Abstract
Schistosomiasis (bilharzia) is a neglected tropical disease caused by parasitic flatworms (blood flukes) of the genus Schistosoma, with considerable morbidity in parts of the Middle East, South America, Southeast Asia and, particularly, in sub-Saharan Africa. Infective larvae grow in an intermediate host (fresh-water snails) before penetrating the skin of the definitive human host. Mature adult worms reside in the mesenteric (Schistosoma mansoni and Schistosoma japonicum) or pelvic (Schistosoma haematobium) veins, where female worms lay eggs, which are secreted in stool or urine. Eggs trapped in the surrounding tissues and organs, such as the liver and bladder, cause inflammatory immune responses (including granulomas) that result in intestinal, hepato-splenic or urogenital disease. Diagnosis requires the detection of eggs in excreta or worm antigens in the serum, and sensitive, rapid, point-of-care tests for populations living in endemic areas are needed. The anti-schistosomal drug praziquantel is safe and efficacious against adult worms of all the six Schistosoma spp. infecting humans; however, it does not prevent reinfection and the emergence of drug resistance is a concern. Schistosomiasis elimination will require a multifaceted approach, including: treatment; snail control; information, education and communication; improved water, sanitation and hygiene; accurate diagnostics; and surveillance-response systems that are readily tailored to social-ecological settings.
Similar content being viewed by others
Introduction
Following an autopsy in Egypt in 1851, the German physician Theodor Bilharz first described a parasitic disease that would later be termed bilharzia1. Currently, the most widely accepted term for this disease is schistosomiasis, although bilharzia is still used2. Schistosomiasis is highly debilitating and is intimately linked to poverty, leading to chronic ill health. The infection is widespread in the tropics and subtropics and is caused by one of six species of trematode worms of the genus Schistosoma3,4,5.
The most common disease-causing species are Schistosoma haematobium, Schistosoma mansoni and Schistosoma japonicum, whereas Schistosoma guineensis, Schistosoma intercalatum and Schistosoma mekongi have lower global prevalence. Adult schistosome parasites dwell in the blood vessels of vertebrate hosts, including humans, but their life cycle requires a phase of asexual multiplication or development within an intermediate host — a snail (Biomphalaria spp. snails for S. mansoni, Bulinus spp. snails for S. haematobium and Oncomelania spp. snails for S. japonicum)6. Schistosome infection in humans occurs by contact with fresh water contaminated by cercariae, the free-swimming, infectious stage of schistosomes that are released by the intermediate host snail (Fig. 1) and that penetrate the intact human skin2,3. In zoonotic schistosomiasis, other mammalian hosts are also involved. Transmission is highly dependent on environmental factors, especially those affecting the intermediate host snail. It is conceivable that climate change will affect aquatic environments, and hence, alter the transmission and distribution of water-borne and water-based diseases, such as schistosomiasis7,8.
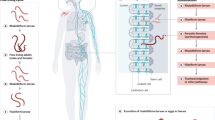
Schistosoma mansoni has a life cycle involving an intermediate fresh-water snail host and the definitive human host. Eggs (excreted in the faeces of the human host) hatch upon contact with water, releasing miracidia (free-swimming ciliated larvae), which penetrate the intermediate host snail (Biomphalaria spp.). Within the snail, the miracidia develop into sporocysts, which in turn produce new daughter sporocysts by asexual reproduction; numerous cercariae (free-swimming larval stage in which the parasites pass from the intermediate to the final host) are then produced again asexually within the daughter sporocysts. After ~30 days, cercariae emerge from the snail in response to sunlight and penetrate the skin of a human host; in the skin, the tails drop off and the larvae transform into schistosomula. The schistosomula enter the venous blood vessels (directly or after invading the lymphatic system) and are transported to the lungs via the right heart, before reaching the left heart, and then enter the arterial circulation. Once they arrive in the hepatic portal system, the schistosomula migrate to the mesenteric veins of the liver, where they mature into separate-sex adults. The male worm grasps the female in the gynaecophoral canal and the worms migrate to the mesenteric veins of the bowel, where the female produces eggs (~5 weeks post-infection). Eggs are released in the bloodstream, pass through the intestinal wall and are excreted in the faeces. Image (top left) reproduced from Oxford Textbook of Medicine 5th Edition edited by Warrell, Cox, and Firth (2010) Figs.7.11.1.3. By permission of Oxford University Press. Image (top right) courtesy of P. Nielsen, Ross University School of Veterinary Medicine, Saint Kitts and Nevis. Image (bottom left) courtesy of A. Azugati. Image (bottom right) courtesy of H. Madsen, University of Copenhagen, Denmark.
There are three distinct phases of clinical disease progression: acute infection, established active infection and late chronic infection. Acute schistosomiasis occurs in travellers to schistosomiasis-endemic areas after a primary infection; common presenting symptoms are myalgia, abdominal pain in the right upper quadrant, diarrhoea (with or without blood), fatigue, malaise, fever and, in case of S. haematobium infection, haematuria (blood in urine)9. Established active and late chronic disease affects mainly individuals from poor rural areas with long-standing infections10. In established active and late chronic infections, immunopathological reactions against schistosome eggs trapped in host tissues lead to inflammatory and obstructive disease; the tissues and organs affected depend on the infecting Schistosoma spp., as the worms nest in different preferential anatomical locations. Late chronic infection with S. guineensis, S. intercalatum, S. mansoni, S. mekongi and S. japonicum (which all reside in the mesenteric veins of the bowel) causes intestinal disease, and advanced disease involves the liver and spleen (hepato-splenic schistosomiasis). Late chronic infection with S. haematobium (which nests in the pelvic venous plexus) causes urogenital schistosomiasis, which mainly involves lesions of the bladder wall4,11,12. Morbidity is particularly severe in high-intensity infections (infections with high worm burden), most importantly with S. mansoni and S. japonicum4,11,12. Schistosomiasis is also associated with undernutrition, exercise intolerance (that is, decreased ability to perform physical exercise at what would be considered the normally expected level or duration), diarrhoea (sometimes bloody), chronic pain and anaemia, and urogenital schistosomiasis may be a factor in the spread of HIV13,14,15.
Although substantial strides have been made in the control of schistosomiasis through programmes of population-based preventive chemotherapy (that is, the periodic administration of praziquantel (an oral anti-schistosomal drug) to at-risk populations without prior diagnosis), additional interventions (including the development of schistosomiasis vaccines) and multisectoral approaches will be required to achieve elimination16.
In this Primer, we describe the main features of schistosome biology and the epidemiology, pathogenesis, clinical features, diagnosis, management and control of schistosomiasis, identifying key areas in which further clinical and basic research are needed for improved management, control and eventual elimination of this human disease.
Epidemiology
The parasites
The six Schistosoma spp. that can infect humans have different geographical distributions. S. haematobium has been reported in 54 countries5 and is the most common species, occurring in sub-Saharan Africa and the Middle East, although a 2013 outbreak of urogenital schistosomiasis was observed in Corsica17. S. mansoni is endemic in sub-Saharan Africa, Brazil, the Caribbean islands, Puerto Rico, Suriname and Venezuela18, and S. japonicum is endemic in the People’s Republic of China and the Philippines, with small foci in Indonesia4. S. japonicum used to be endemic in Japan, but extensive control efforts led to its elimination in the late 1970s (ref.16). The remaining species have lower global prevalence, with S. guineensis and S. intercalatum endemic in West Africa and Central Africa19,20 and S. mekongi restricted to the southern parts of Cambodia and along the Mekong river in Lao People’s Democratic Republic21. Although S. haematobium, S. mansoni, S. guineensis and S. intercalatum primarily infect humans, S. japonicum and S. mekongi are zoonotic species. Indeed, >40 different mammalian species serve as reservoir hosts for S. japonicum, with bovines (water buffalo and cattle) being the most important22. The main reservoir hosts for S. mekongi seem to be dogs and domestic pigs, but other animals, notably bovines, may also be involved in transmission21. Of note, Schistosoma malayensis n. sp., a species related to S. japonicum that was first described in 1988, is responsible for a few autochthonous cases of human schistosomiasis in Peninsular Malaysia23. It is zoonotic, utilizing Robertsiella spp. snails as intermediate hosts and mainly rodents (Rattus muelleri (also known as Sundamys muelleri) and Rattus tiomanicus) as definitive hosts24.
The disease
Prevalence
The WHO considers schistosomiasis a neglected tropical disease that mainly occurs in tropical and subtropical areas (Fig. 2). Globally, an estimated 779 million people are at risk of infection6,25. Over 250 million people are infected with Schistosoma spp. worldwide, with 201.5 million of them living in Africa6,25,26. Over the past decade, considerable progress has been made in mapping and advanced geostatistical modelling, which enables a more-precise display and prediction of the prevalence and intensity of schistosome infection at different scales of analysis (for example, within a single village or at the district, national or continental level)27,28. The application of geographical information system and remote sensing technologies for determining environmental features (such as land surface temperature, rainfall and fresh-water bodies) has been utilized for schistosomiasis risk profiling and estimating treatment needs with the anti-schistosomal drug praziquantel (according to guidelines and treatment thresholds put forth by the WHO)27,29,30. These studies also determined the role of environmental risk factors (for example, living in close proximity to open fresh-water bodies) in explaining patterns and geographical heterogeneity in risk of infection.
A key epidemiological feature of schistosomiasis is its focal distribution (that is, highly variable prevalence and intensity of infection even within a small area, from one village to another), which is governed by the interplay of humans, intermediate host snails and human–water contact patterns. Lack of access to clean water, poor sanitation and hygiene and activities involving contact with water, whether domestic (for example, washing cloths and dishes in open fresh-water bodies), recreational (for example, playing and swimming in ponds, lakes and rivers) or professional (for example, car washing and sand collection), put children, adolescents and adults at risk of schistosome infection when exposed to contaminated water bodies31.
Disease burden
If left untreated, schistosomiasis can result in substantial morbidity and even mortality, although the precise extent is disputed (see Quality of life). According to the Global Burden of Disease Study 2016, the global burden of schistosomiasis is estimated at 1.9 million disability-adjusted life years (DALYs)32. Prior studies estimated the global burden of schistosomiasis at 1.7–4.5 million DALYs33,34,35, whereas a meta-analysis suggested a severalfold higher burden; such higher estimates can be mainly explained by the inclusion in the analysis of undernutrition, anaemia and growth faltering in children, which are partially due to schistosome infection but are usually not attributed to schistosomiasis36. The morbidity and mortality associated with untreated S. japonicum infection are especially high, probably owing to the relatively higher number of eggs produced by this species than by the others37,38.
Mechanisms/pathophysiology
The progression of schistosome infections can be divided into three general overlapping stages influenced by the duration of the individual’s infection: acute, established active and late chronic infection. These stages differ in egg excretion rates in stool or urine as well as in clinical manifestations and symptoms.
Acute stage
Cercarial dermatitis
After cercarial skin penetration of intact skin, a portion of the infectious larvae will die in the skin, whereas the rest enter the venous circulation through a small blood vessel directly or by first entering a lymphatic vessel. The larvae are transported via the blood flow to their maturation site in the liver. In the skin, innate immune responses to dying or dead larvae give rise to hypersensitivity reactions and can cause a maculopapular pruritic reaction, called cercarial dermatitis, on parts of the body that have been exposed to water containing cercariae. Cercarial dermatitis can occur in individuals exposed to human Schistosoma spp. infection for the first time, such as travellers and migrants from a schistosomiasis-endemic area. Swimmer’s itch is a type of cercarial dermatitis caused by cercariae of avian schistosomes9. Swimmer’s itch is more-frequent in some parts of Europe, such as in the Czech Republic, where fresh-water lakes are used for recreational purposes. Whereas the initial exposure to avian cercariae usually causes a relatively mild cutaneous reaction, later exposures in individuals who were previously sensitized generate more-severe symptoms more rapidly. However, infections with avian Schistosoma spp. do not proceed past the initial swimmer’s itch stage.
Acute schistosomiasis
After successful cercarial penetration and maturation of the schistosomula, the infection may proceed to a symptomatic acute stage. Symptomatic acute schistosomiasis is also known as Katayama fever or Katayama syndrome after a district of Hiroshima, Japan, where S. japonicum was detected for the first time in a human39. It usually occurs in naive individuals exposed to Schistosoma spp. for the first time, between 2 weeks and 3 months after exposure9. The symptoms are caused by systemic hypersensitivity reactions and formation of immune complexes in response to antigens released during schistosomula migration or initiation of egg deposition9. Symptoms are often accompanied by eosinophilia and transient pulmonary infiltrates (as observed on chest radiography)3,9,40. Acute schistosomiasis is rarely observed in people living in S. mansoni-endemic or S. haematobium-endemic areas. This lack of susceptibility to acute symptoms may be due to in utero desensitization, resulting in lowered immune responsiveness to schistosome antigens in infants born to infected mothers41, or possibly due to repeated exposure to skin-penetrating cercariae, which induce IL-10 production by CD4+ T cells in the skin, resulting in a regulatory immune response42. However, acute schistosomiasis has been reported in the People’s Republic of China, when flooding has exposed communities living in S. japonicum-endemic areas to new outbreaks of infection38,43.
Established active infection
In most cases, especially in people living in endemic areas, symptomatic acute schistosomiasis is not observed and the disease reaches a stage of established active infection, with mature adult worms and well-established egg production. This stage is characterized by excretion of live eggs in stools or urine. The live adult worms residing in the blood vessels do not stimulate any local inflammation and normally do not directly cause symptoms. This observation can be explained by several factors, such as the fact that adult worms have somatic stem cells that enable the worms to regenerate their surface tegument (the outer protective layer) and binding of host antigens to the surface tegument, thereby hiding the worm antigens on the surface from the host’s immune system44. The main symptoms and organ-related lesions of patent infections are caused by inflammatory responses against parasite eggs (Fig. 3). Schistosome eggs actively secrete antigenic glycoproteins, which have the function of facilitating the passage of the eggs from the blood vessel (where they are laid) to the lumen of the intestine or urinary bladder (thereby promoting transmission) by inducing an inflammatory response. However, these soluble egg antigens also induce the formation of granulomas around eggs that are trapped in the surrounding tissue; granulomas are a collection of inflammatory cells such as eosinophils, neutrophils, lymphocytes and macrophages that accumulate in response to the presence of the eggs.
Granulomas form around eggs trapped in liver tissue. a | Major cellular populations located within and adjacent to the hepatic granuloma induced in either Schistosoma mansoni or Schistosoma japonicum infection. There is a dense population of eosinophils at the core of a S. mansoni-induced hepatic granuloma, whereas in S. japonicum infection the core is composed mainly of neutrophils. Chemokine-binding proteins secreted by S. mansoni eggs can bind neutrophil chemoattractant CXC-chemokine ligand 8 (CXCL8) and block the infiltration of neutrophils to the granuloma; however, these proteins do not bind to eosinophil chemoattractant CC-chemokine ligand 11 (CCL11) and, therefore, do not inhibit the recruitment of eosinophils. b | Hepatic granuloma from a S. mansoni-infected mouse. HSC, hepatic stellate cell. Part a adapted with permission from ref.217, Elsevier. Part b courtesy of A. M. O. Kildemoes, University of Copenhagen, Denmark.
Organ-specific clinical symptoms often positively correlate with infection intensity, as indicated by excreted egg counts, and are mediated by egg-induced inflammation and granulomatous reactions. Established active schistosomiasis is characteristically observed in children in endemic areas and is entirely reversible following treatment and removal of the adult worms3. The high lymphocyte proliferative responses induced by soluble egg antigens at this stage in the infection then progressively decrease as the infection becomes chronic.
Late chronic infection
In most people continuously exposed to infection in schistosomiasis-endemic areas, their worm burdens gradually decline after their early teenage years as a partial immunity to new infections develops, while the number of established worms from earlier infections is reduced over time by natural worm deaths45. This reduction in worms means that fewer new eggs are excreted or deposited in the tissue. At the same time, new granulomas are smaller in size with less inflammation as a result of immunological downregulation, and the previously formed granulomas gradually resolve when eggs within them are destroyed. These granulomas are then replaced by fibrous tissue (scarring), and this process contributes to a reduction in symptom severity.
Immunology and host–parasite interactions
The interaction between the host's immune system and schistosomes is complex and involves several different stages of the life cycle of the parasite, which may be present in the body of the host, such as penetrating cercariae, migrating schistosomula, mature adult worms and parasite eggs trapped in the tissue. Immune responses against cercariae and possibly other parasite stages are important for development of resistance to reinfection, whereas responses towards egg antigens are responsible for the pathology in schistosomiasis. Protective immune responses against schistosomes develop slowly over a period of 10–15 years, and children of <10 years of age in schistosomiasis-endemic areas are susceptible to reinfection after treatment of the schistosome infection, whereas adults are usually resistant. This observation may also explain the characteristic age-prevalence and age-intensity curves observed in schistosomiasis-endemic areas. The human responses linked to resistance to reinfection are T helper 2 (TH2) cell-associated responses characterized by eosinophilia, production of specific immunoglobulin E (IgE) against schistosomes and cytokines such as IL-4 and IL-5. Many studies from a variety of epidemiological settings have shown associations between specific IgE, eosinophils and post-treatment production of IL-4 and IL-5 with resistance to new infections after treatment, whereas specific IgG4 has been associated with susceptibility to infection44. A hypothesis is that death of adult worms – either naturally or after treatment with praziquantel – leads to the release of antigens that may cross-react with larval antigens and stimulate protective IgE responses, suggesting that the more dead worms a person has experienced, the stronger the protective responses46. As the average lifespan of a worm is 3–10 years, this hypothesis could in part explain why children, who have had few dying worms during their lifetime, are susceptible to infection and accumulating more worms through continuous exposure, whereas adults who have experienced many dying worms have developed protective immune responses. This hypothesis may also explain why it takes many years for immunity to develop.
Our knowledge on host–parasite interactions derives from human epidemiological studies analysing immune correlates to resistance to reinfection. Mouse models of schistosome infections cannot be used for this purpose, as mice are naive hosts and do not live long enough to develop protective immune responses after primary infection. However, mouse models are useful for teasing out factors important in immunopathology and formation of granulomas.
The most widely studied experimental animal models of schistosomiasis are murine infections with S. mansoni47 or S. japonicum48. In recent years, these models, particularly using gene knockout or transgenic mice, have substantially advanced the understanding of the immunopathological mechanisms that may be important in various forms of both schistosomiasis and fibrotic disease49.
Pathophysiology
Organ-specific morbidity can develop during established acute and late chronic stages, caused by the accumulation of parasite eggs and development of fibrosis; the severity of symptoms generally correlates with the intensity of the infection. What organs are affected depends on the infecting Schistosoma spp. as the adult worms of different species localize and lay eggs in specific preferred sites. In addition, serious effects result if adult worms locate and lay eggs in aberrant sites9.
Intestinal schistosomiasis
S. mansoni and S. japonicum adult worms live in the mesenteric veins and can cause intestinal schistosomiasis. Symptoms of intestinal schistosomiasis are associated with parasite eggs passing through, or becoming trapped in, intestinal tissues, which induces mucosal granulomatous inflammation with microulcerations, superficial bleeding and, sometimes, pseudopolyposis3,50,51. Pseudopolyps are projecting masses of inflammatory tissue that develop during the healing phase in repeated cycles of ulceration.
Hepato-splenic schistosomiasis
The venous blood flow sweeps a proportion of S. japonicum or S. mansoni eggs from the mesenteric veins into the small portal branches of the liver, via the portal vein, where they are trapped in the pre-sinusoidal periportal tissues. Granulomas form around the eggs (Fig. 3) and, in high-intensity established active infections, this can cause substantial enlargement of the spleen and liver, especially the left liver lobe, characteristic of hepato-splenic schistosomiasis3,52,53. High-intensity childhood infections in particular are often accompanied by hepato-splenomegaly54, and this can be exacerbated by chronic exposure to Plasmodium spp. (the aetiological agent of malaria) infection53. With time and repeated or long-term infections, the worm burden and egg excretion and deposition decrease, and in most adolescents there is an associated decrease in hepato-splenomegaly. However, in some individuals, egg-induced granulomatous responses lead to severe periportal fibrosis, known as Symmer’s pipestem fibrosis, with deposition of collagen around the portal vein, occlusion of the smaller portal branches and severe, often irreversible, pathology55,56. Of note, what factors can contribute or predispose to the development of these complications have yet to be fully understood.
Urogenital schistosomiasis
Adult S. haematobium worms live in the pelvic venous plexus, and the symptoms and pathological changes of urogenital schistosomiasis are closely associated with the passage of parasite eggs through the urinary bladder wall and egg deposition in bladder tissue and genital organs3,55. During an established active infection, clusters of living eggs in the urogenital tissues can be found surrounded by intense inflammatory reactions and intense tissue eosinophilia57,58. The passage of egg clusters into the lumen of the bladder is often accompanied by the sloughing off of the epithelial surface, ulceration and bleeding. The intense egg-induced tissue inflammation can result in bladder wall thickening and development of masses and pseudopolyps59,60. Inflammation and granuloma formation around the ostium (the opening of the ureter in the bladder) blocking the passage of urine can lead to hydronephrosis (swelling of a kidney as a result of a build-up of urine), which may, in some cases, result in a non-functioning kidney61. Urogenital schistosomiasis is associated with squamous cell carcinoma of the urinary bladder, and S. haematobium is now classified by the International Agency for Research on Cancer (IARC) as a carcinogen62,63. The carcinogenic process is closely related to tissue inflammation. Bladder cancer may occur after many years of exposure, infection and urinary tract inflammation63, when an established active infection, with eggs in the urine, is no longer detectable. Generally, late chronic infections are characterized by little or no detectable egg excretion and an accumulation of dead calcified tissue eggs, which may appear as yellow sandy patches in cystoscopic examination of the bladder mucosa58,59.
During established active infection, eggs are frequently deposited in genital organs such as the cervix57,64, seminal vessels and prostate65. Very characteristic cervical lesions are found in S. haematobium infections, including active-stage lesions with intense tissue inflammation with live eggs and chronic-stage sandy patches with clusters of calcified eggs57,66. Analysis of semen samples shows a higher prevalence of leukocytes and increased levels of inflammatory cytokines in men with genital schistosomiasis67. In both women and men, the manifestations of S. haematobium infection may play a part in HIV transmission by increasing the number of inflammatory cells and possibly viral load in semen and causing friable bleeding lesions on the cervical mucosa in women68.
Diagnosis, screening and prevention
Clinical presentations
Acute schistosomiasis
Symptoms may present suddenly and include fever, myalgia, general malaise and fatigue, headache, non-productive cough and intestinal symptoms such as abdominal tenderness or pain. Symptoms are often relatively mild and transient in the weeks or months after exposure. These mild and transient symptoms can lead to misdiagnosis, owing to similarities with many other febrile infectious diseases with acute onset, including malaria, salmonellosis and acute hepatitis40. Many patients recover spontaneously as the symptoms of acute schistosomiasis subside after 2–10 weeks, but the illness follows a more-severe clinical course in some individuals, with weight loss, dyspnoea, diarrhoea and hepatomegaly9,40. Although the clinical manifestations associated with established active and late chronic infections can present in individuals with low-intensity infections (such as naive patients from non-endemic areas), more-severe disease is usually associated with high-intensity infections.
Intestinal schistosomiasis
Intestinal schistosomiasis may be accompanied by intermittent abdominal pain, discomfort, loss of appetite and, sometimes, bloody diarrhoea. These symptoms tend to be more-pronounced with greater intensities of infection51. S. intercalatum and S. guineensis cause generally milder intestinal manifestations than S. mansoni, S. mekongi or S. japonicum.
Hepato-splenic schistosomiasis
Periportal fibrosis is usually seen in adults and, sometimes, in adolescents living in areas with very high schistosomiasis transmission; the severity of periportal fibrosis correlates in part with the intensity and duration of infection69 (Fig. 4). Portal branch occlusion as a result of periportal fibrosis may lead to marked portal hypertension, frequently accompanied by severe enlargement and hardening of the spleen. Of note, whereas portal hypertension is associated with periportal fibrosis in chronic hepato-splenic schistosomiasis in older individuals (often of >15 years of age), in children with severe hepato-splenic schistosomiasis, increased portal pressure may be evident in the absence of detectable periportal fibrosis52.
a | Child with established active schistosome infection, with early inflammatory hepatosplenomegaly. b | Fibrosis around the portal vein (arrow) visualized by ultrasonography. c | Oesophageal varices (arrow) visualized by ultrasonography. d | Severe late chronic fibrotic disease increased portal pressure and ascites in a young adult man. Image in part a courtesy of N. Kabatereine, Imperial College London, UK. Image in part b reproduced from Oxford Textbook of Medicine 5th Edition edited by Warrell, Cox, and Firth (2010) Figs.7.11.1.9. By permission of Oxford University Press. Images in parts c and d courtesy of B. J. Vennervald, University of Copenhagen, Denmark.
In some cases, portal hypertension leads to the development of oesophageal varices, which may rupture with high risk of fatal haematemesis (vomiting of blood)70. Other complications include ascites (accumulation of fluid in the abdominal cavity), delayed growth and puberty and severe anaemia. However, in contrast to toxin-induced and alcohol-induced liver cirrhosis, in schistosomiasis, even with marked periportal fibrosis and portal hypertension, no hepatocyte damage is observed and liver enzymes remain normal70.
Urogenital schistosomiasis
A common characteristic of established active S. haematobium infection is blood in the urine, and in endemic areas many school-aged children present with visible haematuria71. Children may present with dysuria with frequent urination72 as a direct effect of egg-induced urothelial inflammatory responses. However, bladder wall lesions resolve 3–6 months after treatment with praziquantel61,73.
Genital schistosomiasis can cause pelvic discomfort and pain, abnormal vaginal discharge, itch and contact bleeding in girls and women74 and haematospermia and painful ejaculation in men65,75. In both sexes, genital schistosomiasis has been associated with increased risk of HIV infection76.
Other manifestations
Schistosome worms and eggs can sometimes locate in ectopic sites such as the spleen, lungs, skin and central nervous system, causing site-specific manifestations and symptoms.
Neuroschistosomiasis (affection of the central nervous system by Schistosoma spp.) may be one of the most severe clinical outcomes of schistosomiasis and is caused by the inflammatory response around eggs in the cerebral or spinal venous plexus77,78,79. S. mansoni and S. haematobium worms abnormally locate most often in the spinal venous plexus, where they may cause transverse myelitis79, a complication also seen in individuals with acute schistosomiasis40. Neuroschistosomiasis caused by S. japonicum is mainly associated with granulomatous lesions in the brain, which can result in epileptic seizures, encephalopathy with headache, visual impairment, motor deficits and ataxia (reduced coordination of voluntary muscle movements)78.
Pulmonary schistosomiasis is caused by portal-caval shunting, in which venous blood bypasses the liver through collateral veins connecting the portal vein with the vena cava, and eggs are thereby transported to the lung capillaries, where they induce granulomas in the perialveolar area. These granulomas may lead to fibrosis and may result in pulmonary hypertension and cor pulmonale (an enlargement of the right ventricle of the heart due to increased pressure in the lung capillaries)80.
Diagnosis
The diagnosis of acute schistosomiasis requires different diagnostic methods than those used to diagnose established active or late chronic infections40,81. In endemic areas, where individuals typically have established active or late chronic infection, diagnosis is often based on detection of schistosome eggs in faeces or urine by microscopy. However, these methods are often not sensitive enough to detect acute infections, which have low intensity, in returning travellers or recent immigrants from a schistosomiasis-endemic area. In this case, anamnestic information such as recent travels to endemic areas and exposure to fresh-water bodies through recreational or other activities is important and may provide an indication of schistosomiasis82 (Box 1). Eosinophilia is a common finding in these individuals and is often a sign of helminth infection, such as schistosomiasis83.
Parasitological diagnosis
The standard for diagnosing an established active infection is the detection of eggs in faeces, urine or rectal biopsy samples by microscopy81 (Table 1). Parasitological diagnosis of schistosomiasis in populations living in endemic areas most often relies on filtering a standardized small amount of urine or stool sample and microscopically counting all eggs in that volume84,85. The level of infection is then expressed as eggs per 10 ml of urine or eggs per gram of stool (EPG)86. These methods have a low sensitivity (estimated at <50%)86,87, and the observation of 1 egg in a slide corresponds to detection of 20–40 EPG, or 5,000–10,000 eggs per diurnal faecal portion of 250 g (in S. mansoni infections, 1–99 EPG are considered low-intensity infections). However, egg excretion may show a high degree of day-to-day88 as well as intraspecimen and diurnal variation89. This variability warrants collection of multiple biological samples over consecutive days or examination of a single specimen multiple times. Furthermore, as the infection reaches the late chronic stage, egg excretion can be very low or even absent despite severe clinical manifestations3. For travellers or previously exposed people, this diagnostic approach is rarely sensitive enough to detect the often very low levels of infection; thus, larger amounts of urine or stool will have to be examined and other methods employed, such as formol-ether concentration and sedimentation, in which a larger amount of stool is centrifugated81,86.
Schistosome eggs can be detected in tissue biopsy samples66,90 and rectal snip samples, which are very sensitive even for detection of S. haematobium and useful in diagnosing infections in travellers3. S. haematobium can be located in veins around the genital organs in both men and women, and eggs have been detected in semen samples65 and Pap smears64.
Detection of worm antigens, worm DNA and anti-schistosome-specific antibodies
When schistosomes feed on red blood cells, they regurgitate waste products into the bloodstream91. These products are proteoglycans known as circulating anodic (negatively charged) antigens (CAAs) and circulating cathodic (positively charged) antigens (CCAs) and they can be detected in serum and urine by enzyme-linked immunosorbent assay (ELISA) or monoclonal-antibody-based lateral flow assays92. The presence of CAAs or CCAs is an indication of an established active infection with presence of live worms, and circulating antigens can be detected before the worms have started producing eggs93. The levels of antigens correlate well with the intensity of infection94, and the assays for circulating antigens have proved very valuable as research tools in schistosomiasis epidemiology. However, these assays may be less suited for diagnosis in areas with low endemicity and in travellers, who are likely to have very few worms95. There is a commercially available point-of-care (POC) assay (Rapid Medical Diagnostics; Pretoria, South Africa) that detects S. mansoni CCA in urine. This assay is now used for screening of S. mansoni-infected communities in relation to mass drug administration (MDA) programmes96, although the costs of POC-CCA screening may be prohibitive for widespread use in some areas. Concentration of urine samples can improve the sensitivity of the POC-CCA assay97.
PCR-based detection of parasite DNA in stool98 or urine99 is more sensitive100 than parasitological methods and is now increasingly being employed for diagnosis in high-resource settings. Schistosoma spp. DNA can also be detected in vaginal lavage and cerebrospinal fluid samples for diagnosis of urogenital schistosomiasis81 or neuroschistosomiasis, respectively101. Multiplex PCR analysis, which includes detection of several intestinal parasites in a single stool sample, can be an advantage when diagnosing infections in travellers102.
Detection of anti-schistosome-specific antibodies with serological assays can be very useful in travellers who present with clinical symptoms and very low or no egg excretion103. Production of antibodies against adult worm antigens starts ~4–7 weeks after exposure, and in most cases seroconversion takes place within 3 months103. However, serology cannot distinguish between current infection or past exposure in people living in a schistosomiasis-endemic area, where people remain seropositive for several years after treatment104. Antibody detection assays such as rapid diagnostic POC tests105 may prove useful as infection intensities decline following control programmes, as has been the case in the People’s Republic of China106.
Radiology
Ultrasonography can be used to visualize pathological changes associated with established active and late chronic schistosomiasis107. Portable ultrasonography equipment has been used in population-based studies for >20 years to assess the organ-specific pathological changes, such as periportal fibrosis and signs of portal hypertension (such as severe dilatation of the portal vein and presence of collateral veins) in intestinal schistosomiasis108 and bladder wall abnormalities and hydronephrosis in S. haematobium infection108. Ultrasonography is a valuable tool for monitoring the direct effect of interventions on schistosomiasis morbidity109, and standardized protocols for classification and grading of pathological lesions are available110. Radiological examinations such as intravenous pyelography (radiographic examination with injected contrast material) to visualize renal, ureteral and bladder pathology complements ultrasonography for diagnosis of kidney damage59. Endoscopy and cystoscopy can be used to detect oesophageal varices and urinary bladder lesions, respectively111,112 (Fig. 5). In neuroschistosomiasis, CT and especially MRI are the methods of choice113. Of note, these diagnostic tools are generally not available in rural health-care facilities, the primary point of care for many patients with schistosomiasis. Furthermore, they are of limited use in assessing the level of morbidity in endemic populations. However, lesions in the female genital tract, especially cervical lesions, can be detected by colposcopy (direct visualization of the cervix using a special microscope), and a pocket atlas with pictures showing the lesions has been developed for use in health-care facilities in endemic areas57,114.
Active Schistosoma haematobium infection, with egg excretion in urine (parts a–c), and late chronic infection, with calcified eggs (parts d and e). S. haematobium eggs on a nucleopore filter after urine filtration (part a). Urinary bladder wall polyps (arrows) visualized by ultrasonography (part b). Dilatation of ureters and bladder wall thickening (arrows) visualized by ultrasonography (part c). Radiography picture showing bladder calcifications (arrow; part d). Cystoscopy showing yellow sandy patches with calcified S. haematobium eggs (arrow; part e). Images in parts a, c and d courtesy of M. Sacko, Institut National de Recherche en Santé Publique, Mali. Image in part b reproduced from Oxford Textbook of Medicine 5th Edition edited by Warrell, Cox, and Firth (2010) Figs.7.11.1.6. By permission of Oxford University Press. Image in part e reproduced from ref.218, Springer Nature Limited.
Biomarkers
Several biomarkers have been tested for diagnosing or predicting severe schistosomiasis-associated disease. These include different markers for identification of liver fibrosis in S. mansoni or S. japonicum infections10,81 and inflammatory pathological changes in the urogenital tract in S. haematobium infection115. However, such markers are not currently used in routine diagnosis. Several studies have investigated urine biomarkers associated with schistosomiasis and related bladder cancer116, but currently no urine biomarkers have undergone large-scale clinical studies or follow-up studies assessing the cancer-predictive or prognostic value of the tests.
Screening
Screening of populations living in endemic areas is relevant in relation to control programmes, to assess infection prevalence and intensity (that is a proxy for worm burden) before and/or after an intervention, such as MDA81,86. One of the most widely employed, easy-to-use screening methods is testing for S. haematobium-related microhaematuria using a reagent strip test117. This approach has been especially useful in school-aged children, in whom microhaematuria strongly correlates with urogenital schistosomiasis96, and it has also proved to be useful following intervention117. A meta-analysis has shown an overall sensitivity of the reagent strip test for detection of S. haematobium egg-positive urines of 81%, but the overall sensitivity decreased to 72% in previously treated populations117. Direct parasitological methods are inexpensive and easy to use, but a major drawback is their low sensitivity87. The POC-CCA assay may prove useful for detection of S. mansoni infection and has the logistic advantage that it can be performed directly on a urine sample rather than a more-cumbersome stool or blood sample87. The POC-CCA assay is more sensitive than standard parasitological techniques118, but its applicability is limited in low-endemicity areas where people with Schistosoma spp. infection have very few worms.
Tools for detecting Schistosoma spp. transmission in the intermediate host snail and the environment are important to achieve the ultimate goal of schistosomiasis elimination4,81. Traditionally, detection of transmission has been done through collection of intermediate host snails followed by incubation or crushing of the snails to detect shedding of schistosome cercariae119 or sporocysts, respectively119. However, the proportion of collected snails shedding cercariae is generally low, and the direct methods are time consuming with low sensitivity. PCR-based methods or loop-mediated isothermal amplification (LAMP) assays have been used to detect prepatent (that is, sporocysts) or patent (that is, cercariae) infection in intermediate host snails120. Prediction of S. japonicum prevalence and mapping of the risk of infection have been undertaken on the basis of LAMP analysis of its intermediate host snail, Oncomelania hupensis121.
Prevention
Travellers to a schistosomiasis-endemic area should be aware of the risk of infection when engaging in activities involving direct contact with water40. However, for people living in rural areas where schistosomiasis is endemic, it may be very difficult if not impossible to avoid water contact. Schistosomiasis is a poverty-related disease, and preventive measures such as access to clean water and good sanitary facilities are important in reducing the risk of infection but often are not present122. Furthermore, these measures will have little effect on reducing the risk through occupational exposure, such as fishing in the rivers and lakes, and such activities are often the main or only source of income for families123. S. japonicum infection is a zoonosis; thus, preventive measures will have to be directed towards infections in animals as well, particularly water buffaloes, which act as major reservoirs for the parasite124.
Despite intensive development efforts, currently no schistosomiasis vaccines are available125,126.
Management
Management of schistosomiasis involves the targeted treatment of patients diagnosed with schistosomiasis in an endemic area (in a hospital or some other medical facility if available) and in returning travellers or migrants. The aim of treatment with the anti-schistosomal drug praziquantel is curative therapy, and treatment can be repeated several times until the infection is eliminated (that is, no eggs are detected upon microscopic examination of faeces or urine). The recommended dose is generally lower for S. mansoni and S. haematobium infections than for S. japonicum and S. mekongi infections; higher doses are generally split into two administrations a few hours apart55. Global community-based schistosomiasis control programmes, focusing on MDA, are important to reduce morbidity. Of note, in MDA programmes, praziquantel is given only once; thus, as the drug is ineffective against migrating schistosomula, if these larvae are present in an individual, a new patent infection will arise.
Praziquantel
The most effective and widely used compound is praziquantel, an acylated quinoline-pyrazine derivative that has been the mainstay of schistosomiasis treatment for >30 years. Randomized controlled trials showed that oral praziquantel is safe and efficacious against adult worms of all Schistosoma spp., although its precise mechanism of action remains to be established (Box 2), and it is the current therapeutic drug of choice for treating patients diagnosed with schistosomiasis127. Praziquantel is also the cornerstone of global community-based schistosomiasis control programmes, focusing on MDA, to reduce morbidity128,129. Controlled trials (one in Uganda, testing praziquantel against S. mansoni infection, and the other in the Philippines, testing praziquantel against S. japonicum infection) provide support for the use of praziquantel for the treatment of pregnant women after the third trimester, in line with WHO recommendations130.
Praziquantel was discovered in the mid-1970s, commenced trials in humans as an anti-schistosomal drug candidate by 1978 and came on the market as Biltricide (Bayer) in 1988 (ref.131). Subsequently, the efficacy, in terms of cure rate (CR) and egg reduction rate (ERR), and safety of praziquantel have ensured its widespread use. A meta-analysis showed that the WHO-recommended dose of praziquantel (40 mg/kg) achieved CRs of 94.7% for S. japonicum infections, 77.1% for S. haematobium infections, 76.7% for S. mansoni infections and 63.5% for mixed S. haematobium and S. mansoni infections; mean ERR was 95% for S. japonicum, 94.1% for S. haematobium and 86.3% for S. mansoni132. Praziquantel is effective within 1 hour after being ingested; although well absorbed, it undergoes extensive first-pass hepatic clearance (that is, a fraction of the drug (~80%) is metabolized in the liver to inactive metabolites that are excreted in the urine via the kidneys). Praziquantel is also secreted in its active form in breast milk2, but in minute levels; thus, adverse events for the child are not expected, although it is recommended that women should not breastfeed on the day of praziquantel treatment and during the subsequent 72 hours. Praziquantel can be used to treat young children (of >4 years of age) and pregnant women after the first trimester4.
In MDA-based schistosomiasis control programmes, a single dose of praziquantel is recommended by the WHO133, although this regimen has been questioned134 and recent pharmacodynamic and pharmacokinetic data support a higher dose, particularly for children of <6 years of age135. A dose or ‘tablet’ pole is used in the field to estimate the number of tablets given on the basis of height rather than weight of the patient136, and this has been modified recently for treating preschool-aged children (<5 years of age)137 as CRs in this age group are low138.
Co-infections of Schistosoma spp. and soil-transmitted helminths are very common in areas where these worms are co-endemic, such as in Africa and Asia. In these areas, joint preventive therapy of albendazole (another anthelminthic drug for treating soil-transmitted helminth infections) and praziquantel is recommended by the WHO, with schedules related to community prevalence of the two infections139.
Adverse events associated with praziquantel therapy are generally regarded as minimal, including dizziness, headache, abdominal pain, transient nausea, pruritus (itch) and rash, and are likely due to the consequences of antigenic release from dead or dying worms and not the drug itself3,55,127. Allergic reactions to praziquantel are rare140. In individuals with schistosomiasis and concurrent cysticercosis (Taenia solium (pork tapeworm) infection that results in the development of cysts in the central nervous system, skin, eyes and muscle), praziquantel can induce seizures and/or cerebral infarction and permanent eye lesions as a result of the severe inflammatory reactions caused by the dying T. solium worms141. Currently there is no monitoring or evaluation of MDA for schistosomiasis in African communities where schistosomiasis and T. solium cysticercosis are co-endemic; thus, the risk of adverse events in individuals with Schistosoma spp. and T. solium co-infections could be substantially underestimated142.
Limitations of praziquantel
Praziquantel has no effect on immature schistosomes and cannot prevent reinfection or alter the schistosome life cycle134,143. Furthermore, there have been some reports of S. mansoni and S. haematobium infections that are either poorly or not responsive to praziquantel in areas where there has been heavy use of the drug2,3,127,144. In addition, some experimental evidence in mice indicates that schistosome parasites residing in mice that received repeated in vivo praziquantel treatments matured into adult worms that had reduced drug sensitivity; this phenomenon seems to be amplified over each generation of worms145. Furthermore, drug-tolerant schistosome worms have increased levels of P-glycoprotein146, which may correlate with reduced drug efficacy, as increased expression of this member of the ATP-binding cassette superfamily of proteins is known to be associated with multidrug resistance in tumour cells. However, there is no clear confirmation that clinically relevant praziquantel resistance has developed, on the basis of a considerable number of studies in different endemic settings, which have indicated no loss in efficacy in individuals with schistosomiasis who have received multiple courses of treatment over many years147. In addition, there are no reports to date of the development of praziquantel-resistant S. japonicum parasites following extensive use of the drug in the People’s Republic of China148. Notwithstanding, praziquantel resistance cannot be discounted in the future, and there is also widespread concern of the risk associated with relying on a single drug, particularly as MDA is being further scaled up.
The potential for the development of drug resistance and the incomplete efficacy of praziquantel further highlight the need to continue research into developing new compounds and for improved formulations of praziquantel. A series of endoperoxide and praziquantel conjugates had in vitro activity against both adults and schistosomula of S. japonicum and against adult S. mansoni149. Furthermore, a combination of praziquantel and the proton pump inhibitor omeprazole had a synergistic effect against adult worms of S. mansoni in vitro150. By contrast, praziquantel, in combination with the anti-malarial Synriam (Ranbaxy), did not increase efficacy compared with praziquantel alone in a randomized, exploratory phase II trial in adolescents infected with S. mansoni in West Africa151.
The large size of praziquantel tablets and the characteristic bitter taste of the drug mean that treating preschool-aged children is challenging as they may be unable to swallow the whole tablet and, if they chew it, vomiting can occur. The drug is manufactured and administered as a racemic mixture of (S) and (R) stereoisomers, and although the anti-schistosomal properties are associated with (R)-praziquantel, the bitterness is mostly due to (S)-praziquantel. Thus, there are approaches to change the synthesis of praziquantel to remove the (S) isomer152, and another programme (the Pediatric Praziquantel Consortium, a public–private partnership established in 2012) is developing a paediatric formulation suitable for treating schistosomiasis in preschool-aged children153, which, once available, could be included as a primary health-care option through the Integrated Management of Childhood Illnesses (IMCI) platform developed by the WHO and UNICEF (United Nations Children’s Fund)154.
Artemisinin and its derivatives
Despite its effectiveness against adult schistosomes, praziquantel cannot be used as a chemoprophylactic drug (chemoprophylaxis refers to the use of drugs (given at prophylactic doses) to temporarily protect individuals entering an area of high endemicity) owing to its short half-life (1–1.5 h) and because 3–21-day-old migrating schistosomula are refractory to the drug. The potent anti-malarials artesunate and artemether, derived from artemisinin, the active ingredient of the medicinal plant Artemisia annua, are effective against schistosomula during the first 21 days of infection in humans and animals and are capable of killing all immature schistosomula if given every 2 weeks. The mechanism of action of these schistosomicides is not known, although artemether interacts with haemin (an iron-containing molecule that can be formed from haem, the cofactor of human haemoglobin) to exert a toxic effect on schistosomes155. The anti-schistosomal activity of the artemisinin derivatives on schistosome infections in animals and in clinical trials has been extensively reviewed156. Artemether has been used effectively in the chemoprophylaxis of high-risk groups, such as fishermen and flood relief workers, in schistosomiasis-endemic areas of the People’s Republic of China157 and might be useful for individuals travelling to schistosomiasis-endemic areas, yet further evidence is required to support this claim158. Adverse events such as fever, giddiness, sweating and abdominal discomfort are minimal and temporary. The dose required for schistosomiasis chemoprophylaxis is lower than that used for the treatment of malaria; nevertheless, the use of artemether and artemisinin-based combinations for schistosomiasis chemoprophylaxis is not recommended in malaria-endemic areas because of the possible selection of artemisinin-resistant Plasmodium spp. parasites.
It has been suggested that, in areas where continuous schistosomiasis transmission occurs, artemether and other artemisinins can be used as a combined therapy with praziquantel to improve CRs. In support of this approach, controlled trials in animals indicated that the combination of praziquantel plus artemether was safe, resulting in higher CRs than therapy with praziquantel alone157, and a meta-analysis indicated twice higher CRs in humans with the combination therapy than with praziquantel monotherapy159. By contrast, however, in a randomized, placebo-controlled double-blind trial in the People’s Republic of China, combinations of artemether and praziquantel for the treatment of acute schistosomiasis japonica (caused by S. japonicum) did not result in increased levels of efficacy compared with praziquantel monotherapy160. Praziquantel monotherapy was also more efficacious in the treatment of children with S. mansoni infection in western Kenya than another combination therapy comprising artemether with sulfalene plus pyrimethamine, an effective artemisinin-based anti-malarial drug combination161. Furthermore, the addition of mefloquine (a quinine-derived anti-malarial) or mefloquine–artemether (fixed-dose combination) did not increase the efficacy of praziquantel against chronic S. haematobium infection in school-aged children in Côte d’Ivoire162. Thus, overall, the role of artemether-based combination therapy in the treatment of schistosomiasis is still unclear163.
Adjuvant care
In addition to worm elimination (or at least reduction of the intensity of infection), the management of schistosomiasis includes treatment of the complications that may arise following infection.
Corticosteroids, such as prednisone, are used to treat acute schistosomiasis (within 2 months of cercarial exposure)9. If the diagnosis and treatment of neuroschistosomiasis are timely, the prognosis is generally good. In individuals with neuroschistosomiasis, corticosteroids and anticonvulsants may be required in addition to praziquantel2,55. Corticosteroids help to reduce acute allergic reactions, should they occur, and prevent or reduce damage induced by excessive inflammation associated with schistosome egg granulomas in the central nervous system. Anticonvulsants are used to treat seizures associated with neuroschistosomiasis; however, lifelong use is generally unnecessary. Corticosteroids are also used to treat intracranial hypertension and hydrocephalus (accumulation of cerebrospinal fluid within the brain) in neuroschistosomiasis164. Surgery (that is, a ventriculoperitoneal shunt, connecting a brain ventricle with the peritoneal cavity) should be undertaken only on patients who deteriorate, despite clinical management, and for special cases such as those with symptoms of medullary compression164.
Approximately 5–10% of patients with intestinal schistosomiasis progress to the most severe form, hepato-splenic schistosomiasis165. This form is associated with portal hypertension and splenomegaly and can result in the formation of oesophageal varices with episodes of gastrointestinal bleeding, even with liver function preserved. Treatment of variceal bleeding includes band ligation, endoscopic sclerotherapy, splenectomy, portal systemic shunts and combinations of gastro-oesophageal devascularization166. Praziquantel should always be given to patients with advanced disease to prevent cardiopulmonary schistosomiasis (a severe clinical manifestation occurring when prolonged and extensive obstruction of the pulmonary arterioles by schistosome eggs leads to pulmonary hypertension and cor pulmonale) and to arrest fibrosis166.
Quality of life
The assessment of the quality of life of individuals with schistosomiasis is a difficult endeavour because the life cycles of the parasites are complex, the disease shows considerable spatial and temporal heterogeneity and there is a paucity of readily available tools for accurate diagnosis in the field, particularly in settings where infection intensities are low87. For example, the sensitivity of the Kato-Katz technique for the diagnosis of S. mansoni and S. japonicum infections in epidemiological surveys might be <25% in low-endemicity areas167,168. Moreover, there is considerable day-to-day and intraspecimen variation of faecal egg output, which makes reaching a definitive diagnosis difficult167. Of note, infection occurs several weeks before Schistosoma spp. eggs are detected in stool or urine, and it might take many months and multiple infections until people feel that quality of life is affected169. Clearly, this has ramifications on people’s perception of the disease and its morbidity and how to measure and quantify quality of life.
Being a debilitating disease, schistosomiasis has negative implications for human socio-economic activities in endemic areas. Late chronic infection in children compromises cognitive development, growth and physical fitness. In adults, late chronic infection may affect work efficiency, resulting in reduced economic productivity. Schistosomiasis can lead to nonspecific morbidity such as anaemia, impaired childhood growth and malnutrition170,171 as a result of continued inflammation during established active infection. This morbidity is common among school-aged children living in endemic areas, where schistosomiasis may also result in substandard school performance4. Indeed, evidence from randomized drug intervention trials suggests that children had increased physical fitness 1–2 months post-treatment, their skin-fold thickness (a measure that estimates subcutaneous body fat) improved 1–8 months post-treatment, they gained weight 3–12 months post-treatment and they had improved cognition 3 months post-treatment. Meanwhile, levels of perceived pain and anaemia were lower at a 6-month treatment follow-up (C. King, personal communication).
Published estimates show considerable variation with regard to the number of at-risk population, number of people infected with Schistosoma spp., annual mortality and the global burden of disease. In 1998, the first published estimate of the burden of schistosomiasis was 1.7 million DALYs172. Another estimate by the WHO, in 2002, gave a threefold higher estimate of 4.5 million DALYs33. A systematic review, coupled with an analysis of performance-related symptoms and disability-associated outcomes for the human infections associated with the different schistosome species, concluded that the 4.5 million DALY estimate was probably a serious underestimation of the ‘true’ burden36. The highest burden estimate for schistosomiasis reported in the peer-reviewed literature is 70 million DALYs173. New efforts by hundreds of disease experts who systematically review the literature and employ mathematical models to obtain prevalence, incidence, morbidity and mortality data for more than 300 diseases, injuries and risk factors resulted in refined, internally consistent global burden of disease estimates; in 2016, the burden of schistosomiasis was estimated at 1.9 million DALYs32. This most recent trend of declining DALYs is indicative of progress made in schistosomiasis control, most likely as a result of large-scale MDA campaigns27. Overall, the variability of these estimates underscores the challenge of precisely measuring and quantifying the quality of life of individuals with schistosomiasis.
Outlook
The WHO set a target of potential global elimination of schistosomiasis as a public health problem by 2025 (ref.174). To this end, there has been an increased emphasis on reducing schistosomiasis prevalence and intensity of infection, and hence morbidity, mainly through the use of large-scale administration of praziquantel to school-aged children and sometimes other high-risk groups. However, the introduction of such programmes has not been a totally unqualified success on its own despite important health benefits in terms of achieving decreased morbidity in several settings175. Furthermore, these large-scale preventive treatment programmes may, in the longer term, profoundly affect Schistosoma spp. biology and immunoepidemiology with potentially important consequences for public health; thus, careful surveillance and monitoring will be required in the future as MDA programmes are rolled out176. Furthermore, the development of new complementary interventions and new approaches to control are required.
To achieve the WHO target, the 2007 comprehensive schistosomiasis research agenda should be revisited177. The agenda comprised elements ranging from basic science to public health and categorized the research into four themes: tools and interventions for schistosomiasis (including drugs, diagnostics and control programmes and their implementation); ecological, biological and societal aspects of schistosomiasis transmission; schistosomiasis disease burden and epidemiology; and basic science of relevance to schistosomiasis, including vaccines, immunology and pathology. We summarize the most important outstanding areas and emphasize some of the pressing research needs in Box 3.
Diagnosis and prevention
Precise diagnosis of Schistosoma spp. infections, in both snail and mammalian hosts, will be crucial in achieving morbidity reduction and eventually disease elimination, particularly in areas where extensive control has reduced schistosomiasis prevalence and infection intensity to very low levels. Thus, the development of improved, more-cost-effective and field-friendly diagnostic tests represents a key area for future applied schistosomiasis research81,87.
Vaccines
No schistosomiasis vaccine has yet been accepted for public use. One vaccine candidate, monovalent recombinant protein S. haematobium glutathione (S)-transferase (Sh28GST) with adjuvant (Bilhvax, Eurogentec)178, has been tested in a clinical phase III trial, the results of which are still pending125. Schistosomiasis vaccine development is hindered not only by current limited funding but also by the complicated immunology associated with the disease179. Protective responses in natural settings favour IgE production; a schistosomiasis vaccine that is dependent on IgE would be potentially problematic and would likely be affected by regulatory and safety factors owing to potential vaccine-induced anaphylaxis (as IgE is associated with allergic responses)179,180. A step-by-step approach involving identification of protective antigens, selection and cloning of the most promising among them and then ensuring the feasibility of large-scale production has resulted in the identification of a large number of putative, protective antigens as vaccine candidates181. Support for development of schistosomiasis vaccines (both a human schistosomiasis vaccine and a veterinary vaccine for bovine use) and their evaluation in public health programmes has been reinforced by the National Institute of Allergy and Infectious Diseases of the NIH in the United States and the Bill & Melinda Gates Foundation; although in some areas schistosomiasis control could be achieved through current MDA programmes, global control would require an approach integrated with additional interventions, including suitably adjuvanted vaccines125,126. Indeed mathematical modelling supports the view that even partially protective schistosomiasis vaccines would contribute to reducing schistosome infections and interrupting transmission in endemic areas182.
Snail control
Elimination of a human parasitic disease such as schistosomiasis requires interruption of a complex pathway of transmission. In the case of S. japonicum-associated schistosomiasis, control is especially challenging as S. japonicum can persist in a range of nonhuman mammalian hosts.
Control efforts are hindered by rapid reinfection of previously treated individuals. The removal of infected snails would reduce the number of water-borne infectious cercariae, thereby reducing the risk of reinfection. Snail control through the use of chemicals, such as niclosamide, released directly into water, has been implemented in many areas. However, to be most effective, chemicals should be used at least biannually, making the strategy time consuming and expensive, especially when large areas are involved. Additional concerns are the toxicity of molluscicides to larger animals such as fish and the general pollution they cause. Other snail control approaches are environmental methods, such as digging water drainage tunnels or ditches to flood and bury the snail habitats, and the use of biological and ecological measures, including using snail predators such as fish and crustacea183 or introducing schistosome-resistant snail strains into the wild snail population184. Despite the concerns, affordable snail control, combined with MDA using praziquantel, could be part of the optimum strategy leading to schistosomiasis elimination185.
New anti-schistosomal drugs
No anti-schistosomal drug candidates are presently undergoing human clinical trials, a fact that reflects the general current drug discovery and development landscape for parasitic helminth infections186. Nevertheless, although it is unlikely that a new anti-schistosomal drug will be developed to compete with praziquantel in the near future131, owing to the incomplete efficacy of praziquantel and the possibility of resistance developing, there is an urgent need to find an alternative or complementary treatment. Research should continue to develop new compounds or repurposed drugs as an alternative for treatment187 (Box 4).
The availability of the annotated genomic sequences for the three major Schistosoma spp. infecting humans188,189,190 has been pivotal in re-stimulating the search for new anti-schistosomal drug targets. In addition, in vitro and in vivo drug screening has been undertaken of anticancer compounds and other agents with potential for repurposing to treat schistosomiasis191,192. Currently, approaches used in schistosomiasis drug discovery programmes include molecular modelling methods, drug repurposing studies, structure-based drug design and target-focused compound screening189,193,194. These approaches have enabled biochemical mapping of metabolic pathways to predict metabolic rate-limiting steps in schistosomes, which can help to identify molecules that could be new drug targets or are known drug targets in other organisms.
One of the major signalling pathways in schistosomes is apoptosis, and the key molecular components have been identified through advances in genomics and transcriptomics sequencing195. Apoptosis has been extensively targeted with drugs, particularly those used in cancer treatment. Some of these drugs target the BCL-2 family of proteins, which regulate the intrinsic apoptotic pathway, and these compounds are now considered as potentially important anti-schistosomal agents196. The epigenome and epigenetic components represent further anti-schistosomal drug targets in view of the tight control of gene transcription associated with the complexity of the schistosome life cycle197.
The large number of putative drug targets identified through these approaches will require extensive screening to determine which ones are most capable of yielding suitable downstream drug candidates. Subsequently, selected compounds, such as organometallic agents198, or those derived from natural products199, will require rigorous, costly scrutiny and testing for toxicity and efficacy200 before full-scale clinical application.
Disease burden
A precise evidence-based consensus should be developed on how to evaluate and implement the available data on schistosomiasis disease burden locally, nationally and globally. Control efforts have been successful, notably in the People’s Republic of China, but it is now crucial to extend these achievements to areas with limited resources, in particular many countries in sub-Saharan Africa. The battle against schistosomiasis will not be won solely through the distribution and deployment of praziquantel. The creation of strong and efficient health-care systems and the implementation of integrated, cost-effective schistosomiasis control measures that are sustainable in the long term represent important challenges for the public health community well into the 21st century. At the same time, climate change and elevated temperatures may increase the geographical distribution of schistosomiasis through expansion of suitable environments for snails into higher altitudes and into further northern latitudes. When the consequences of climate change become more-pronounced, an increase in the number of migrants will likely result, governed by individuals’ vulnerability and resilience strategies, and it is recognized that several aspects of population movements are directly linked to the spread of schistosomiasis201. Similar to many other neglected tropical diseases, schistosomiasis is a disease of poverty, and strong local and international governmental involvement and support will be needed to finally consign this disease to history.
References
Jordan, P. From Katayama to the Dakhla Oasis: the beginning of epidemiology and control of bilharzia. Acta Trop. 77, 9–40 (2000).
Ross, A. G. et al. Schistosomiasis. N. Engl. J. Med. 346, 1212–1220 (2002). This review article describes the latest updates at the turn of the new millennium with an emphasis on the pathophysiology, diagnosis, genomics, host infection susceptibility, epidemiology, treatment, control and vaccine development for human schistosomiasis.
Gryseels, B., Polman, K., Clerinx, J. & Kestens, L. Human schistosomiasis. Lancet 368, 1106–1118 (2006).
Colley, D. G., Bustinduy, A. L., Secor, W. E. & King, C. H. Human schistosomiasis. Lancet 383, 2253–2264 (2014). This authoritative review pertains to all aspects of human schistosomiasis, including diagnosis, epidemiology, immunology, mapping and surveillance, pathogenesis, morbidity and comorbidities, treatment and control and elimination.
World Health Organization. Schistosomiasis (WHO, 2017).
Steinmann, P., Keiser, J., Bos, R., Tanner, M. & Utzinger, J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6, 411–425 (2006). This highly cited paper presents global estimates of people at risk of schistosomiasis, number of people infected and evidence of changing risk patterns due to water resource developments, specifically the construction and management of large dams and irrigation systems.
McCreesh, N., Nikulin, G. & Booth, M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit. Vectors 8, 4 (2015).
Zhou, X.-N. et al. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 78, 188–194 (2008).
Ross, A. G., Vickers, D., Olds, G. R., Shah, S. M. & McManus, D. P. Katayama syndrome. Lancet Infect. Dis. 7, 218–224 (2007).
Olveda, D. U. et al. The chronic enteropathogenic disease schistosomiasis. Int. J. Infect. Dis. 28, 193–203 (2014).
Hatz, C. F. The use of ultrasound in schistosomiasis. Adv. Parasitol. 48, 225–284 (2001).
van der Werf, M. J. et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 86, 125–139 (2003). This paper reviews and quantifies clinical morbidity due to S. haematobium and S. mansoni and puts forward annual mortality estimates of schistosomiasis in excess of 200,000 in Africa alone.
King, C. H. Parasites and poverty: the case of schistosomiasis. Acta Trop. 113, 95–104 (2010).
Secor, W. E. The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr. Opin. HIV AIDS 7, 254–259 (2012).
Kjetland, E. F. et al. Genital schistosomiasis and its unacknowledged role on HIV transmission in the STD intervention studies. Int. J. STD AIDS 25, 705–715 (2014).
Rollinson, D. et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 128, 423–440 (2013). This paper reviews schistosomiasis control and elimination efforts in different parts of the world and puts forward a schistosomiasis elimination agenda.
Boissier, J. et al. Outbreak of urogenital schistosomiasis in Corsica (France): an epidemiological case study. Lancet Infect. Dis. 16, 971–979 (2016). The study describes the epidemiology of an outbreak of urogenital schistosomiasis in Corsica, France, showing that the causative parasite (that is, S. haematobium) was imported by individuals infected in West Africa, that suitable intermediate hosts were present to close the life cycle and that hybridization between S. haematobium and the cattle schistosome Schistosoma bovis had a putative role in this outbreak. The study recommends future monitoring to offset the potential risk of schistosomiasis outbreaks elsewhere in Europe.
Kurup, R. & Hunjan, G. S. Epidemiology and control of schistosomiasis and other intestinal parasitic infections among school children in three rural villages of south Saint Lucia. J. Vector Borne Dis. 47, 228–234 (2010).
Tchuem Tchuenté, L.-A., Southgate, V. R., Jourdane, J., Webster, B. L. & Vercruysse, J. Schistosoma intercalatum: an endangered species in Cameroon? Trends Parasitol. 19, 389–393 (2003).
Ekpo, U. F. et al. Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat. Health 7, 355 (2013).
Muth, S. et al. Schistosoma mekongi in Cambodia and Lao People’s Democratic Republic. Adv. Parasitol. 72, 179–203 (2010).
Zhou, X.-N. et al. Schistosomiasis japonica control and research needs. Adv. Parasitol. 72, 145–178 (2010).
Latif, B., Heo, C. C., Razuin, R., Shamalaa, D. V. & Tappe, D. Autochthonous human schistosomiasis, Malaysia. Emerg. Infect. Dis. 19, 1340–1341 (2013).
Greer, G. J., Ow-Yang, C. K. & Yong, H.-S. Schistosoma malayensis n. sp.: a Schistosoma japonicum-complex schistosome from Peninsular Malaysia. J. Parasitol. 74, 471 (1988).
Utzinger, J. et al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology 136, 1859 (2009).
Hotez, P. J. et al. The Global Burden of Disease Study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 8, e2865 (2014).
Lai, Y.-S. et al. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect. Dis. 15, 927–940 (2015). This systematic review and geostatistical meta-analysis describes the use of advanced Bayesian-based geostatistical modelling to produce high-resolution risk estimates of infection with Schistosoma spp. in sub-Saharan Africa. Additionally, through the use of gridded population estimates, the authors determined the annualized numbers of doses of praziquantel treatment needed to prevent morbidity in sub-Saharan Africa countries for spatial targeting of schistosomiasis control interventions.
Walz, Y., Wegmann, M., Dech, S., Raso, G. & Utzinger, J. Risk profiling of schistosomiasis using remote sensing: approaches, challenges and outlook. Parasit. Vectors 8, 163 (2015).
Simoonga, C. et al. Remote sensing, geographical information system and spatial analysis for schistosomiasis epidemiology and ecology in Africa. Parasitology 136, 1683–1693 (2009).
Schur, N., Vounatsou, P. & Utzinger, J. Determining treatment needs at different spatial scales using geostatistical model-based risk estimates of schistosomiasis. PLoS Negl. Trop. Dis. 6, e1773 (2012).
Grimes, J. E. T. et al. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 8, e3296 (2014).
GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1260–1344 (2017).
WHO Expert Committee on the Control of Schistosomiasis. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: WHO Technical Report Series N°912, (WHO, 2002).
Utzinger, J. & Keiser, J. Schistosomiasis and soil-transmitted helminthiasis: common drugs for treatment and control. Expert Opin. Pharmacother. 5, 263–285 (2004).
World Health Organization. The World Health Report 2004 – changing history (WHO, 2004).
King, C. H., Dickman, K. & Tisch, D. J. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 365, 1561–1569 (2005).This systematic review of data on disability-associated outcomes for all forms of schistosomiasis shows that the disease is significantly associated with anaemia, chronic pain, diarrhoea, exercise intolerance and undernutrition, suggesting that the disability burden previously assigned to schistosomiasis by the WHO is an underestimate and indicating a need to reassess priorities for treating this silent pandemic.
Gillespie, S. H. & Pearson, R. D. Principles and Practice of Clinical Parasitology (John Wiley & Sons, Ltd, 2001).
Ross, A. G. P. et al. Schistosomiasis in the People’s Republic of China: prospects and challenges for the 21st century. Clin. Microbiol. Rev. 14, 270–295 (2001).
Ishii, A., Tsuji, M. & Tada, I. History of Katayama disease: schistosomiasis japonica in Katayama district, Hiroshima, Japan. Parasitol. Int. 52, 313–319 (2003).
Clerinx, J. & Van Gompel, A. Schistosomiasis in travellers and migrants. Travel Med. Infect. Dis. 9, 6–24 (2011).
King, C. L. et al. B cell sensitization to helminthic infection develops in utero in humans. J. Immunol. 160, 3578–3584 (1998).
Sanin, D. E., Prendergast, C. T., Bourke, C. D. & Mountford, A. P. Helminth infection and commensal microbiota drive early IL-10 production in the skin by CD4+ T cells that are functionally suppressive. PLoS Pathog. 11, e1004841 (2015). This study reports the use of a murine model of repeated infection with S. mansoni larvae, showing that the site of infection in the skin becomes rich in regulatory IL-10, whereas in its absence, inflammation, neutrophil recruitment and local lymphocyte proliferation are increased, and suggests how tolerance and pathogen clearance are co-regulated early after exposure to an infectious agent.
Wu, X.-H. et al. Effect of floods on the transmission of schistosomiasis in the Yangtze River valley, People’s Republic of China. Parasitol. Int. 57, 271–276 (2008).
Colley, D. G. & Secor, W. E. Immunology of human schistosomiasis. Parasite Immunol. 36, 347–357 (2014). This paper comprehensively summarizes the range of immunological studies that have been carried out on immunopathogenesis mechanisms, resistance to reinfection and diagnostics in experimental and human schistosomiasis.
Wilkins, H. A., Goll, P. H., de Marshall, C. T. F. & Moore, P. J. Dynamics of Schistosoma haematobium infection in a Gambian community. III. Acquisition and loss of infection. Trans. R. Soc. Trop. Med. Hyg. 78, 227–232 (1984).
Fitzsimmons, C. M. et al. Progressive cross-reactivity in IgE responses: an explanation for the slow development of human immunity to schistosomiasis? Infect. Immun. 80, 4264–4270 (2012). This important study provides a possible explanation of why individuals in S. mansoni -endemic areas slowly acquire immunity to schistosomiasis over many years.
Barron, L. & Wynn, T. A. Macrophage activation governs schistosomiasis-induced inflammation and fibrosis. Eur. J. Immunol. 41, 2509–2514 (2011).
Chen, X. et al. Follicular helper T cells promote liver pathology in mice during Schistosoma japonicum infection. PLoS Pathog. 10, e1004097 (2014).
Cook, P. C. et al. A dominant role for the methyl-CpG-binding protein Mbd2 in controlling Th2 induction by dendritic cells. Nat. Commun. 6, 6920 (2015).
Chen, M. Assessment of morbidity due to Schistosoma japonicum infection in China. Infect. Dis. Poverty 3, 6 (2014).
Lambertucci, J. R., Voieta, I. & Barbosa, A. J. A. Colonic polyps in hepatosplenic schistosomiasis mansoni. Rev. Soc. Bras. Med. Trop. 38, 80–81 (2005).
Vennervald, B. J. et al. Detailed clinical and ultrasound examination of children and adolescents in a Schistosoma mansoni endemic area in Kenya: hepatosplenic disease in the absence of portal fibrosis. Trop. Med. Int. Health 9, 461–470 (2004).
Wilson, S., Vennervald, B. J. & Dunne, D. W. Chronic hepatosplenomegaly in African school children: a common but neglected morbidity associated with schistosomiasis and malaria. PLOS Negl. Trop. Dis. 5, e1149 (2011).
Wilson, S. et al. Health implications of chronic hepatosplenomegaly in Kenyan school-aged children chronically exposed to malarial infections and Schistosoma mansoni. Trans. R. Soc. Trop. Med. Hyg. 104, 110–116 (2010).
Gray, D. J., Ross, A. G., Li, Y.-S. & McManus, D. P. Diagnosis and management of schistosomiasis. BMJ 342, d2651 (2011).
Lambertucci, J. R., Voieta, I. & Resende, V. Mild, moderate and intense Symmers’s fibrosis in hepatosplenic schistosomiasis mansoni. Rev. Soc. Bras. Med. Trop. 42, 611–612.
Randrianasolo, B. S. et al. Gynecological manifestations, histopathological findings, and schistosoma-specific polymerase chain reaction results among women with Schistosoma haematobium infection: a cross-sectional study in Madagascar. J. Infect. Dis. 212, 275–284 (2015).
Ghoneim, M. A. Bilharziasis of the genitourinary tract. BJU Int. 89, 22–30 (2002).
Burki, A. et al. Comparison of ultrasonography, intravenous pyelography and cystoscopy in detection of urinary tract lesions due to Schistosoma haematobium. Acta Trop. 43, 139–151 (1986).
Hatz, C. et al. Measurement of schistosomiasis-related morbidity at community level in areas of different endemicity. Bull. World Health Organ. 68, 777–787 (1990).
Kayange, N. M. et al. Kidney disease among children in sub-Saharan Africa: systematic review. Pediatr. Res. 77, 272–281 (2014).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 100, 1–441 (2012).
Vennervald, B. J. & Polman, K. Helminths and malignancy. Parasite Immunol. 31, 686–696 (2009).
Leutscher, P. et al. Schistosoma haematobium induced lesions in the female genital tract in a village in Madagascar. Acta Trop. 66, 27–33 (1997).
Leutscher, P. et al. Community-based study of genital schistosomiasis in men from Madagascar. Lancet 355, 117–118 (2000).
Kjetland, E. F. et al. Classification of the lesions observed in female genital schistosomiasis. Int. J. Gynecol. Obstet. 127, 227–228 (2014).
Leutscher, P. D. C. et al. Increased prevalence of leukocytes and elevated cytokine levels in semen from Schistosoma haematobium–infected individuals. J. Infect. Dis. 191, 1639–1647 (2005).
Midzi, N., Mduluza, T., Mudenge, B., Foldager, L. & Leutscher, P. D. C. Decrease in seminal HIV-1 RNA load after praziquantel treatment of urogenital schistosomiasis coinfection in HIV-Positive men-an observational study. Open Forum Infect. Dis. 4, ofx199 (2017).
Booth, M. et al. Hepatosplenic morbidity in two neighbouring communities in Uganda with high levels of Schistosoma mansoni infection but very different durations of residence. Trans. R. Soc. Trop. Med. Hyg. 98, 125–136 (2004).
Strauss, E. Hepatosplenic schistosomiasis: a model for the study of portal hypertension. Ann. Hepatol. 1, 6–11 (2002).
Ganapathi, L. & Somers, M. A. Child with gross hematuria. N. Engl. J. Med. 373, e11 (2015).
Ismail, H. et al. Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Parasit. Vectors 7, 478 (2014).
Wagatsuma, Y. et al. Resolution and resurgence of Schistosoma haematobium-induced pathology after community-based chemotherapy in Ghana, as detected by ultrasound. J. Infect. Dis. 179, 1515–1522 (1999).
Hegertun, I. E. A. et al. S. haematobium as a common cause of genital morbidity in girls: a cross-sectional study of children in South Africa. PLoS Negl. Trop. Dis. 7, e2104 (2013).
van Delft, F., Visser, L., Polderman, A. & van Lieshout, L. Cough and alterations in semen after a tropical swim. Neth. J. Med. 65, 304–306 (2007).
Kjetland, E. F. et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 20, 593–600 (2006).
Ferrari, T. C. A. & Moreira, P. R. R. Neuroschistosomiasis: clinical symptoms and pathogenesis. Lancet Neurol. 10, 853–864 (2011).
Ross, A. G. et al. Neuroschistosomiasis. J. Neurol. 259, 22–32 (2011).
Vale, T. C., de Sousa-Pereira, S. R., Ribas, J. G. R. & Lambertucci, J. R. Neuroschistosomiasis mansoni: literature review and guidelines. Neurologist 18, 333–342 (2012).
Graham, B. B., Bandeira, A. P., Morrell, N. W., Butrous, G. & Tuder, R. M. Schistosomiasis-associated pulmonary hypertension: pulmonary vascular disease: the global perspective. Chest 137, 20S–29S (2010).
Weerakoon, K. G., Gobert, G. N., Cai, P. & McManus, D. P. Advances in the diagnosis of human schistosomiasis. Clin. Microbiol. Rev. 28, 939–967 (2015). This authoritative review considers some of the earlier approaches in the search for new diagnostics for schistosomiasis but emphasizes the more-recent developments that have practical applications in the laboratory, the clinic and the field.
Coltart, C. E. et al. Schistosomiasis presenting in travellers: a 15 year observational study at the Hospital for Tropical Diseases, London. Trans. R. Soc. Trop. Med. Hyg. 109, 214–220 (2015).
Checkley, A. M. et al. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J. Infect. 60, 1–20 (2010).
Katz, N., Chaves, A. & Pellegrino, J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14, 397–400.
Feldmeier, H., Doehring, E. & Daffalla, A. A. Simultaneous use of a sensitive filtration technique and reagent strips in urinary schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 76, 416–421 (1982).
Feldmeier, H. & Poggensee, G. Diagnostic techniques in schistosomiasis control. A review. Acta Trop. 52, 205–220 (1993).
Utzinger, J., Becker, S. L., van Lieshout, L., van Dam, G. J. & Knopp, S. New diagnostic tools in schistosomiasis. Clin. Microbiol. Infect. 21, 529–542 (2015). This comprehensive review of past, current and potential future diagnostic tools for schistosomiasis emphasizes target product profiles that are required for different stages of control and elimination efforts.
Polman, K., Deelder, A. M., Fathers, L., Gryseels, B. & Engels, D. Day-to-day fluctuation of schistosome circulating antigen levels in serum and urine of humans infected with Schistosoma mansoni in Burundi. Am. J. Trop. Med. Hyg. 59, 150–154 (1998).
Doehring, E., Feldmeier, H. & Daffalla, A. A. Day-to-day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan. Ann. Trop. Med. Parasitol. 77, 587–594 (1983).
Fritzsche, C. et al. Confocal laser scanning microscopy, a new in vivo diagnostic tool for schistosomiasis. PLoS ONE 7, e34869 (2012).
Deelder, A. M., Kornelis, D., Van Marck, E. A. E., Eveleigh, P. C. & Van Egmond, J. G. Schistosoma mansoni: characterization of two circulating polysaccharide antigens and the immunological response to these antigens in mouse, hamster, and human infections. Exp. Parasitol. 50, 16–32 (1980).
van Dam, G. J. et al. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp. Parasitol. 135, 274–282 (2013).
van Dam, G. J., Bogitsh, B. J., van Zeyl, R. J. M., Rotmans, J. P. & Deelder, A. M. Schistosoma mansoni: in vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J. Parasitol. 82, 557 (1996).
Van Lieshout, L. et al. Analysis of worm burden variation in human Schistosoma mansoni infections by determination of serum levels of circulating anodic antigen and circulating cathodic antigen. J. Infect. Dis. 172, 1336–1342 (1995).
van Lieshout, L., Polderman, A. M., Visser, L. G., Verwey, J. J. & Deelder, A. M. Detection of the circulating antigens CAA and CCA in a group of Dutch travellers with acute schistosomiasis. Trop. Med. Int. Health 2, 551–557 (1997).
Ochodo, E. A. et al. Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas. Cochrane Database Syst. Rev. 3, CD009579 https://doi.org/10.1002/14651858.cd009579.pub2 (2015). This systematic review shows that microhaematuria correctly detected the largest proportions of infections and non-infections identified by microscopy for S. haematobium infections, whereas the POC-CCA urine cassette test for S. mansoni detected a high proportion of infections identified by microscopy but misclassified a large number of microscopy negatives as positives in endemic areas with a moderate to high prevalence of infection.
Grenfell, R. F. Q. et al. Innovative methodology for point-of-care circulating cathodic antigen with rapid urine concentration for use in the field for detecting low Schistosoma mansoni infection and for control of cure with high accuracy. Trans. R. Soc. Trop. Med. Hyg. 112, 1–7 (2018).
Meurs, L. et al. Is PCR the next reference standard for the diagnosis of Schistosoma in stool? A comparison with microscopy in Senegal and Kenya. PLoS Negl. Trop. Dis. 9, e0003959 (2015).
Obeng, B. B. et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobiumin urine samples from Ghana. Ann. Trop. Med. Parasitol. 102, 625–633 (2008).
He, P. et al. Real-time PCR diagnosis of Schistosoma japonicum in low transmission areas of China. Infect. Dis. Poverty 7, 8 (2018).
Härter, G. et al. Diagnosis of neuroschistosomiasis by antibody specificity index and semi-quantitative real-time PCR from cerebrospinal fluid and serum. J. Med. Microbiol. 63, 309–312 (2014).
Cnops, L., Tannich, E., Polman, K., Clerinx, J. & Van Esbroeck, M. Schistosoma real-time PCR as diagnostic tool for international travellers and migrants. Trop. Med. Int. Health 17, 1208–1216 (2012).
Whitty, C. J. M., Mabey, D. C., Armstrong, M., Wright, S. G. & Chiodini, P. L. Presentation and outcome of 1107 cases of schistosomiasis from Africa diagnosed in a non-endemic country. Trans. R. Soc. Trop. Med. Hyg. 94, 531–534 (2000).
Doenhoff, M. J., Chiodini, P. L. & Hamilton, J. V. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies? Trends Parasitol. 20, 35–39 (2004).
Nausch, N. et al. Field evaluation of a new antibody-based diagnostic for Schistosoma haematobium and S. mansoni at the point-of-care in northeast Zimbabwe. BMC Infect. Dis. 14, 165 (2014).
Cai, Y.-C. et al. Field comparison of circulating antibody assays versus circulating antigen assays for the detection of schistosomiasis japonica in endemic areas of China. Parasit. Vectors 7, 138 (2014).
Barata, C. H., Pinto-Silva, R. A. & Lambertucci, J. R. Abdominal ultrasound in acute schistosomiasis mansoni. Br. J. Radiol. 72, 949–952 (1999).
Akpata, R. et al. The WHO ultrasonography protocol for assessing morbidity due to Schistosoma haematobium. Acceptance and evolution over 14 years. Syst. Rev. Parasitol. Res. 114, 1279–1289 (2015).
Chigusa, Y. et al. Effects of repeated praziquantel treatment on schistosomiasis mekongi morbidity as detected by ultrasonography. Parasitol. Int. 55, 261–265 (2006).
World Health Organization. Ultrasound in schistosomiasis: a practical guide to the standard use of ultrasonography for assessment of schistosomiasis-related morbidity (WHO, 2016).
Chofle, A. A. et al. Oesophageal varices, schistosomiasis, and mortality among patients admitted with haematemesis in Mwanza, Tanzania: a prospective cohort study. BMC Infect. Dis. 14, 303 (2014).
Ahmed, F. O., Hamdan, H. Z., Abdelgalil, H. B. & Sharfi, A. A. A comparison between transabdominal ultrasonographic and cystourethroscopy findings in adult Sudanese patients presenting with haematuria. Int. Urol. Nephrol. 47, 223–228 (2014).
Henriques-Souza, A. M. & Valença, M. M. Schistosomal myelopathy in childhood: findings of magnetic resonance imaging in 26 patients. Pediatr. Neurol. 45, 373–376 (2011).
Norseth, H. M. et al. The colposcopic atlas of schistosomiasis in the lower female genital tract based on studies in Malawi, Zimbabwe, Madagascar and South Africa. PLoS Negl. Trop. Dis. 8, e3229 (2014).
Ramarokoto, C. E. et al. Eosinophil granule proteins ECP and EPX as markers for a potential early-stage inflammatory lesion in female genital schistosomiasis (FGS). PLoS Negl. Trop. Dis. 8, e2974 (2014).
Shiff, C., Naples, J. M., Isharwal, S., Bosompem, K. M. & Veltri, R. W. Non-invasive methods to detect schistosome-based bladder cancer: is the association sufficient for epidemiological use? Trans. R. Soc. Trop. Med. Hyg. 104, 3–5 (2010).
King, C. H. & Bertsch, D. Meta-analysis of urine heme dipstick diagnosis of Schistosoma haematobium infection, including low-prevalence and previously-treated populations. PLoS Negl. Trop. Dis. 7, e2431 (2013).
Kittur, N., Castleman, J. D., Campbell, C. H., King, C. H. & Colley, D. G. Comparison of Schistosoma mansoni prevalence and intensity of infection, as determined by the circulating cathodic antigen urine assay or by the Kato-Katz fecal assay: a systematic review. Am. J. Trop. Med. Hyg. 94, 605–610 (2016).
Sturrock, R. F., Karamsadkar, S. J. & Ouma, J. Schistosome infection rates in field snails: Schistosoma mansoni in Biomphalaria pfeifferi from Kenya. Ann. Trop. Med. Parasitol. 73, 369–375 (1979).
Hamburger, J. et al. Evaluation of loop-mediated isothermal amplification suitable for molecular monitoring of schistosome-infected snails in field laboratories. Am. J. Trop. Med. Hyg. 88, 344–351 (2013).
Tong, Q.-B. et al. A new surveillance and response tool: risk map of infected Oncomelania hupensis detected by loop-mediated isothermal amplification (LAMP) from pooled samples. Acta Trop. 141, 170–177 (2015).
Grimes, J. E. T. et al. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit. Vectors 8, 156 (2015).
Olsen, A., Kinung’hi, S. & Magnussen, P. Schistosoma mansoni infection along the coast of Lake Victoria in Mwanza region, Tanzania. Am. J. Trop. Med. Hyg. 92, 1240–1244 (2015).
Wang, L.-D. et al. China’s new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop. Med. Int. Health 14, 1475–1483 (2009).
Mo, A. X., Gordon, L., Hall, B. F., Walson, J. L. & Agosti, J. M. Schistosomiasis elimination strategies and potential role of a vaccine in achieving global health goals. Am. J. Trop. Med. Hyg. 90, 54–60 (2014). This paper describes the outcomes of a 2013 meeting co-sponsored by the National Institute of Allergy and Infectious Diseases and the Bill & Melinda Gates Foundation and concludes that an integrated, multifaceted approach involving chemotherapy; water, sanitation and hygiene (WASH); snail control; vaccines and other innovative tools will be necessary to have a permanent effect on schistosomiasis.
Mo, A. X. & Colley, D. G. Workshop report: schistosomiasis vaccine clinical development and product characteristics. Vaccine 34, 995–1001 (2016).
Doenhoff, M. J., Cioli, D. & Utzinger, J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 21, 659–667 (2008).
Knopp, S., Becker, S. L., Ingram, K. J., Keiser, J. & Utzinger, J. Diagnosis and treatment of schistosomiasis in children in the era of intensified control. Expert Rev. Anti. Infect. Ther. 11, 1237–1258 (2013).
Stothard, J. R., Sousa-Figueiredo, J. C. & Navaratnam, A. M. D. Advocacy, policies and practicalities of preventive chemotherapy campaigns for African children with schistosomiasis. Expert Rev. Anti. Infect. Ther. 11, 733–752 (2013).
Friedman, J. F., Olveda, R. M., Mirochnick, M. H., Bustinduy, A. L. & Elliott, A. M. Praziquantel for the treatment of schistosomiasis during human pregnancy. Bull. World Health Organization 96, 59–65 (2018).
Fenwick, A. Praziquantel: do we need another antischistosoma treatment? Future Med. Chem. 7, 677–680 (2015).
Zwang, J. & Olliaro, P. L. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis—a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl. Trop. Dis. 8, e3286 (2014).
World Health Organization. Preventive chemotherapy in human helminthiasis – coordinated use of anthelminthic drugs in control interventions (WHO, 2006).
Ross, A. G. P., Olveda, R. M. & Li, Y. An audacious goal: the elimination of schistosomiasis in our lifetime through mass drug administration. Lancet 385, 2220–2221 (2015).
Bustinduy, A. L. et al. Population pharmacokinetics and pharmacodynamics of praziquantel in Ugandan children with intestinal schistosomiasis: higher dosages are required for maximal efficacy. MBio 7, e00227–00216 (2016).
Montresor, A. et al. Development and validation of a ‘tablet pole’ for the administration of praziquantel in sub-Saharan Africa. Trans. R. Soc. Trop. Med. Hyg. 95, 542–544 (2001).
Sousa-Figueiredo, J. C., Betson, M. & Stothard, J. R. Treatment of schistosomiasis in African infants and preschool-aged children: downward extension and biometric optimization of the current praziquantel dose pole. Int. Health 4, 95–102 (2012).
Sousa-Figueiredo, J. C. et al. Performance and safety of praziquantel for treatment of intestinal schistosomiasis in infants and preschool children. PLoS Negl. Trop. Dis. 6, e1864 (2012).
World Health Organization. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. (WHO, 2006).
Patel, T. A., Bailey, R. L., Lukawska, J. & Rowe, J. Treatment of schistosomiasis in a patient allergic to praziquantel: a desensitization and treatment protocol. Am. J. Trop. Med. Hyg. 95, 1041–1043 (2016).
Fong, G. C. & Cheung, R. T. Caution with praziquantel in neurocysticercosis. Stroke 28, 1648–1649 (1997).
Braae, U. et al. Taenia solium taeniosis/cysticercosis and the co-distribution with schistosomiasis in Africa. Parasit. Vectors 8, 323 (2015).
Gray, D. J. et al. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect. Dis. 10, 733–736 (2010). This personal view argues that current praziquantel-based schistosomiasis control programmes are not effective or sustainable in the long term, whereas multifaceted, integrated control options would have a greater and longer lasting effect in reducing morbidity and on disease transmission.
Crellen, T. et al. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin. Infect. Dis. 63, ciw506 (2016).
Pica-Mattoccia, L. et al. Genetic analysis of decreased praziquantel sensitivity in a laboratory strain of Schistosoma mansoni. Acta Trop. 111, 82–85 (2009).
Messerli, S. M., Kasinathan, R. S., Morgan, W., Spranger, S. & Greenberg, R. M. Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility. Mol. Biochem. Parasitol. 167, 54–59 (2009).
Wang, W., Wang, L. & Liang, Y.-S. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol. Res. 111, 1871–1877 (2012).
McManus, D. P. et al. Schistosomiasis in the People’s Republic of China: the era of the Three Gorges dam. Clin. Microbiol. Rev. 23, 442–466 (2010).
Yang, J. et al. Design, synthesis and biological evaluation of praziquantel and endoperoxide conjugates as antischistosomal agents. Future Med. Chem. 7, 713–725 (2015).
Almeida, G. T. et al. Synergy of omeprazole and praziquantel in vitro treatment against Schistosoma mansoni adult worms. PLoS Negl. Trop. Dis. 9, e0004086 (2015).
Barda, B. et al. Efficacy and safety of moxidectin, synriam, synriam-praziquantel versus praziquantel against Schistosoma haematobium and S. mansoni infections: a randomized, exploratory phase 2 trial. PLoS Negl. Trop. Dis. 10, e0005008 (2016).
Cupit, P. M. & Cunningham, C. What is the mechanism of action of praziquantel and how might resistance strike? Future Med. Chem. 7, 701–705 (2015). This paper discusses current thinking regarding the mechanism of action of praziquantel and the potential for widespread drug resistance.
Stothard, J. R., Sousa-Figueiredo, J. C., Betson, M., Bustinduy, A. & Reinhard-Rupp, J. Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 29, 197–205 (2013).
Bustinduy, A. L. et al. Expanding praziquantel (PZQ) access beyond mass drug administration programs: paving a way forward for a pediatric PZQ formulation for schistosomiasis. PLoS Negl. Trop. Dis. 10, e0004946 (2016).
Xiao, S. H., Binggui, S., Chollet, J., Utzinger, J. & Tanner, M. Tegumental alterations in juvenile Schistosoma haematobium harboured in hamsters following artemether treatment. Parasitol. Int. 50, 175–183 (2001).
Saeed, M. E. M., Krishna, S., Greten, H. J., Kremsner, P. G. & Efferth, T. Antischistosomal activity of artemisinin derivatives in vivo and in patients. Pharmacol. Res. 110, 216–226 (2016).
Xiao, S. H. Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemisinins. Acta Trop. 96, 153–167 (2005).
Utzinger, J. et al. Oral artemether for prevention of Schistosoma mansoni infection: randomised controlled trial. Lancet 355, 1320–1325 (2000).
Pérez del Villar, L., Burguillo, F. J., López-Abán, J. & Muro, A. Systematic review and meta-analysis of artemisinin based therapies for the treatment and prevention of schistosomiasis. PLoS ONE 7, e45867 (2012).
Hou, X. Y. et al. A randomized, double-blind, placebo-controlled trial of safety and efficacy of combined praziquantel and artemether treatment for acute schistosomiasis japonica in China. Bull. World Health Organ. 86, 788–795 (2008).
Obonyo, C. O., Muok, E. M. O. & Mwinzi, P. N. Efficacy of artesunate with sulfalene plus pyrimethamine versus praziquantel for treatment of Schistosoma mansoni in Kenyan children: an open-label randomised controlled trial. Lancet Infect. Dis. 10, 603–611 (2010).
Keiser, J. et al. Praziquantel, mefloquine-praziquantel, and mefloquine-artesunate-praziquantel against Schistosoma haematobium: a randomized, exploratory, open-label trial. PLoS Negl. Trop. Dis. 8, e2975 (2014).
Utzinger, J., Tanner, M. & Keiser, J. ACTs for schistosomiasis: do they act? Lancet Infect. Dis. 10, 579–581 (2010).
Carod-Artal, F. J. Neuroschistosomiasis. Expert Rev. Anti. Infect. Ther. 8, 1307–1318 (2010).
Leite, L. A. C. et al. Splenectomy improves hemostatic and liver functions in hepatosplenic schistosomiasis mansoni. PLoS ONE 10, e0135370 (2015).
Richter, J. et al. Severe liver fibrosis caused by Schistosoma mansoni: management and treatment with a transjugular intrahepatic portosystemic shunt. Lancet Infect. Dis. 15, 731–737 (2015).
Bergquist, R., Johansen, M. V. & Utzinger, J. Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol. 25, 151–156 (2009).
Bärenbold, O. et al. Estimating sensitivity of the Kato-Katz technique for the diagnosis of Schistosoma mansoni and hookworm in relation to infection intensity. PLoS Negl. Trop. Dis. 11, e0005953 (2017).
Stothard, J. R. et al. Schistosoma mansoni infections in young children: when are schistosome antigens in urine, eggs in stool and antibodies to eggs first detectable? PLoS Negl. Trop. Dis. 5, e938 (2011).
Chami, G. F. et al. Influence of Schistosoma mansoni and hookworm infection intensities on anaemia in Ugandan villages. PLoS Negl. Trop. Dis. 9, e0004193 (2015).
Olveda, D. U. et al. Bilharzia in the Philippines: past, present, and future. Int. J. Infect. Dis. 18, 52–56 (2014).
World Health Organization. The world health report 1999 – making a difference (WHO, 2013).
Hotez, P. J. & Fenwick, A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl. Trop. Dis. 3, e485 (2009).
World Health Organization. Schistosomiasis: progress report 2001–2011, strategic plan 2012–2020 (WHO, 2013).
Secor, W. E. Early lessons from schistosomiasis mass drug administration programs. F1000Res. 4 (2015).
Mutapi, F., Maizels, R., Fenwick, A. & Woolhouse, M. Human schistosomiasis in the post mass drug administration era. Lancet Infect. Dis. 17, e42–e48 (2017). This paper considers the potential consequences of the current extensive MDA programmes for schistosomiasis, which, although curing infection, could have profound effects in the future on schistosome biology, immunoepidemiology and public health.
Colley, D. G. & Secor, W. E. A. Schistosomiasis research agenda. PLoS Negl. Trop. Dis. 1, e32 (2007).
Riveau, G. et al. Safety and immunogenicity of rSh28GST antigen in humans: phase 1 randomized clinical study of a vaccine candidate against urinary schistosomiasis. PLoS Negl. Trop. Dis. 6, e1704 (2012).
Bergquist, R. & McManus, D. P. in Schistosoma: biology, pathology, and control (ed. Jamieson, B.G.M.) 462–478 (CRC Press,2016).
McManus, D. P. & Loukas, A. Current status of vaccines for schistosomiasis. Clin. Microbiol. Rev. 21, 225–242 (2008).
Tebeje, B. M., Harvie, M., You, H., Loukas, A. & McManus, D. P. Schistosomiasis vaccines: where do we stand? Parasit. Vectors 9, 528 (2016).
Alsallaq, R. A., Gurarie, D., Ndeffo Mbah, M., Galvani, A. & King, C. Quantitative assessment of the impact of partially protective anti-schistosomiasis vaccines. PLoS Negl. Trop. Dis. 11, e0005544 (2017).
Sokolow, S. H. et al. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc. Natl Acad. Sci. USA 112, 9650–9655 (2015).
Marques, D. P. & de, A. et al. Reduced susceptibility of a Biomphalaria tenagophila population to Schistosoma mansoni after introducing the resistant Taim/RS strain of B. tenagophila into Herivelton Martins stream. PLoS ONE 9, e99573 (2014).
Sokolow, S. H. et al. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl. Trop. Dis. 10, e0004794 (2016).
Trainor-Moss, S. & Mutapi, F. Schistosomiasis therapeutics: whats in the pipeline? Expert Rev. Clin. Pharmacol. 9, 157–160 (2015).
Cioli, D., Pica-Mattoccia, L., Basso, A. & Guidi, A. Schistosomiasis control: praziquantel forever? Mol. Biochem. Parasitol. 195, 23–29 (2014).
Schistosoma japonicum Genome Sequencing & Functional Analysis Consortium. et al. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature 460, 345–351 (2009). This paper presents a draft genomic sequence for S. japonicum, along with S. mansoni, the first reported for any flatworm, and provides a unique resource for facilitating the development of new control interventions against schistosomiasis.
Berriman, M. et al. The genome of the blood fluke Schistosoma mansoni. Nature 460, 352–358 (2009). This study describes the sequence and analysis of the S. mansoni genome and identifies targets to accelerate drug discovery, leading to new treatments for the control and elimination of schistosomiasis.
Young, N. D. et al. Whole-genome sequence of Schistosoma haematobium. Nat. Genet. 44, 221–225 (2012). This article describes the genome of S. haematobium, providing new insights for studying parasite development, host–parasite interactions and schistosome-associated bladder cancer, and offers a resource for the design and development of new anti-schistosomal drugs, vaccines and diagnostic tools.
Cowan, N. & Keiser, J. Repurposing of anticancer drugs: in vitro and in vivo activities against Schistosoma mansoni. Parasit. Vectors 8, 417 (2015).
Gouveia, M., Brindley, P., Gärtner, F., Costa, J. & Vale, N. Drug repurposing for schistosomiasis: combinations of drugs or biomolecules. Pharmaceuticals 11, 15 (2018).
Mansour, N. R. et al. High throughput screening identifies novel lead compounds with activity against larval, juvenile and adult Schistosoma mansoni. PLoS Negl. Trop. Dis. 10, e0004659 (2016).
Mafud, A. C., Ferreira, L. G., Mascarenhas, Y. P., Andricopulo, A. D. & de Moraes, J. Discovery of novel antischistosomal agents by molecular modeling approaches. Trends Parasitol. 32, 874–886 (2016).
Lee, E. F., Young, N. D., Lim, N. T. Y., Gasser, R. B. & Fairlie, W. D. Apoptosis in schistosomes: toward novel targets for the treatment of schistosomiasis. Trends Parasitol. 30, 75–84 (2014).
Lee, E. F. & Fairlie, W. D. Repurposing apoptosis-inducing cancer drugs to treat schistosomiasis. Future Med. Chem. 7, 707–711 (2015).
Cabezas-Cruz, A., Lancelot, J., Caby, S., Oliveira, G. & Pierce, R. J. Epigenetic control of gene function in schistosomes: a source of therapeutic targets? Front. Genet. 5, 317 (2014).
Hess, J., Keiser, J. & Gasser, G. Toward organometallic antischistosomal drug candidates. Future Med. Chem. 7, 821–830 (2015).
Wangchuk, P., Giacomin, P. R., Pearson, M. S., Smout, M. J. & Loukas, A. Identification of lead chemotherapeutic agents from medicinal plants against blood flukes and whipworms. Sci. Rep. 6, 32101 (2016).
Julé, A. M., Vaillant, M., Lang, T. A., Guérin, P. J. & Olliaro, P. L. The schistosomiasis clinical trials landscape: a systematic review of antischistosomal treatment efficacy studies and a case for sharing individual participant-level data (IPD). PLoS Negl. Trop. Dis. 10, e0004784 (2016).
Aagaard-Hansen, J., Nombela, N. & Alvar, J. Population movement: a key factor in the epidemiology of neglected tropical diseases. Trop. Med. Int. Health 15, 1281–1288 (2010).
Gnanasekar, M., Salunkhe, A. M., Mallia, A. K., He, Y. X. & Kalyanasundaram, R. Praziquantel affects the regulatory myosin light chain of Schistosoma mansoni. Antimicrob. Agents Chemother. 53, 1054–1060 (2008).
Angelucci, F. et al. The anti-schistosomal drug praziquantel is an adenosine antagonist. Parasitology 134, 1215 (2007).
Thomas, C. M., Fitzsimmons, C. M., Dunne, D. W. & Timson, D. J. Comparative biochemical analysis of three members of the Schistosoma mansoni TAL family: differences in ion and drug binding properties. Biochimie 108, 40–47 (2015).
Taylor, A. B. et al. Structural and functional characterization of the enantiomers of the antischistosomal drug oxamniquine. PLoS Negl. Trop. Dis. 9, e0004132 (2015).
Rojo-Arreola, L. et al. Chemical and genetic validation of the statin drug target to treat the helminth disease, schistosomiasis. PLoS ONE 9, e87594 (2014).
Buro, C. et al. Imatinib treatment causes substantial transcriptional changes in adult Schistosoma mansoni in vitro exhibiting pleiotropic effects. PLoS Negl. Trop. Dis. 8, e2923 (2014).
Anderson, L. et al. Histone deacetylase inhibition modulates histone acetylation at gene promoter regions and affects genome-wide gene transcription in Schistosoma mansoni. PLoS Negl. Trop. Dis. 11, e0005539 (2017).
Lancelot, J. et al. Schistosome sirtuins as drug targets. Future Med. Chem. 7, 765–782 (2015).
Ishida, K. & Jolly, E. R. Hsp70 may be a molecular regulator of schistosome host invasion. PLoS Negl. Trop. Dis. 10, e0004986 (2016).
Johann, L. et al. Synthesis and evaluation of 1,4-naphthoquinone ether derivatives asSmTGR inhibitors and new anti-schistosomal drugs. FEBS J. 282, 3199–3217 (2015).
Fajtová, P. et al. Prolyl oligopeptidase from the blood fluke Schistosoma mansoni: from functional analysis to anti-schistosomal inhibitors. PLoS Negl. Trop. Dis. 9, e0003827 (2015).
Cabezas-Cruz, A., Valdés, J. J., Lancelot, J. & Pierce, R. J. Fast evolutionary rates associated with functional loss in class I glucose transporters of Schistosoma mansoni. BMC Genomics 16, 980 (2015).
Chan, J. D. et al. A miniaturized screen of a Schistosoma mansoni serotonergic G protein-coupled receptor identifies novel classes of parasite-selective inhibitors. PLoS Pathog. 12, e1005651 (2016).
Bais, S. & Greenberg, R. M. TRP channels in schistosomes. Int. J. Parasitol. Drugs Drug Resist. 6, 335–342 (2016).
Sundaraneedi, M. K. et al. Polypyridylruthenium(II) complexes exert anti-schistosome activity and inhibit parasite acetylcholinesterases. PLoS Negl. Trop. Dis. 11, e0006134 (2017).
Chuah, C., Jones, M. K., Burke, M. L., McManus, D. P. & Gobert, G. N. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 30, 141–150 (2014).
Harder, A. & Mehlhorn, H. in Treatment of Human Parasitosis in Traditional Chinese Medicine. (eds Mehlhorn, H., Wu, Z. & Ye, B.) 79–115 (Springer, 2014).
Reviewer information
Nature Reviews Disease Primers thanks P. M. Z. Coelho, J. Friedman, N. Midzi, E. Secor and the other anonymous referee(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (D.P.M. and J.U.); Epidemiology (X.-N.Z. and J.U.); Mechanisms/pathophysiology (B.J.V., D.W.D. and M.S.); Diagnosis, screening and prevention (B.J.V., M.S., D.W.D. and D.P.M.); Management (D.P.M.); Quality of life (J.U.); Outlook (D.P.M. and B.J.V.); Overview of Primer (D.P.M.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McManus, D.P., Dunne, D.W., Sacko, M. et al. Schistosomiasis. Nat Rev Dis Primers 4, 13 (2018). https://doi.org/10.1038/s41572-018-0013-8
Published:
DOI: https://doi.org/10.1038/s41572-018-0013-8
This article is cited by
-
Prevalence of diverse colorectal polyps and risk factors for colorectal carcinoma in situ and neoplastic polyps
Journal of Translational Medicine (2024)
-
Circulating cell-free DNA as a biomarker for diagnosis of Schistosomiasis japonica
Parasites & Vectors (2024)
-
Advances in the study of the interaction between schistosome infections and the host's intestinal microorganisms
Parasites & Vectors (2024)
-
Subtelomeric plasticity contributes to gene family expansion in the human parasitic flatworm Schistosoma mansoni
BMC Genomics (2024)
-
Yolk proteins of the schistosomiasis vector snail Biomphalaria glabrata revealed by multi-omics analysis
Scientific Reports (2024)