviral liver disorders and related physical conditions and life-style. Dig. Dis. Sci. 47, 549–555 (2002).
Mueller, S. et al. Inflammation-adapted liver stiffness values for improved fibrosis staging in patients with hepatitis C virus and alcoholic liver disease. Liver Int. 35, 2514–2521 (2015).
Mueller, S. et al. Caspase-cleaved keratin-18 fragments increase during alcohol withdrawal and predict liver-related death in patients with alcoholic liver disease. Hepatology 66, 96–107 (2017).
Lucey, M. R., Mathurin, P. & Morgan, T. R. Alcoholic Hepatitis. N. Engl. J. Med. 360, 2758–2769 (2009).
Altamirano, J. et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 146, 1231–1239 (2014).
Crabb, D. W. et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 150, 785–790 (2016).
Bedossa, P. et al. Apolipoprotein A1 is a serum and tissue marker of liver fibrosis in alcoholic patients. Alcohol. Clin. Exp. Res. 13, 829–833 (1989).
Parés, A. et al. Serum hyaluronate reflects hepatic fibrogenesis in alcoholic liver disease and is useful as a marker of fibrosis. Hepatology 24, 1399–1403 (1996).
Oberti, F. et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 113, 1609–1616 (1997).
Plevris, J. N. et al. Serum hyaluronan - a non-invasive test for diagnosing liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 12, 1121–1127 (2000).
Croquet, V. et al. Prothrombin index is an indirect marker of severe liver fibrosis. Eur. J. Gastroenterol. Hepatol. 14, 1133–1141 (2002).
Nøjgaard, C. et al. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J. Hepatol. 39, 179–186 (2003).
Stickel, F. et al. Serum hyaluronate correlates with histological progression in alcoholic liver disease. Eur. J. Gastroenterol. Hepatol. 15, 945–950 (2003).
Rosenberg, W. M. C. et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 127, 1704–1713 (2004).
Stickel, F. et al. Serum collagen type VI and XIV and hyaluronic acid as early indicators for altered connective tissue turnover in alcoholic liver disease. Dig. Dis. Sci. 46, 2025–2032 (2001).
Naveau, S. et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology 49, 97–105 (2009).
Stickel, F., Datz, C., Hampe, J. & Bataller, R. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver 11, 173–188 (2017).
Joshi, K., Kohli, A., Manch, R. & Gish, R. Alcoholic liver disease: high risk or low risk for developing hepatocellular carcinoma? Clin. Liver Dis. 20, 563–580 (2016).
Trojan, J., Zangos, S. & Schnitzbauer, A. A. Diagnostics and treatment of hepatocellular carcinoma in 2016: standards and developments. Visc. Med. 32, 116–120 (2016).
Filingeri, V. et al. A retrospective analysis of 1.011 percutaneous liver biopsies performed in patients with liver transplantation or liver disease: ultrasonography can reduce complications? Eur. Rev. Med. Pharmacol. Sci. 20, 3609–3617 (2016).
Mathurin, P. & Bataller, R. Trends in the management and burden of alcoholic liver disease. J. Hepatol. 62, S38–S46 (2015).
Mookerjee, R. P. et al. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J. Hepatol. 55, 1103–1111 (2011).
Hardy, T. et al. White cell count and platelet count associate with histological alcoholic hepatitis in jaundiced harmful drinkers. BMC Gastroenterol. 13, 55 (2013).
Bissonnette, J. et al. A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology 66, 555–563 (2017).
Levin, D. M., Baker, A. L., Riddell, R. H., Rochman, H. & Boyer, J. L. Nonalcoholic liver disease. Am. J. Med. 66, 429–434 (1979).
Louvet, A. & Mathurin, P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 12, 231–242 (2015).
Yip, W. W. & Burt, A. D. Alcoholic liver disease. Semin. Diagn. Pathol. 23, 149–160 (2006).
[No authors listed.]. Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1, 707–711 (1981).
Katoonizadeh, A. et al. Early features of acute-on-chronic alcoholic liver failure: A prospective cohort study. Gut 59, 1561–1569 (2010).
Desmet, V. J. & Roskams, T. Cirrhosis reversal: a duel between dogma and myth. J. Hepatol. 40, 860–867 (2004).
Bohn, M. J., Babor, T. F. & Kranzler, H. R. The alcohol use disorders identification test (AUDIT): validation of a screening instrument for use in medical settings. J. Stud. Alcohol 56, 423–432 (1995).
Bush, K., Kivlahan, D. R., McDonell, M. B., Fihn, S. D. & Bradley, K. A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Arch. Intern. Med. 158, 1789–1795 (1998).
Casswell, S. & Thamarangsi, T. Reducing harm from alcohol: call to action. Lancet 373, 2247–2257 (2009).
Anderson, P., Chisholm, D. & Fuhr, D. C. Effectiveness and cost-effectiveness of policies and programmes to reduce the harm caused by alcohol. Lancet 373, 2234–2246 (2009).
Mueller, S. Does pressure cause liver cirrhosis? The sinusoidal pressure hypothesis. World J. Gastroenterol. 22, 10482–10501 (2016).
Mueller, S. et al. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J. Gastroenterol. 16, 966–972 (2010).
Parkes, J. et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 59, 1245–1251 (2010).
Anton, R. F. et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. JAMA 295, 2003 (2006).
Nahler, G. Dictionary of Pharmaceutical Medicine. 69 (Springer, Vienna, 2009).
Sobell, L. C., Brown, J., Leo, G. I. & Sobell, M. B. The reliability of the alcohol timeline followback when administered by telephone and by computer. Drug Alcohol Depend. 42, 49–54 (1996).
Addolorato, G., Mirijello, A., Barrio, P. & Gual, A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J. Hepatol. 65, 618–630 (2016).
Stephens, J. R. et al. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J. Gen. Intern. Med. 29, 587–593 (2014).
Bird, R. D. & Makela, E. H. Alcohol withdrawal: what is the benzodiazepine of choice? Ann. Pharmacother. 28, 67–71 (1994).
Chedid, A. et al. Prognostic factors in alcoholic liver disease. VA Cooperative Study Group. Am. J. Gastroenterol. 86, 210–216 (1991).
Jepsen, P., Ott, P., Andersen, P. K., Sørensen, H. T. & Vilstrup, H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 51, 1675–1682 (2010).
Parés, A., Caballería, J., Bruguera, M., Torres, M. & Rodés, J. Histological course of alcoholic hepatitis. Influence of abstinence, sex and extent of hepatic damage. J. Hepatol. 2, 33–42 (1986).
Maddrey, W. C. et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 75, 193–199 (1978).
Carithers, R. L. et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis: a randomized multicenter trial. Ann. Intern. Med. 110, 685–691 (1989).
Dunn, W. et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 41, 353–358 (2005).
Dominguez, M. et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am. J. Gastroenterol. 103, 2747–2756 (2008).
Forrest, E. H. et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 54, 1174–1179 (2005).
Louvet, A. et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 45, 1348–1354 (2007). This study is important in predicting response to therapy and prognosis of alcoholic hepatitis.
Mathurin, P. et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 60, 255–260 (2011).
Louvet, A. et al. Combining data from liver disease scoring systems better predicts outcomes of patients with alcoholic hepatitis. Gastroenterology 149, 398–406 (2015).
Thursz, M. R. et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N. Engl. J. Med. 372, 1619–1628 (2015).
Singh, S. et al. Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: a systematic review and network meta-analysis. Gastroenterology 149, 958–970 (2015).
Mathurin, P. et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis. JAMA 310, 1033 (2013).
Nguyen-Khac, E. et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N. Engl. J. Med. 365, 1781–1789 (2011). This study shows a better short-term survival of patients with severe alcoholic hepatitis receiving steroids plus N -acetyl cysteine, which is important because it adds another drug to the potential therapies.
Louvet, A. et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 137, 541–548 (2009).
Vergis, N. et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology 152, 1068–1077.e4 (2017).
Mathurin, P. et al. Early liver transplantation for severe alcoholic hepatitis. N. Engl. J. Med. 365, 1790–1800 (2011). This paper presents a pioneer study on liver transplantation in severe alcoholic hepatitis.
Im, G. Y. et al. Early liver transplantation for severe alcoholic hepatitis in the United States-a single-center experience. Am. J. Transplant. 16, 841–849 (2016).
Lee, B. P. et al. Three-year results of a pilot program in early liver transplantation for severe alcoholic hepatitis. Ann. Surg. 265, 20–29 (2017).
Stroh, G., Rosell, T., Dong, F. & Forster, J. Early liver transplantation for patients with acute alcoholic hepatitis: public views and the effects on organ donation. Am. J. Transplant. 15, 1598–1604 (2015).
Ge, P. S. & Runyon, B. A. Treatment of patients with cirrhosis. N. Engl. J. Med. 375, 767–777 (2016).
Galle, P. R. et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Beresford, T. P. & Lucey, M. R. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 53, 135–144 (2018).
Kumar, S. et al. Orthotopic liver transplantation for alcoholic liver disease. Hepatology 11, 159–164 (1990).
Seitz, H. K. Commentary: alcohol and alcoholism special issue on ‘alcohol and liver transplantation’. Alcohol Alcohol. 53, 133–134 (2018).
Foster, P. F. et al. Prediction of abstinence from ethanol in alcoholic recipients following liver transplantation. Hepatology 25, 1469–1477 (1997).
Schütte, K. et al. Delayed diagnosis of HCC with chronic alcoholic liver disease. Liver Cancer 1, 257–266 (2012).
Heimbach, J. K. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67, 358–380 (2018).
Vaughn-Sandler, V., Sherman, C., Aronsohn, A. & Volk, M. L. Consequences of perceived stigma among patients with cirrhosis. Dig. Dis. Sci. 59, 681–686 (2014).
Burra, P. et al. Liver transplantation for alcoholic liver disease in europe: a study from the ELTR (European Liver Transplant Registry). Am. J. Transplant. 10, 138–148 (2010).
Berlakovich, G. A. et al. General compliance after liver transplantation for alcoholic cirrhosis. Transpl. Int. 13, 129–135 (2000).
Cowling, T. et al. Societal Reintegration After Liver Transplantation. Ann. Surg. 239, 93–98 (2004).
Ruppert, K., Kuo, S., DiMartini, A. & Balan, V. In a 12-year study, sustainability of quality of life benefits after liver transplantation varies with pretransplantation diagnosis. Gastroenterology 139, 1619–1629 (2010).
Anderson, P. & Baumberg, B. Alcohol in Europe A Public Health Perspective. (Institute of Alcohol Studies, 2006).
Rehm, J. et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373, 2223–2233 (2009).
Edwards, G. Alcohol policy and the public good. Addiction 92 (Suppl. 1), 73–79 (1997).
Xu, M.-J., Zhou, Z., Parker, R. & Gao, B. Targeting inflammation for the treatment of alcoholic liver disease. Pharmacol. Ther. 180, 77–89 (2017).
Kong, X., Feng, D., Mathews, S. & Gao, B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J. Gastroenterol. Hepatol. 28, 56–60 (2013).
Gao, B. & Shah, V. H. Combination therapy: new hope for alcoholic hepatitis? Clin. Res. Hepatol. Gastroenterol. 39, S7–S11 (2015).
Spahr, L. et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: A randomized trial. Hepatology 48, 221–229 (2008).
Singh, V. et al. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am. J. Gastroenterol. 109, 1417–1423 (2014).
Morgan, T. R. Is granulocyte colony stimulating factor a new treatment for alcoholic hepatitis? Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2018.06.013 (2018).
Spahr, L. et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLOS One 8, e53719 (2013).
Thompson, J. et al. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: a multinational, prospective, controlled, randomized trial. Liver Transpl. 24, 380–393 (2018).
WHO. Global status report on alcohol and health 2014. (WHO, 2016).
Alatalo, P. I. et al. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am. J. Clin. Nutr. 88, 1097–1103 (2008).
Loomba, R., Bettencourt, R. & Barrett-Connor, E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment. Pharmacol. Ther. 30, 1137–1149 (2009).
Raynard, B. et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology 35, 635–638 (2002).
Ekstedt, M. et al. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 44, 366–374 (2009).
Ruhl, C. E. & Everhart, J. E. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin. Gastroenterol. Hepatol. 3, 1260–1268 (2005).
Kawamura, Y. et al. Effects of alcohol consumption on hepatocarcinogenesis in Japanese patients with fatty liver disease. Clin. Gastroenterol. Hepatol. 14, 597–605 (2016).
Liangpunsakul, S. & Chalasani, N. What should we recommend to our patients with NAFLD regarding alcohol use? Am. J. Gastroenterol. 107, 976–978 (2012).
Seitz, H. K. et al. Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology 98, 406–413 (1990).
Simanowski, U. A. et al. Increased rectal cell proliferation following alcohol abuse. Gut 49, 418–422 (2001).
Basuroy, S., Sheth, P., Mansbach, C. M. & Rao, R. K. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G367–375 (2005).
Hartmann, P., Seebauer, C. T. & Schnabl, B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol. Clin. Exp. Res. 39, 763–775 (2015). The paper describes in an excellent way the mechanisms by which gut microbiota contribute to the development of ALD.
Elamin, E., Masclee, A., Troost, F., Dekker, J. & Jonkers, D. Activation of the epithelial-to-mesenchymal transition factor snail mediates acetaldehyde-induced intestinal epithelial barrier disruption. Alcohol. Clin. Exp. Res. 38, 344–353 (2014).
Szabo, G. & Csak, T. Inflammasomes in liver diseases. J. Hepatol. 57, 642–654 (2012).
Adachi, Y., Bradford, B. U., Gao, W., Bojes, H. K. & Thurman, R. G. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology 20, 453–460 (1994).
Adachi, Y., Moore, L. E., Bradford, B. U., Gao, W. & Thurman, R. G. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108, 218–224 (1995).
Mookerjee, R. P. et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology 46, 831–840 (2007).
Acknowledgements
The authors thank H. Engel for her help in managing administration, English language correction and the reference list during the writing of this article.
Reviewer information
Nature Reviews Disease Primers thanks H. Tilg, M. Neuman, A. Cederbaum, T. Morgan and R. Williams for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (H.K.S.); Epidemiology (H.K.S. and H.C.-P.); Mechanisms/pathophysiology (H.K.S., R.B., G.S. and H.T.); Diagnosis, screening and prevention (H.K.S., A.G., C.L. and S.M.); Management (R.B., A.G. and P.M.); Quality of life (H.C.-P.); Outlook (R.B. and B.G.); Overview of Primer (H.K.S.).
Corresponding author
Ethics declarations
Competing interests
H.K.S. has received lecture fees from the Falk Foundation and research grants from Octapharma. R.B. has no conflicts of interest. H.C.-P. has received lecture fees and advisory board fees from Genfit, Gilead Sciences, Intercept Pharmaceuticals and Merck. She is also the Policy Councillor for the European Association for the Study of the Liver. B.G. has no conflicts of interest. A.G. has received honoraria and grants for research from D&A Pharma SAS and Lundbeck Limited. He was also principal investigator in one of the nalmefene pivotal studies, investigator in the sodium oxybate trial and Spanish coordinator of the acamprosate trial (Adisa study). He is a past president of the European Federation of Addiction Societies and vice president of the International Network on Brief Interventions for Alcohol and Drugs. C.L. has no conflicts of interest. P.M. consults for Gilead Sciences and Verlyx Pharma. S.M. has previously been an adviser for Echosens. G.S. has received US NIH grant funding from the National Institute on Alcohol Abuse and Alcoholism, is employed by the University of Massachusetts Medical School and is the Editor-in-Chief of Hepatology Communications of the American Association for the Study of Liver Disease. H.T. has received grants and donations from EA Pharma, Gilead Sciences and Otsuka Pharmaceutical.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seitz, H.K., Bataller, R., Cortez-Pinto, H. et al. Alcoholic liver disease. Nat Rev Dis Primers 4, 16 (2018). https://doi.org/10.1038/s41572-018-0014-7
Published:
DOI: https://doi.org/10.1038/s41572-018-0014-7
This article is cited by
-
The micro-743a-3p–GSTM1 pathway is an endogenous protective mechanism against alcohol-related liver disease in mice
Cellular & Molecular Biology Letters (2024)
-
Golden bile powder prevents drunkenness and alcohol-induced liver injury in mice via the gut microbiota and metabolic modulation
Chinese Medicine (2024)
-
Association of urinary chlorpyrifos, paraquat, and cyproconazole levels with the severity of fatty liver based on MRI
BMC Public Health (2024)
-
Global burden of liver cirrhosis and other chronic liver diseases caused by specific etiologies from 1990 to 2019
BMC Public Health (2024)
-
GSDMD induces hepatocyte pyroptosis to trigger alcoholic hepatitis through modulating mitochondrial dysfunction
Cell Division (2024)