Abstract
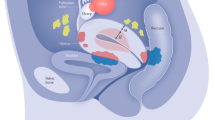
Endometriosis is a common inflammatory disease characterized by the presence of tissue outside the uterus that resembles endometrium, mainly on pelvic organs and tissues. It affects ~5–10% of women in their reproductive years — translating to 176 million women worldwide — and is associated with pelvic pain and infertility. Diagnosis is reliably established only through surgical visualization with histological verification, although ovarian endometrioma and deep nodular forms of disease can be detected through ultrasonography and MRI. Retrograde menstruation is regarded as an important origin of the endometrial deposits, but other factors are involved, including a favourable endocrine and metabolic environment, epithelial–mesenchymal transition and altered immunity and inflammatory responses in genetically susceptible women. Current treatments are dictated by the primary indication (infertility or pelvic pain) and are limited to surgery and hormonal treatments and analgesics with many adverse effects that rarely provide long-term relief. Endometriosis substantially affects the quality of life of women and their families and imposes costs on society similar to those of other chronic conditions such as type 2 diabetes mellitus, Crohn’s disease and rheumatoid arthritis. Future research must focus on understanding the pathogenesis, identifying disease subtypes, developing non-invasive diagnostic methods and targeting non-hormonal treatments that are acceptable to women who wish to conceive.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Marsh, E. E. & Laufer, M. R. Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly. Fertil. Steril. 83, 758–760 (2005).
Halme, J., Hammond, M. G., Hulka, J. F., Raj, S. G. & Talbert, L. M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 64, 151–154 (1984).
Horton, J. D., Dezee, K. J., Ahnfeldt, E. P. & Wagner, M. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am. J. Surg. 196, 207–212 (2008).
Goldberg, J. & Davis, A. Extrapelvic endometriosis. Semin. Reprod. Med. 35, 98–101 (2016).
Hirsch, M. et al. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. BJOG 125, 556–564 (2018).
American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 67, 817–821 (1997).
Vercellini, P. et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum. Reprod. 22, 266–271 (2006).
Nnoaham, K. E. et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil. Steril. 96, 366–373.e8 (2011). This paper presents the largest multicentre prospective study to date describing the effects of endometriosis; in women undergoing their first laparoscopy, a significantly reduced physical (but not mental) HRQOL, which was associated with delay in diagnosis, was reported in those with endometriosis compared with endometriosis-free symptomatic and asymptomatic women.
Peres, L. C. et al. Racial/ethnic differences in the epidemiology of ovarian cancer: a pooled analysis of 12 case-control studies. Int. J. Epidemiol. 47, 460–472 (2018).
Eskenazi, B. & Warner, M. L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. North Am. 24, 235–258 (1997).
Buck Louis, G. M. et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil. Steril. 96, 360–365 (2011).
Janssen, E. B., Rijkers, A. C. M., Hoppenbrouwers, K., Meuleman, C. & D’Hooghe, T. M. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum. Reprod. Update 19, 570–582 (2013).
Zondervan, K. T., Cardon, L. R. & Kennedy, S. H. What makes a good case–control study? Hum. Reprod. 17, 1415–1423 (2002).
Adamson, G. D., Kennedy, S. & Hummelshoj, L. Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. J. Endometr. Pelvic Pain Disord. 2, 3–6 (2010).
Gemmell, L. C. et al. The management of menopause in women with a history of endometriosis: a systematic review. Hum. Reprod. Update 23, 481–500 (2017).
Leibson, C. L. et al. Incidence and characterization of diagnosed endometriosis in a geographically defined population. Fertil. Steril. 82, 314–321 (2004).
Missmer, S. A. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am. J. Epidemiol. 160, 784–796 (2004).
Nnoaham, K. E., Webster, P., Kumbang, J., Kennedy, S. H. & Zondervan, K. T. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil. Steril. 98, 702–712.e6 (2012).
Missmer, S. A. et al. Reproductive history and endometriosis among premenopausal women. Obstet. Gynecol. 104, 965–974 (2004).
Peterson, C. M. et al. Risk factors associated with endometriosis: importance of study population for characterizing disease in the ENDO Study. Am. J. Obstet. Gynecol. 208, 451.e1–451.e11 (2013).
Prescott, J. et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum. Reprod. 31, 1475–1482 (2016).
Buck Louis, G. M. et al. Women’s reproductive history before the diagnosis of incident endometriosis. J. Womens Health 25, 1021–1029 (2016).
Farland, L. V. et al. History of breast feeding and risk of incident endometriosis: prospective cohort study. BMJ 358, j3778 (2017).
Leeners, B., Damaso, F., Ochsenbein-Kölble, N. & Farquhar, C. The effect of pregnancy on endometriosis — facts or fiction? Hum. Reprod. Update 24, 290–299 (2018).
Shah, D. K., Correia, K. F., Vitonis, A. F. & Missmer, S. A. Body size and endometriosis: results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum. Reprod. 28, 1783–1792 (2013).
Farland, L. V. et al. Associations among body size across the life course, adult height and endometriosis. Hum. Reprod. 32, 1732–1742 (2017).
McCann, S. E. et al. Endometriosis and body fat distribution. Obstet. Gynecol. 82, 545–549 (1993).
Rahmioglu, N. et al. Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum. Mol. Genet. 24, 1185–1199 (2015).
de Ridder, C. M. et al. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J. Clin. Endocrinol. Metab. 70, 888–893 (1990).
Cramer, D. W. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 255, 1904–1908 (1986).
Baron, J. A., La Vecchia, C. & Levi, F. The antiestrogenic effect of cigarette smoking in women. Am. J. Obstet. Gynecol. 162, 502–514 (1990).
Ohtake, F., Fujii-Kuriyama, Y., Kawajiri, K. & Kato, S. Cross-talk of dioxin and estrogen receptor signals through the ubiquitin system. J. Steroid Biochem. Mol. Biol. 127, 102–107 (2011).
Parazzini, F. Selected food intake and risk of endometriosis. Hum. Reprod. 19, 1755–1759 (2004).
Trabert, B., Peters, U., De Roos, A. J., Scholes, D. & Holt, V. L. Diet and risk of endometriosis in a population-based case–control study. Br. J. Nutr. 105, 459–467 (2010).
Missmer, S. A. et al. A prospective study of dietary fat consumption and endometriosis risk. Hum. Reprod. 25, 1528–1535 (2010).
Savaris, A. L. & do Amaral, V. F. Nutrient intake, anthropometric data and correlations with the systemic antioxidant capacity of women with pelvic endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 158, 314–318 (2011).
Mozaffarian, D. et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 79, 606–612 (2004).
Lebovic, D. I., Mueller, M. D. & Taylor, R. N. Immunobiology of endometriosis. Fertil. Steril. 75, 1–10 (2001).
Rier, S. E. et al. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 21, 433–441 (1993).
Smarr, M. M., Kannan, K. & Buck Louis, G. M. Endocrine disrupting chemicals and endometriosis. Fertil. Steril. 106, 959–966 (2016).
Kvaskoff, M. et al. Endometriosis: a high-risk population for major chronic diseases? Hum. Reprod. Update 21, 500–516 (2015). This article presents a meta-analysis of all data and a critical methodologic review suggesting that patients with endometriosis are at higher risk of ovarian and breast cancers, cutaneous melanoma, asthma and some autoimmune, cardiovascular and atopic diseases and are at decreased risk of cervical cancer.
Benagiano, G., Brosens, I. & Habiba, M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum. Reprod. Update 20, 386–402 (2014).
Kunz, G. et al. Adenomyosis in endometriosis — prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum. Reprod. 20, 2309–2316 (2005).
Pearce, C. L. et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case–control studies. Lancet Oncol. 13, 385–394 (2012).
Kim, H. S., Kim, T. H., Chung, H. H. & Song, Y. S. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br. J. Cancer 110, 1878–1890 (2014).
Farland, L. V. et al. Endometriosis and the risk of skin cancer: a prospective cohort study. Cancer Causes Control 28, 1011–1019 (2017).
Sinaii, N. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum. Reprod. 17, 2715–2724 (2002).
Nielsen, N. M., Jorgensen, K. T., Pedersen, B. V., Rostgaard, K. & Frisch, M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum. Reprod. 26, 1555–1559 (2011).
Harris, H. R. et al. Endometriosis and the risks of systemic lupus erythematosus and rheumatoid arthritis in the Nurses’ Health Study II. Ann. Rheum. Dis. 75, 1279–1284 (2015).
Mu, F. et al. Association between endometriosis and hypercholesterolemia or hypertensionnovelty and significance. Hypertension 70, 59–65 (2017).
Nyholt, D. R. et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat. Genet. 44, 1355–1359 (2012).
Borghese, B., Zondervan, K. T., Abrao, M. S., Chapron, C. & Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 91, 254–264 (2017).
Treloar, S. A. et al. Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am. J. Hum. Genet. 77, 365–376 (2005).
Zondervan, K. T. et al. Significant evidence of one or more susceptibility loci for endometriosis with near-Mendelian inheritance on chromosome 7p13–15. Hum. Reprod. 22, 717–728 (2006).
Rahmioglu, N., Montgomery, G. W. & Zondervan, K. T. Genetics of endometriosis. Womens Health 11, 577–586 (2015).
Sapkota, Y. et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat. Commun. 8, 15539 (2017).
Lee, S. H. et al. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer’s disease, multiple sclerosis and endometriosis. Hum. Mol. Genet. 22, 832–841 (2012).
Uimari, O. et al. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum. Reprod. 32, 780–793 (2017).
Lu, Y. et al. Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum. Mol. Genet. 24, 5955–5964 (2015).
Painter, J. N. et al. Genetic overlap between endometriosis and endometrial cancer: evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 7, 1978–1987 (2018).
Sapkota, Y. et al. Analysis of potential protein-modifying variants in 9000 endometriosis patients and 150000 controls of European ancestry. Sci. Rep. 7, 11380 (2017).
Visscher, P. M., Brown, M. A., McCarthy, M. I. & Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 90, 7–24 (2012).
Fung, J. N. et al. The genetic regulation of transcription in human endometrial tissue. Hum. Reprod. 32, 1–12 (2017).
Becker, C. M. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil. Steril. 102, 1213–1222 (2014).
Vitonis, A. F. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil. Steril. 102, 1223–1232 (2014).
Rahmioglu, N. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil. Steril. 102, 1233–1243 (2014).
Fassbender, A. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil. Steril. 102, 1244–1253 (2014).
Wiegand, K. C. et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 363, 1532–1543 (2010).
Yamamoto, S., Tsuda, H., Takano, M., Tamai, S. & Matsubara, O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod. Pathol. 25, 615–624 (2011).
Anglesio, M. S. et al. Cancer-associated mutations in endometriosis without cancer. N. Engl. J. Med. 376, 1835–1848 (2017).
Kato, S., Lippman, S. M., Flaherty, K. T. & Kurzrock, R. The conundrum of genetic ‘drivers’ in benign conditions. J. Natl Cancer Inst. 108, djw036 (2016).
Guo, S.-W. Epigenetics of endometriosis. Mol. Hum. Reprod. 15, 587–607 (2009).
Wu, Y., Starzinski-Powitz, A. & Guo, S.-W. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod. Sci. 14, 374–382 (2007).
Dyson, M. T. et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 10, e1004158 (2014).
Burney, R. O. et al. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 15, 625–631 (2009).
Saare, M. et al. Challenges in endometriosis miRNA studies — from tissue heterogeneity to disease specific miRNAs. Biochim. Biophys. Acta 1863, 2282–2292 (2017).
Sampson, J. A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 14, 422–469 (1927).
Vercellini, P. et al. Asymmetry in distribution of diaphragmatic endometriotic lesions: evidence in favour of the menstrual reflux theory. Hum. Reprod. 22, 2359–2367 (2007).
D’Hooghe, T. M., Bambra, C. S., Raeymaekers, B. M. & Koninckx, P. R. Increased prevalence and recurrence of retrograde menstruation in baboons with spontaneous endometriosis. Hum. Reprod. 11, 2022–2025 (1996).
Witz, C. A., Cho, S., Centonze, V. E., Montoya-Rodriguez, I. A. & Schenken, R. S. Time series analysis of transmesothelial invasion by endometrial stromal and epithelial cells using three-dimensional confocal microscopy. Fertil. Steril. 79(Suppl. 1), 770–778 (2003).
Reis, F. M., Petraglia, F. & Taylor, R. N. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 19, 406–418 (2013).
Sanchez, A. M. et al. The endometriotic tissue lining the internal surface of endometrioma: hormonal, genetic, epigenetic status, and gene expression profile. Reprod. Sci. 22, 391–401 (2015).
Borghese, B., Zondervan, K. T., Abrao, M. S., Chapron, C. & Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 91, 254–264 (2016).
Meyer, R. Zur Frage der heterotopen Epithelwucherung, insbesondere des Peritonealepithels und in die Ovarien [German]. Virch Arch. Path. Anat. Phys. 250, 595–610 (1924).
Ferguson, B. R., Bennington, J. L. & Haber, S. L. Histochemistry of mucosubstances and histology of mixed müllerian pelvic lymph node glandular inclusions. Evidence for histogenesis by müllerian metaplasia of coelomic epithelium. Obstet. Gynecol. 33, 617–625 (1969).
Figueira, P. G. M., Abrão, M. S., Krikun, G. & Taylor, H. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. NY Acad. Sci. 1221, 10–17 (2011).
Du, H. & Taylor, H. S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 25, 2082–2086 (2007).
Gargett, C. E. & Masuda, H. Adult stem cells in the endometrium. Mol. Hum. Reprod. 16, 818–834 (2010).
Matsuzaki, S. & Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 27, 712–721 (2012).
Somigliana, E. et al. Association rate between deep peritoneal endometriosis and other forms of the disease: pathogenetic implications. Hum. Reprod. 19, 168–171 (2004).
Zheng, W. et al. Initial endometriosis showing direct morphologic evidence of metaplasia in the pathogenesis of ovarian endometriosis. Int. J. Gynecol. Pathol. 24, 164–172 (2005).
Troncon, J. K. et al. Endometriosis in a patient with mayer-rokitansky-küster-hauser syndrome. Case Rep. Obstet. Gynecol. 2014, 376231 (2014).
Taguchi, S., Enomoto, Y. & Homma, Y. Bladder endometriosis developed after long-term estrogen therapy for prostate cancer. Int. J. Urol. 19, 964–965 (2012).
Halban, J. Hysteroadenosis metastatica. Zentralbl. Gyndkoi 7, 387–391 (1925).
Mechsner, S. et al. Estrogen and progestogen receptor positive endometriotic lesions and disseminated cells in pelvic sentinel lymph nodes of patients with deep infiltrating rectovaginal endometriosis: a pilot study. Hum. Reprod. 23, 2202–2209 (2008).
Gargett, C. E. et al. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 20, 591–598 (2014).
Witz, C. A. et al. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil. Steril. 75, 385–390 (2001).
Zeitoun, K. M. & Bulun, S. E. Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil. Steril. 72, 961–969 (1999).
Pellegrini, C. et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil. Steril. 98, 1200–1208 (2012).
Plante, B. J. et al. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod. Sci. 19, 684–693 (2012).
Han, S. J. et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 163, 960–974 (2015).
Vercellini, P., Viganò, P., Somigliana, E. & Fedele, L. Endometriosis: pathogenesis and treatment. Nat. Rev. Endocrinol. 10, 261–275 (2013). For this review, the best-quality evidence was selected to describe the performance of diagnostic tools and the effectiveness of approaches to address endometriosis-associated symptoms and infertility.
Patel, B. et al. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 21, 155–173 (2014).
Al-Sabbagh, M., Lam, E. W.-F. & Brosens, J. J. Mechanisms of endometrial progesterone resistance. Mol. Cell. Endocrinol. 358, 208–215 (2012).
La Marca, A., Carducci Artenisio, A., Stabile, G., Rivasi, F. & Volpe, A. Evidence for cycle-dependent expression of follicle-stimulating hormone receptor in human endometrium. Gynecol. Endocrinol. 21, 303–306 (2005).
Zondervan, K. et al. Beyond endometriosis genome-wide association study: from genomics to phenomics to the patient. Semin. Reprod. Med. 34, 242–254 (2016).
Jiang, Y., Chen, L., Taylor, R. N., Li, C. & Zhou, X. Physiological and pathological implications of retinoid action in the endometrium. J. Endocrinol. 236, R169–R188 (2018).
Orvis, G. D. & Behringer, R. R. Cellular mechanisms of Müllerian duct formation in the mouse. Dev. Biol. 306, 493–504 (2007).
Vigano, P. et al. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 33, 347–352 (2018).
Yu, J. et al. Endometrial stromal decidualization responds reversibly to hormone stimulation and withdrawal. Endocrinology 157, 2432–2446 (2016).
Yin, X., Pavone, M. E., Lu, Z., Wei, J. & Kim, J. J. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J. Clin. Endocrinol. Metab. 97, E35–E43 (2012).
Du, Y., Liu, X. & Guo, S.-W. Platelets impair natural killer cell reactivity and function in endometriosis through multiple mechanisms. Hum. Reprod. 32, 1–17 (2017).
Lessey, B., Lebovic, D. & Taylor, R. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin. Reprod. Med. 31, 109–124 (2013).
Cominelli, A. et al. Matrix metalloproteinase-27 is expressed in CD163+/CD206+ M2 macrophages in the cycling human endometrium and in superficial endometriotic lesions. Mol. Hum. Reprod. 20, 767–775 (2014).
Takebayashi, A. et al. Subpopulations of macrophages within eutopic endometrium of endometriosis patients. Am. J. Reprod. Immunol. 73, 221–231 (2015).
Nisenblat, V. et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 5, CD012179 (2016).
Wang, X.-Q. et al. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J. Mol. Endocrinol. 45, 291–299 (2010).
McKinnon, B. D., Kocbek, V., Nirgianakis, K., Bersinger, N. A. & Mueller, M. D. Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Hum. Reprod. Update 22, 382–403 (2016).
Morotti, M., Vincent, K. & Becker, C. M. Mechanisms of pain in endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 209, 8–13 (2017).
Berkley, K. J. The pains of endometriosis. Science 308, 1587–1589 (2005).
Taylor, R. N. & Lebovic, D. I. in Yen and Jaffe’s Reproductive Endocrinology (eds Strauss, J. F. & Barbieri, R. L.) 565–585 (Saunders Elsevier, 2014). This book chapter discusses the diagnosis and management of endometriosis, with a particularly well-referenced review of theories of aetiology and pathogenesis
Gnecco, J. S. et al. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann. Biomed. Eng. 45, 1758–1769 (2017).
Sanchez, A. M. et al. The endometriotic tissue lining the internal surface of endometrioma. Reprod. Sci. 22, 391–401 (2014).
Ryan, I. P., Schriock, E. D. & Taylor, R. N. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J. Clin. Endocrinol. Metab. 78, 642–649 (1994).
Greaves, E., Critchley, H. O. D., Horne, A. W. & Saunders, P. T. K. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstet. Gynecol. Scand. 96, 644–658 (2017).
Korch, C. et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol. Oncol. 127, 241–248 (2012).
Grümmer, R. Translational animal models to study endometriosis-associated infertility. Semin. Reprod. Med. 31, 125–132 (2013). Given the ethical limitations of clinical studies, this is a comprehensive review of the use of animal models to investigate factors contributing to the fertility-compromising effects of endometriosis.
Bruner-Tran, K. L., McConaha, M. E. & Osteen, K. G. in Endometriosis. Science and Practice (eds Giudice, L. C., Evers, J. L. H. & Healy, D. L.) 270–283 (Wiley-Blackwell, 2012).
Mariani, M. et al. The selective vitamin D receptor agonist, elocalcitol, reduces endometriosis development in a mouse model by inhibiting peritoneal inflammation. Hum. Reprod. 27, 2010–2019 (2012).
Fazleabas, A. in Endometriosis: Science and Practice (eds Giudice, L. C., Evers, J. L. & Healy, D. L.) 285–291 (Wiley-Blackwell, 2012).
Ngô, C. et al. Antiproliferative effects of anastrozole, methotrexate, and 5-fluorouracil on endometriosis in vitro and in vivo. Fertil. Steril. 94, 1632–1638.e1 (2010).
Bruner-Tran, K. L., Osteen, K. G. & Duleba, A. J. Simvastatin protects against the development of endometriosis in a nude mouse model. J. Clin. Endocrinol. Metab. 94, 2489–2494 (2009).
Pullen, N. et al. The translational challenge in the development of new and effective therapies for endometriosis: a review of confidence from published preclinical efficacy studies. Hum. Reprod. Update 17, 791–802 (2011).
Greaves, E. et al. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am. J. Pathol. 184, 1930–1939 (2014).
Stilley, J. A. W., Woods-Marshall, R., Sutovsky, M., Sutovsky, P. & Sharpe-Timms, K. L. Reduced fecundity in female rats with surgically induced endometriosis and in their daughters: a potential role for tissue inhibitors of metalloproteinase 11. Biol. Reprod. 80, 649–656 (2009).
Zondervan, K. T. Familial aggregation of endometriosis in a large pedigree of rhesus macaques. Hum. Reprod. 19, 448–455 (2004).
Lebovic, D. I. et al. Peroxisome proliferator-activated receptor-γ receptor ligand partially prevents the development of endometrial explants in baboons: a prospective, randomized, placebo-controlled study. Endocrinology 151, 1846–1852 (2010).
D’Hooghe, T. M. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil. Steril. 68, 613–625 (1997).
Fazleabas, A. Progesterone resistance in a baboon model of endometriosis. Semin. Reprod. Med. 28, 75–80 (2010).
D’Hooghe, T. M. et al. Nonhuman primate models for translational research in endometriosis. Reprod. Sci. 16, 152–161 (2009).
Chapron, C. et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum. Reprod. 13, 867–872 (1998).
Chung, M. K., Chung, R. R., Gordon, D. & Jennings, C. The evil twins of chronic pelvic pain syndrome: endometriosis and interstitial cystitis. JSLS 6, 311–314 (2002).
Redwine, D. B. Diaphragmatic endometriosis: diagnosis, surgical management, and long-term results of treatment. Fertil. Steril. 77, 288–296 (2002).
Fedele, L. et al. Ileocecal endometriosis: clinical and pathogenetic implications of an underdiagnosed condition. Fertil. Steril. 101, 750–753 (2014).
DiVasta, A. D., Vitonis, A. F., Laufer, M. R. & Missmer, S. A. Spectrum of symptoms in women diagnosed with endometriosis during adolescence versus adulthood. Am. J. Obstet. Gynecol. 218, 324.e1–324.e11 (2018).
Ballard, K., Lane, H., Hudelist, G., Banerjee, S. & Wright, J. Can specific pain symptoms help in the diagnosis of endometriosis? A cohort study of women with chronic pelvic pain. Fertil. Steril. 94, 20–27 (2010).
Becker, C. M., Gattrell, W. T., Gude, K. & Singh, S. S. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil. Steril. 108, 125–136 (2017).
Brawn, J., Morotti, M., Zondervan, K. T., Becker, C. M. & Vincent, K. Central changes associated with chronic pelvic pain and endometriosis. Hum. Reprod. Update 20, 737–747 (2014).
Morotti, M., Vincent, K., Brawn, J., Zondervan, K. T. & Becker, C. M. Peripheral changes in endometriosis-associated pain. Hum. Reprod. Update 20, 717–736 (2014).
Brosens, I., Gordts, S. & Benagiano, G. Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion. Hum. Reprod. 28, 2026–2031 (2013).
Laufer, M. R., Sanfilippo, J. & Rose, G. Adolescent endometriosis: diagnosis and treatment approaches. J. Pediatr. Adolesc. Gynecol. 16, S3–S11 (2003).
Davis, G. D., Thillet, E. & Lindemann, J. Clinical characteristics of adolescent endometriosis. J. Adolesc. Health 14, 362–368 (1993).
Saraswat, L. et al. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG 124, 444–452 (2017).
Sanchez, A. M. et al. Is the oocyte quality affected by endometriosis? A review of the literature. J. Ovarian Res. 10, 43 (2017).
Simón, C. et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum. Reprod. 9, 725–729 (1994).
Senapati, S., Sammel, M. D., Morse, C. & Barnhart, K. T. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertil. Steril. 106, 164–171.e1 (2016).
Taniguchi, F. et al. Analysis of pregnancy outcome and decline of anti-Müllerian hormone after laparoscopic cystectomy for ovarian endometriomas. J. Obstet. Gynaecol. Res. 42, 1534–1540 (2016).
Lessey, B. A. & Kim, J. J. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil. Steril. 108, 19–27 (2017).
Miravet-Valenciano, J., Ruiz-Alonso, M., Gómez, E. & Garcia-Velasco, J. A. Endometrial receptivity in eutopic endometrium in patients with endometriosis: it is not affected, and let me show you why. Fertil. Steril. 108, 28–31 (2017).
Díaz, I. et al. Impact of stage iii–iv endometriosis on recipients of sibling oocytes: matched case-control study. Fertil. Steril. 74, 31–34 (2000).
Prapas, Y. et al. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod. Biomed. Online 25, 543–548 (2012).
Burney, R. O. et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148, 3814–3826 (2007).
Dunselman, G. A. J. et al. ESHRE guideline: management of women with endometriosis. Hum. Reprod. 29, 400–412 (2014). This guideline discusses the management of general endometriosis.
Weijenborg, P. T. M., ter Kuile, M. M. & Jansen, F. W. Intraobserver and interobserver reliability of videotaped laparoscopy evaluations for endometriosis and adhesions. Fertil. Steril. 87, 373–380 (2007).
Tuttlies, F. et al. ENZIAN-score, a classification of deep infiltrating endometriosis [German]. Zentralbl. Gynakol. 127, 275–281 (2005).
Johnson, N. P. et al. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 32, 315–324 (2017).
Nisolle, M. & Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 68, 585–596 (1997).
Fukuda, S. et al. Thoracic endometriosis syndrome: comparison between catamenial pneumothorax or endometriosis-related pneumothorax and catamenial hemoptysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 225, 118–123 (2018).
Rousset-Jablonski, C. et al. Catamenial pneumothorax and endometriosis-related pneumothorax: clinical features and risk factors. Hum. Reprod. 26, 2322–2329 (2011).
Redwine, D. B. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil. Steril. 72, 310–315 (1999).
Hudelist, G. et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 37, 257–263 (2011).
Holland, T. K. et al. Ultrasound mapping of pelvic endometriosis: does the location and number of lesions affect the diagnostic accuracy? A multicentre diagnostic accuracy study. BMC Womens Health 13, 43 (2013).
Noventa, M. et al. Ultrasound techniques in the diagnosis of deep pelvic endometriosis: algorithm based on a systematic review and meta-analysis. Fertil. Steril. 104, 366–383.e2 (2015).
Bazot, M. et al. European Society of Urogenital Radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur. Radiol. 27, 2765–2775 (2016).
Exacoustos, C., Manganaro, L. & Zupi, E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 28, 655–681 (2014).
Stratton, P. Diagnostic accuracy of laparoscopy, magnetic resonance imaging, and histopathologic examination for the detection of endometriosis. Fertil. Steril. 79, 1078–1085 (2003).
Kennedy, S. et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 20, 2698–2704 (2005).
Wykes, C. B., Clark, T. J. & Khan, K. S. REVIEW: Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG 111, 1204–1212 (2004).
Balasch, J. et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum. Reprod. 11, 387–391 (1996).
Lessey, B. A., Higdon, H. L., Miller, S. E. & Price, T. A. Intraoperative detection of subtle endometriosis: a novel paradigm for detection and treatment of pelvic pain associated with the loss of peritoneal integrity. J. Vis. Exp. https://doi.org/10.3791/4313 (2012).
Lue, J. R., Pyrzak, A. & Allen, J. Improving accuracy of intraoperative diagnosis of endometriosis: role of firefly in minimal access robotic surgery. J. Minim. Access Surg. 12, 186–189 (2016).
Practice Committee of American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil. Steril. 90, S260–S269 (2008). This article is a guideline for the management of pelvic pain associated with endometriosis.
Duffy, J. M. N. et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 4, CD011031 (2014).
Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil. Steril. 98, 591–598 (2012). This paper is a guideline for the management of infertility associated with endometriosis.
Tanbo, T. & Fedorcsak, P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 96, 659–667 (2017).
Osuga, Y. et al. Role of laparoscopy in the treatment of endometriosis-associated infertility. Gynecol. Obstet. Invest. 53, 33–39 (2002).
Hart, R. J., Hickey, M., Maouris, P., Buckett, W. & Garry, R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004992.pub2 (2005).
Benaglia, L. et al. Rate of severe ovarian damage following surgery for endometriomas. Hum. Reprod. 25, 678–682 (2010).
Vercellini, P. et al. Effect of patient selection on estimate of reproductive success after surgery for rectovaginal endometriosis: literature review. Reprod. Biomed. Online 24, 389–395 (2012).
Nyangoh Timoh, K., Ballester, M., Bendifallah, S., Fauconnier, A. & Darai, E. Fertility outcomes after laparoscopic partial bladder resection for deep endometriosis: retrospective analysis from two expert centres and review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 220, 12–17 (2018).
Adamson, G. D. & Pasta, D. J. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil. Steril. 94, 1609–1615 (2010).
Werbrouck, E., Spiessens, C., Meuleman, C. & D’Hooghe, T. No difference in cycle pregnancy rate and in cumulative live-birth rate between women with surgically treated minimal to mild endometriosis and women with unexplained infertility after controlled ovarian hyperstimulation and intrauterine insemination. Fertil. Steril. 86, 566–571 (2006).
National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. NICE https://www.nice.org.uk/guidance/cg156/chapter/Recommendations#intrauterine-insemination (2013).
de Ziegler, D., Borghese, B. & Chapron, C. Endometriosis and infertility: pathophysiology and management. Lancet 376, 730–738 (2010).
Steures, P. et al. Prediction of an ongoing pregnancy after intrauterine insemination. Fertil. Steril. 82, 45–51 (2004).
Reindollar, R. H. et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil. Steril. 94, 888–899 (2010).
Eijkemans, M. J. C. et al. Cost-effectiveness of ‘immediate IVF’ versus ‘delayed IVF’: a prospective study. Hum. Reprod. 32, 999–1008 (2017).
Hamdan, M., Omar, S. Z., Dunselman, G. & Cheong, Y. Influence of endometriosis on assisted reproductive technology outcomes. Obstet. Gynecol. 125, 79–88 (2015).
Benschop, L., Farquhar, C., van der Poel, N. & Heineman, M. J. Interventions for women with endometrioma prior to assisted reproductive technology. Cochrane Database Syst. Rev. 11, CD008571 (2010).
de Ziegler, D. et al. Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcomes. Fertil. Steril. 94, 2796–2799 (2010).
Donnez, J., García-Solares, J. & Dolmans, M.-M. Fertility preservation in women with ovarian endometriosis. Minerva Ginecol. https://doi.org/10.23736/S0026-4784.18.04229-6 (2018).
Somigliana, E. et al. Surgical excision of endometriomas and ovarian reserve: a systematic review on serum antimüllerian hormone level modifications. Fertil. Steril. 98, 1531–1538 (2012).
Bianchi, P. H. M. et al. Extensive excision of deep infiltrative endometriosis before in vitro fertilization significantly improves pregnancy rates. J. Minim. Invasive Gynecol. 16, 174–180 (2009).
Zullo, F. et al. Endometriosis and obstetrics complications: a systematic review and meta-analysis. Fertil. Steril. 108, 667–672.e5 (2017).
Vigano, P., Corti, L. & Berlanda, N. Beyond infertility: obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil. Steril. 104, 802–812 (2015).
Donnez, J. & Squifflet, J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum. Reprod. 25, 1949–1958 (2010).
Zanelotti, A. & Decherney, A. H. Surgery and endometriosis. Clin. Obstet. Gynecol. 60, 477–484 (2017).
Olive, D. L. Medical therapy of endometriosis. Semin. Reprod. Med. 21, 209–222 (2003).
Harada, T., Momoeda, M., Taketani, Y., Hoshiai, H. & Terakawa, N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil. Steril. 90, 1583–1588 (2008).
Vercellini, P. et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen–progestogen combination versus low-dose norethindrone acetate. Fertil. Steril. 84, 1375–1387 (2005).
Harada, T. et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis — a randomized, double-blind, multicenter, controlled trial. Fertil. Steril. 91, 675–681 (2009).
Casper, R. F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 107, 533–536 (2017).
Abou-Setta, A. M., Houston, B., Al-Inany, H. G. & Farquhar, C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst. Rev. 1, CD005072 (2013).
Brown, J., Pan, A. & Hart, R. J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 12, CD008475 (2010).
Sagsveen, M. et al. Gonadotrophin-releasing hormone analogues for endometriosis: bone mineral density. Cochrane Database Syst. Rev. 4, CD001297 (2003).
Bedaiwy, M. A., Allaire, C. & Alfaraj, S. Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy. Fertil. Steril. 107, 537–548 (2017).
Taylor, H. S. et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N. Engl. J. Med. 377, 28–40 (2017).
Hornstein, M. D. An oral GnRH antagonist for endometriosis — a new drug for an old disease. N. Engl. J. Med. 377, 81–83 (2017).
Bedaiwy, M. A., Alfaraj, S., Yong, P. & Casper, R. New developments in the medical treatment of endometriosis. Fertil. Steril. 107, 555–565 (2017).
Shakiba, K., Bena, J. F., McGill, K. M., Minger, J. & Falcone, T. Surgical treatment of endometriosis. Obstet. Gynecol. 111, 1285–1292 (2008).
Vercellini, P. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis: results of a randomized, controlled trial. Fertil. Steril. 68, S3 (1997).
Meuleman, C. et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum. Reprod. Update 17, 311–326 (2011).
Koga, K., Takamura, M., Fujii, T. & Osuga, Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil. Steril. 104, 793–801 (2015).
Gao, X. et al. Health-related quality of life burden of women with endometriosis: a literature review. Curr. Med. Res. Opin. 22, 1787–1797 (2006).
Simoens, S. et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 27, 1292–1299 (2012). This prospective study calculates the average annual costs and HRQOL per woman with endometriosis-associated symptoms, showing it to be similar to other chronic conditions.
Jones, G., Kennedy, S., Barnard, A., Wong, J. & Jenkinson, C. Development of an endometriosis quality-of-life instrument. Obstet. Gynecol. 98, 258–264 (2001). This study describes the only validated endometriosis-specific quality-of-life outcome tool developed, measuring endometriosis-related health status on five scales. The tool has since been shown to be sensitive to changes in symptoms, making it a useful tool in endometriosis-specific clinical trials.
Jones, G., Jenkinson, C. & Kennedy, S. Development of the short form endometriosis health profile questionnaire: the EHP-5. Qual. Life Res. 13, 695–704 (2004).
Jones, G., Jenkinson, C. & Kennedy, S. Evaluating the responsiveness of the endometriosis health profile questionnaire: the EHP-30. Qual. Life Res. 13, 705–713 (2004).
Hirsch, M. et al. Variation in outcome reporting in endometriosis trials: a systematic review. Am. J. Obstet. Gynecol. 214, 452–464 (2016).
Khan, K. The CROWN Initiative: journal editors invite researchers to develop core outcomes in women’s health. BJOG 123, 103–104 (2016).
van Nooten, F. E., Cline, J., Elash, C. A., Paty, J. & Reaney, M. Development and content validation of a patient-reported endometriosis pain daily diary. Health Qual. Life Outcomes 16, 3 (2018).
Rogers, P. A. W. et al. Research priorities for endometriosis. Reprod. Sci. 24, 202–226 (2017).
Butrick, C. W. Patients with chronic pelvic pain: endometriosis or interstitial cystitis/painful bladder syndrome? JSLS 11, 182–189 (2007).
Sørlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100, 8418–8423 (2003).
Cancer Genome Atlas Network. et al. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Gupta, D. et al. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 4, CD012165 (2016).
Nisenblat, V. et al. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst. Rev. 7, CD012281 (2016).
Nisenblat, V., Bossuyt, P. M. M., Farquhar, C., Johnson, N. & Hull, M. L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2, CD009591 (2016).
May, K. E. et al. Peripheral biomarkers of endometriosis: a systematic review. Hum. Reprod. Update 16, 651–674 (2010).
May, K. E., Villar, J., Kirtley, S., Kennedy, S. H. & Becker, C. M. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum. Reprod. Update 17, 637–653 (2011).
van der Zanden, M. & Nap, A. W. Knowledge of, and treatment strategies for, endometriosis among general practitioners. Reprod. Biomed. Online 32, 527–531 (2016).
Seear, K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc. Sci. Med. 69, 1220–1227 (2009).
Greene, R., Stratton, P., Cleary, S. D., Ballweg, M. L. & Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 91, 32–39 (2009).
Horne, A. W., Saunders, P. T. K., Abokhrais, I. M. & Hogg, L. Top ten endometriosis research priorities in the UK and Ireland. Lancet 389, 2191–2192 (2017).
Hans Evers, J. L. H. Is adolescent endometriosis a progressive disease that needs to be diagnosed and treated? Hum. Reprod. 28, 2023 (2013).
Neal, D. M. & McKenzie, P. J. Putting the pieces together: endometriosis blogs, cognitive authority, and collaborative information behavior. J. Med. Libr. Assoc. 99, 127–134 (2011).
Uno, S. et al. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat. Genet. 42, 707–710 (2010).
Painter, J. N. et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat. Genet. 43, 51–54 (2011).
Albertsen, H. M., Chettier, R., Farrington, P. & Ward, K. Genome-wide association study link novel loci to endometriosis. PLoS ONE 8, e58257 (2013).
Steinthorsdottir, V. et al. Common variants upstream of KDR encoding VEGFR2 and in TTC39B associate with endometriosis. Nat. Commun. 7, 12350 (2016).
Sobalska-Kwapis, M. et al. New variants near RHOJ and C2, HLA-DRA region and susceptibility to endometriosis in the Polish population — the genome-wide association study. Eur. J. Obstet. Gynecol. Reprod. Biol. 217, 106–112 (2017).
Acknowledgements
R.N.T. acknowledges funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development through grants R01-HD33238, U54-HD37321, U54-HD55787, R01-HD55379, U01-HD66439 and R21-HD78818. S.A.M. is also affiliated with the Boston Center for Endometriosis, Boston Children’s Hospital and Brigham and Women’s Hospital and the Division of Adolescent and Young Adult Medicine, Department of Medicine, Boston Children’s Hospital and Harvard Medical School. The authors thank N. Moore (Oxford University Hospitals Foundation Trust, UK) for providing MRI images, D. Barber (Oxford Endometriosis CaRe Centre, UK) for providing ultrasonography pictures and J. Malzahn (Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, UK) for providing the picture of the histology slide in Fig. 5.
Reviewer information
Nature Reviews Disease Primers thanks I. Brosens, M. J. Canis, S. Ferrero, C. Nezhat, V. Remorgida, M. Simões Abrão and other anonymous referee(s) for the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (K.T.Z.); Epidemiology (S.A.M.); Mechanisms/pathophysiology (R.N.T. and P.V.); Diagnosis, screening and prevention (C.M.B.); Management (K.K.); Quality of life (K.T.Z.); Outlook (K.T.Z.); Overview of the Primer (K.T.Z.).
Corresponding author
Ethics declarations
Competing interests
K.T.Z. has received grant funding from the Wellcome Trust, Medical Research Council UK, the US NIH, the European Union and the World Endometriosis Research Foundation (WERF). She also has scientific collaborations with, and has received grant funding from, Bayer AG, MDNA Life Sciences, Roche Diagnostics and Volition Rx and has served as a scientific consultant to AbbVie and Roche Diagnostics. She is Secretary of the World Endometriosis Society (WES), the European Society of Human Reproduction and Embryology (ESHRE) Special Interest Group in Endometriosis and Endometrial Disorders and Wellbeing of Women, and she is Chair of the WES Research Directions Working Group. C.M.B. is a member of the independent data monitoring group for a clinical endometriosis trial by ObsEva. He has received research grants from Bayer AG, MDNA Life Sciences, Volition Rx and Roche Diagnostics as well as from Wellbeing of Women, Medical Research Council UK, the NIH, the UK National Institute for Health Research and the European Union. He is the current Chair of the Endometriosis Guideline Development Group of the ESHRE and was a co-opted member of the Endometriosis Guideline Group by the UK National Institute for Health and Care Excellence (NICE). K.K. has received grant funding from the Ministry of Education, Culture, Sports Science and Technology Japan, the Ministry of Health, Labour and Welfare Japan, Takeda Research Support and MSD. She has also served as a scientific consultant to Bayer AG. She is an ambassador of the WES and a member of the Guideline Development Group of the Japan Society of Obstetrics and Gynecology. S.A.M. has received grant funding from the NIH and the Marriott family foundations and has served as an adviser to and has scientific collaborations with AbbVie, Celmatix and Oratel Diagnostics. She is a treasurer of the WES, Secretary of the WERF, Chair of the American Society of Reproductive Medicine Endometriosis Special Interest Group and a member of the NIH Reproductive Medicine Network Data Safety and Monitoring Board. R.N.T. has received grant funding from Bayer AG, Ferring Research Institute, the NIH and Pfizer and has served as a scientific consultant or adviser to AbbVie, Allergan, the NIH, ObsEva SA and the Population Council. He is the immediate past honorary secretary of the WES. P.V. has received grant funding from Bayer AG and Merck Serono and has served as a scientific consultant to Ferring Pharmaceuticals and Roche Diagnostics. She is a board member of the WES.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
WERF Endometriosis Phenome and Biobanking Harmonisation Project: https://endometriosisfoundation.org/ephect/
Rights and permissions
About this article
Cite this article
Zondervan, K.T., Becker, C.M., Koga, K. et al. Endometriosis. Nat Rev Dis Primers 4, 9 (2018). https://doi.org/10.1038/s41572-018-0008-5
Published:
DOI: https://doi.org/10.1038/s41572-018-0008-5
This article is cited by
-
Impact of oil-based contrast agents in hysterosalpingography on fertility outcomes in endometriosis: a retrospective cohort study
Reproductive Biology and Endocrinology (2024)
-
Efficacy and safety of a novel pain management device, AT-04, for endometriosis-related pain: study protocol for a phase III randomized controlled trial
Reproductive Health (2024)
-
Surge in endometriosis research after decades of underfunding could herald new era for women’s health
Nature Medicine (2024)
-
Mining phase separation-related diagnostic biomarkers for endometriosis through WGCNA and multiple machine learning techniques: a retrospective and nomogram study
Journal of Assisted Reproduction and Genetics (2024)
-
The roles of chromatin regulatory factors in endometriosis
Journal of Assisted Reproduction and Genetics (2024)