Abstract
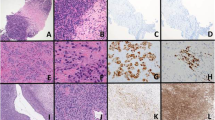
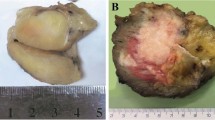
Ewing sarcoma is the second most frequent bone tumour of childhood and adolescence that can also arise in soft tissue. Ewing sarcoma is a highly aggressive cancer, with a survival of 70–80% for patients with standard-risk and localized disease and ~30% for those with metastatic disease. Treatment comprises local surgery, radiotherapy and polychemotherapy, which are associated with acute and chronic adverse effects that may compromise quality of life in survivors. Histologically, Ewing sarcomas are composed of small round cells expressing high levels of CD99. Genetically, they are characterized by balanced chromosomal translocations in which a member of the FET gene family is fused with an ETS transcription factor, with the most common fusion being EWSR1–FLI1 (85% of cases). Ewing sarcoma breakpoint region 1 protein (EWSR1)–Friend leukaemia integration 1 transcription factor (FLI1) is a tumour-specific chimeric transcription factor (EWSR1–FLI1) with neomorphic effects that massively rewires the transcriptome. Additionally, EWSR1–FLI1 reprogrammes the epigenome by inducing de novo enhancers at GGAA microsatellites and by altering the state of gene regulatory elements, creating a unique epigenetic signature. Additional mutations at diagnosis are rare and mainly involve STAG2, TP53 and CDKN2A deletions. Emerging studies on the molecular mechanisms of Ewing sarcoma hold promise for improvements in early detection, disease monitoring, lower treatment-related toxicity, overall survival and quality of life.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gaspar, N. et al. Ewing sarcoma: current management and future approaches through collaboration. J. Clin. Oncol. 33, 3036–3046 (2015).
Pappo, A. S. & Dirksen, U. Rhabdomyosarcoma, Ewing sarcoma, and other round cell sarcomas. J. Clin. Oncol. 36, 168–179 (2018).
Stahl, M. et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr. Blood Cancer 57, 549–553 (2011).
Kovar, H. Ewing’s sarcoma and peripheral primitive neuroectodermal tumors after their genetic union. Curr. Opin. Oncol. 10, 334–342 (1998).
de Alava, E., Lessnick, S. L. & Sorensen, P. H. in WHO Classification of Tumours of Soft Tissue and Bone (eds Fletcher, C. M., Bridge, J. A., Hogendoorn, P. C. W., Mertens, F.) 305–309 (IARC, Lyon, 2013).
Doyle, L. A. Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer 120, 1763–1774 (2014).
Watson, S. et al. Transcriptomic definition of molecular subgroups of small round cell sarcomas. J. Pathol. 245, 29–40 (2018).
Sankar, S. & Lessnick, S. L. Promiscuous partnerships in Ewing’s sarcoma. Cancer Genet. 204, 351–365 (2011).
Ewing, J. Diffuse endothelioma of bone. Proc. N. Y. Pathol. Soc. 21, 17 (1921).
Toomey, E. C., Schiffman, J. D. & Lessnick, S. L. Recent advances in the molecular pathogenesis of Ewing’s sarcoma. Oncogene 29, 4504–4516 (2010).
Delattre, O. et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 359, 162–165 (1992). This paper is the first description of the EWSR1–FLI1 gene fusion in Ewing sarcoma.
Gangwal, K., Close, D., Enriquez, C. A., Hill, C. P. & Lessnick, S. L. Emergent properties of EWS/FLI regulation via GGAA microsatellites in Ewing’s sarcoma. Genes Cancer 1, 177–187 (2010).
Gangwal, K. et al. Microsatellites as EWS/FLI response elements in Ewing’s sarcoma. Proc. Natl Acad. Sci. USA 105, 10149–10154 (2008). This study shows for the first time that an oncogenic transcription factor (EWSR1–FLI1) uses so-called ‘junk DNA’ (that is, GGAA microsatellites) as DNA response elements.
Guillon, N. et al. The oncogenic EWS-FLI1 protein binds in vivo GGAA microsatellite sequences with potential transcriptional activation function. PLOS ONE 4, e4932 (2009).
Brohl, A. S. et al. The genomic landscape of the Ewing sarcoma family of tumors reveals recurrent STAG2 mutation. PLOS Genet. 10, e1004475 (2014).
Crompton, B. D. et al. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 4, 1326–1341 (2014).
Solomon, D. A. et al. Mutational inactivation of STAG2 causes aneuploidy in human cancer. Science 333, 1039–1043 (2011).
Tirode, F. et al. Genomic landscape of Ewing sarcoma defines an aggressive subtype with co-association of STAG2 and TP53 mutations. Cancer Discov. 4, 1342–1353 (2014). This paper describes the largest series of Ewing sarcoma tumours in which matched whole-genome sequencing of germline and tumoural DNA has been carried out.
Jawad, M. U. et al. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: an analysis of 1631 cases from the SEER database, 1973–2005. Cancer 115, 3526–3536 (2009).
Fraumeni, J. F. & Glass, A. G. Rarity of Ewing’s sarcoma among U. S. Negro children. Lancet 1, 366–367 (1970).
Jensen, R. D. & Drake, R. M. Rarity of Ewing’s tumour in Negroes. Lancet 1, 777 (1970).
Worch, J. et al. Racial differences in the incidence of mesenchymal tumors associated with EWSR1 translocation. Cancer Epidemiol. Biomark. Prev. 20, 449–453 (2011).
Joyce, M. J. et al. Ewing’s sarcoma in female siblings. A clinical report and review of the literature. Cancer 53, 1959–1962 (1984).
Valery, P. C., McWhirter, W., Sleigh, A., Williams, G. & Bain, C. Farm exposures, parental occupation, and risk of Ewing’s sarcoma in Australia: a national case-control study. Cancer Causes Control 13, 263–270 (2002).
Postel-Vinay, S. et al. Common variants near TARDBP and EGR2 are associated with susceptibility to Ewing sarcoma. Nat. Genet. 44, 323–327 (2012). This paper describes the first Ewing sarcoma GWAS, which highlighted the contribution of common variants to susceptibility of Ewing sarcoma in people of European descent.
Grünewald, T. G. P. et al. Chimeric EWSR1-FLI1 regulates the Ewing sarcoma susceptibility gene EGR2 via a GGAA microsatellite. Nat. Genet. 47, 1073–1078 (2015). This paper describes how EWSR1–FLI1 and inherited germline variants located in a Ewing sarcoma susceptibility locus cooperate to promote tumorigenesis.
Brohl, A. S. et al. Frequent inactivating germline mutations in DNA repair genes in patients with Ewing sarcoma. Genet. Med. 19, 955–958 (2017).
Chang, W. et al. MultiDimensional clinomics for precision therapy of children and adolescent young adults with relapsed and refractory cancer: a report from the Center for Cancer Research. Clin. Cancer Res. 22, 3810–3820 (2016).
Rahman, N. Realizing the promise of cancer predisposition genes. Nature 505, 302–308 (2014).
Beck, R. et al. EWS/FLI-responsive GGAA microsatellites exhibit polymorphic differences between European and African populations. Cancer Genet. 205, 304–312 (2012).
Rocchi, A. et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis. J. Clin. Invest. 120, 668–680 (2010).
Kreppel, M. et al. Suppression of KCMF1 by constitutive high CD99 expression is involved in the migratory ability of Ewing’s sarcoma cells. Oncogene 25, 2795–2800 (2006).
Manara, M. C., Pasello, M. & Scotlandi, K. CD99: a cell surface protein with an oncojanus role in tumors. Genes 9, 159 (2018).
Pasello, M., Manara, M. C. & Scotlandi, K. CD99 at the crossroads of physiology and pathology. J. Cell Commun. Signal. 12, 55–68 (2018).
Martinelli, M. et al. CD99 polymorphisms significantly influence the probability to develop Ewing sarcoma in earlier age and patient disease progression. Oncotarget 7, 77958–77967 (2016).
Aurias, A., Rimbaut, C., Buffe, D., Zucker, J. M. & Mazabraud, A. Translocation involving chromosome 22 in Ewing’s sarcoma. A cytogenetic study of four fresh tumors. Cancer Genet. Cytogenet. 12, 21–25 (1984).
Zucman, J. et al. Combinatorial generation of variable fusion proteins in the Ewing family of tumours. EMBO J. 12, 4481–4487 (1993).
Sorensen, P. H. et al. A second Ewing’s sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat. Genet. 6, 146–151 (1994). This paper provided one of the first descriptions of genetic redundancy of fusion genes in human solid tumours.
Ginsberg, J. P. et al. EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing’s sarcoma. J. Clin. Oncol. 17, 1809–1814 (1999).
Jeon, I. S. et al. A variant Ewing’s sarcoma translocation (7;22) fuses the EWS gene to the ETS gene ETV1. Oncogene 10, 1229–1234 (1995).
Urano, F. et al. Molecular analysis of Ewing’s sarcoma: another fusion gene, EWS-E1AF, available for diagnosis. Jpn J. Cancer Res. 89, 703–711 (1998).
Peter, M. et al. A new member of the ETS family fused to EWS in Ewing tumors. Oncogene 14, 1159–1164 (1997).
Ng, T. L. et al. Ewing sarcoma with novel translocation t(2;16) producing an in-frame fusion of FUS and FEV. J. Mol. Diagn. 9, 459–463 (2007).
de Alava, E. et al. EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing’s sarcoma. J. Clin. Oncol. 16, 1248–1255 (1998).
Le Deley, M.-C. et al. Impact of EWS-ETS fusion type on disease progression in Ewing’s sarcoma/peripheral primitive neuroectodermal tumor: prospective results from the cooperative Euro-E.W.I.N.G. 99 trial. J. Clin. Oncol. 28, 1982–1988 (2010).
van Doorninck, J. A. et al. Current treatment protocols have eliminated the prognostic advantage of type 1 fusions in Ewing sarcoma: a report from the Children’s Oncology Group. J. Clin. Oncol. 28, 1989–1994 (2010).
Xiao, T., Wallace, J. & Felsenfeld, G. Specific sites in the C terminus of CTCF interact with the SA2 subunit of the cohesin complex and are required for cohesin-dependent insulation activity. Mol. Cell. Biol. 31, 2174–2183 (2011).
van der Lelij, P. et al. Synthetic lethality between the cohesin subunits STAG1 and STAG2 in diverse cancer contexts. eLife 6, e26980 (2017).
Brownhill, S. C., Taylor, C. & Burchill, S. A. Chromosome 9p21 gene copy number and prognostic significance of p16 in ESFT. Br. J. Cancer 96, 1914–1923 (2007).
Huang, H.-Y. et al. Ewing sarcomas with p53 mutation or p16/p14ARF homozygous deletion: a highly lethal subset associated with poor chemoresponse. J. Clin. Oncol. 23, 548–558 (2005).
Worst, B. C. et al. Next-generation personalised medicine for high-risk paediatric cancer patients – the INFORM pilot study. Eur. J. Cancer 65, 91–101 (2016).
Harttrampf, A. C. et al. Molecular Screening for Cancer Treatment Optimization (MOSCATO-01) in pediatric patients: a single-institutional prospective molecular stratification trial. Clin. Cancer Res. 23, 6101–6112 (2017).
Mugneret, F., Lizard, S., Aurias, A. & Turc-Carel, C. Chromosomes in Ewing’s sarcoma. II. Nonrandom additional changes, trisomy 8 and der(16)t(1;16). Cancer Genet. Cytogenet. 32, 239–245 (1988).
Hattinger, C. M. et al. Prognostic impact of chromosomal aberrations in Ewing tumours. Br. J. Cancer 86, 1763–1769 (2002).
Roberts, P. et al. Ploidy and karyotype complexity are powerful prognostic indicators in the Ewing’s sarcoma family of tumors: a study by the United Kingdom Cancer Cytogenetics and the Children’s Cancer and Leukaemia Group. Genes Chromosomes Cancer 47, 207–220 (2008).
Mackintosh, C. et al. 1q gain and CDT2 overexpression underlie an aggressive and highly proliferative form of Ewing sarcoma. Oncogene 31, 1287–1298 (2012).
May, W. A. et al. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc. Natl Acad. Sci. USA 90, 5752–5756 (1993). This paper describes for the first time that EWSR1–FLI1 acts as an oncogenic transcription factor.
Lessnick, S. L., Braun, B. S., Denny, C. T. & May, W. A. Multiple domains mediate transformation by the Ewing’s sarcoma EWS/FLI-1 fusion gene. Oncogene 10, 423–431 (1995).
Ohno, T., Rao, V. N. & Reddy, E. S. EWS/Fli-1 chimeric protein is a transcriptional activator. Cancer Res. 53, 5859–5863 (1993).
Cidre-Aranaz, F. & Alonso, J. EWS/FLI1 target genes and therapeutic opportunities in Ewing sarcoma. Front. Oncol. 5, 162 (2015).
Riggi, N. et al. EWS-FLI1 utilizes divergent chromatin remodeling mechanisms to directly activate or repress enhancer elements in Ewing sarcoma. Cancer Cell 26, 668–681 (2014). This study yields mechanistic insight into EWSR1–FLI1-mediated epigenetic reprogramming in Ewing sarcoma cell lines and primary tumours.
Tomazou, E. M. et al. Epigenome mapping reveals distinct modes of gene regulation and widespread enhancer reprogramming by the oncogenic fusion protein EWS-FLI1. Cell Rep. 10, 1082–1095 (2015).
Sheffield, N. C. et al. DNA methylation heterogeneity defines a disease spectrum in Ewing sarcoma. Nat. Med. 23, 386–395 (2017). This paper constitutes the largest epigenetic characterization of Ewing sarcoma tumours to date, which suggests that Ewing sarcoma arises from a specific cellular lineage with different epigenetic states at the time of cellular transformation.
Gorthi, A. et al. EWS–FLI1 increases transcription to cause R-loops and block BRCA1 repair in Ewing sarcoma. Nature 555, 387–391 (2018).
Patel, M. et al. Tumor-specific retargeting of an oncogenic transcription factor chimera results in dysregulation of chromatin and transcription. Genome Res. 22, 259–270 (2012).
Johnson, K. M. et al. Role for the EWS domain of EWS/FLI in binding GGAA-microsatellites required for Ewing sarcoma anchorage independent growth. Proc. Natl Acad. Sci. USA 114, 9870–9875 (2017).
Boulay, G. et al. Cancer-specific retargeting of BAF complexes by a prion-like domain. Cell 171, 163–178 (2017). This paper shows for the first time how EWSR1–FLI1 remodels the epigenome as a pioneer factor by recruiting the BAF complex to EWSR1–FLI1 binding sites such as GGAA microsatellites.
Katschnig, A. M. et al. EWS-FLI1 perturbs MRTFB/YAP-1/TEAD target gene regulation inhibiting cytoskeletal autoregulatory feedback in Ewing sarcoma. Oncogene 36, 5995–6005 (2017).
Sankar, S. et al. Mechanism and relevance of EWS/FLI-mediated transcriptional repression in Ewing sarcoma. Oncogene 32, 5089–5100 (2013).
Le Deley, M.-C. et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: results of the randomized noninferiority Euro-EWING99-R1 trial. J. Clin. Oncol. 32, 2440–2448 (2014).
Peschansky, V. J. & Wahlestedt, C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics 9, 3–12 (2014).
Dylla, L., Moore, C. & Jedlicka, P. MicroRNAs in Ewing sarcoma. Front. Oncol. 3, 65 (2013).
Tirode, F. et al. Mesenchymal stem cell features of Ewing tumors. Cancer Cell 11, 421–429 (2007). This study highlights the transcriptional and functional relationship of MSCs and EWSR1–FLI1-silenced Ewing sarcoma cell lines, suggesting MSCs as cells of Ewing sarcoma origin.
Hancock, J. D. & Lessnick, S. L. A transcriptional profiling meta-analysis reveals a core EWS-FLI gene expression signature. Cell Cycle 7, 250–256 (2008).
Riggi, N. et al. Development of Ewing’s sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 65, 11459–11468 (2005).
Riggi, N. et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 24, 916–932 (2010).
von Levetzow, C. et al. Modeling initiation of Ewing sarcoma in human neural crest cells. PLOS ONE 6, e19305 (2011).
Castillero-Trejo, Y., Eliazer, S., Xiang, L., Richardson, J. A. & Ilaria, R. L. Expression of the EWS/FLI-1 oncogene in murine primary bone-derived cells results in EWS/FLI-1-dependent, Ewing sarcoma-like tumors. Cancer Res. 65, 8698–8705 (2005).
Tanaka, M. et al. Ewing’s sarcoma precursors are highly enriched in embryonic osteochondrogenic progenitors. J. Clin. Invest. 124, 3061–3074 (2014).
Gomez, N. C. et al. Widespread chromatin accessibility at repetitive elements links stem cells with human cancer. Cell Rep. 17, 1607–1620 (2016).
Staege, M. S. et al. DNA microarrays reveal relationship of Ewing family tumors to both endothelial and fetal neural crest-derived cells and define novel targets. Cancer Res. 64, 8213–8221 (2004).
Schuetz, A. N. et al. Intercellular junctions in Ewing sarcoma/primitive neuroectodermal tumor: additional evidence of epithelial differentiation. Mod. Pathol. 18, 1403–1410 (2005).
Bailey, K. M., Airik, M., Krook, M. A., Pedersen, E. A. & Lawlor, E. R. Micro-environmental stress induces Src-dependent activation of invadopodia and cell migration in Ewing sarcoma. Neoplasia 18, 480–488 (2016).
Krook, M. A. et al. Stress-induced CXCR4 promotes migration and invasion of ewing sarcoma. Mol. Cancer Res. 12, 953–964 (2014).
Hong, S.-H. et al. High neuropeptide Y release associates with Ewing sarcoma bone dissemination - in vivo model of site-specific metastases. Oncotarget 6, 7151–7165 (2015).
Aryee, D. N. T. et al. Hypoxia modulates EWS-FLI1 transcriptional signature and enhances the malignant properties of Ewing’s sarcoma cells in vitro. Cancer Res. 70, 4015–4023 (2010).
Mendoza-Naranjo, A. et al. ERBB4 confers metastatic capacity in Ewing sarcoma. EMBO Mol. Med. 5, 1087–1102 (2013).
Eirew, P. et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature 518, 422–426 (2015).
Kovar, H. et al. The second European interdisciplinary Ewing sarcoma research summit—a joint effort to deconstructing the multiple layers of a complex disease. Oncotarget 7, 8613–8624 (2016).
Leprivier, G., Rotblat, B., Khan, D., Jan, E. & Sorensen, P. H. Stress-mediated translational control in cancer cells. Biochim. Biophys. Acta 1849, 845–860 (2015).
El-Naggar, A. M. et al. Translational activation of HIF1α by YB-1 promotes sarcoma metastasis. Cancer Cell 27, 682–697 (2015).
Somasekharan, S. P. et al. YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1. J. Cell Biol. 208, 913–929 (2015).
Miller, I. V. et al. First identification of Ewing’s sarcoma-derived extracellular vesicles and exploration of their biological and potential diagnostic implications. Biol. Cell 105, 289–303 (2013).
Villasante, A. et al. Recapitulating the size and cargo of tumor exosomes in a tissue-engineered model. Theranostics 6, 1119–1130 (2016).
Toretsky, J. A., Kalebic, T., Blakesley, V., LeRoith, D. & Helman, L. J. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J. Biol. Chem. 272, 30822–30827 (1997).
Scotlandi, K. et al. Insulin-like growth factor I receptor-mediated circuit in Ewing’s sarcoma/peripheral neuroectodermal tumor: a possible therapeutic target. Cancer Res. 56, 4570–4574 (1996).
Strammiello, R. et al. Impact of IGF-I/IGF-IR circuit on the angiogenetic properties of Ewing’s sarcoma cells. Horm. Metab. Res. 35, 675–684 (2003).
Uren, A. et al. Beta-platelet-derived growth factor receptor mediates motility and growth of Ewing’s sarcoma cells. Oncogene 22, 2334–2342 (2003).
Kamura, S. et al. Basic fibroblast growth factor in the bone microenvironment enhances cell motility and invasion of Ewing’s sarcoma family of tumours by activating the FGFR1-PI3K-Rac1 pathway. Br. J. Cancer 103, 370–381 (2010).
Cidre-Aranaz, F. et al. EWS-FLI1-mediated suppression of the RAS-antagonist Sprouty 1 (SPRY1) confers aggressiveness to Ewing sarcoma. Oncogene 36, 766–776 (2017).
Lissat, A. et al. IL6 secreted by Ewing sarcoma tumor microenvironment confers anti-apoptotic and cell-disseminating paracrine responses in Ewing sarcoma cells. BMC Cancer 15, 552 (2015).
Hahm, K. B. et al. Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat. Genet. 23, 222–227 (1999).
Ban, J. et al. EWS-FLI1 suppresses NOTCH-activated p53 in Ewing’s sarcoma. Cancer Res. 68, 7100–7109 (2008).
Ban, J. et al. Suppression of deacetylase SIRT1 mediates tumor-suppressive NOTCH response and offers a novel treatment option in metastatic Ewing sarcoma. Cancer Res. 74, 6578–6588 (2014).
Potratz, J. et al. Receptor tyrosine kinase gene expression profiles of Ewing sarcomas reveal ROR1 as a potential therapeutic target in metastatic disease. Mol. Oncol. 10, 677–692 (2016).
Zhou, Z., Stewart, K. S., Yu, L. & Kleinerman, E. S. Bone marrow cells participate in tumor vessel formation that supports the growth of Ewing’s sarcoma in the lung. Angiogenesis 14, 125–133 (2011).
Hauer, K. et al. DKK2 mediates osteolysis, invasiveness, and metastatic spread in Ewing sarcoma. Cancer Res. 73, 967–977 (2013).
Lagares-Tena, L. et al. Caveolin-1 promotes Ewing sarcoma metastasis regulating MMP-9 expression through MAPK/ERK pathway. Oncotarget 7, 56889–56903 (2016).
Richter, G. H. S. et al. EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc. Natl Acad. Sci. USA 106, 5324–5329 (2009).
Richter, G. H. S. et al. G-protein coupled receptor 64 promotes invasiveness and metastasis in Ewing sarcomas through PGF and MMP1. J. Pathol. 230, 70–81 (2013).
Grünewald, T. G. P. et al. The Zyxin-related protein thyroid receptor interacting protein 6 (TRIP6) is overexpressed in Ewing’s sarcoma and promotes migration, invasion and cell growth. Biol. Cell 105, 535–547 (2013).
Grünewald, T. G. P. et al. STEAP1 is associated with the invasive and oxidative stress phenotype of Ewing tumors. Mol. Cancer Res. 10, 52–65 (2012).
Sannino, G., Marchetto, A., Kirchner, T. & Grünewald, T. G. P. Epithelial-to-mesenchymal and mesenchymal-to-epithelial transition in mesenchymal tumors: a paradox in sarcomas? Cancer Res. 77, 4556–4561 (2017).
Chaturvedi, A. et al. Molecular dissection of the mechanism by which EWS/FLI expression compromises actin cytoskeletal integrity and cell adhesion in Ewing sarcoma. Mol. Biol. Cell 25, 2695–2709 (2014).
Franzetti, G.-A. et al. Cell-to-cell heterogeneity of EWSR1-FLI1 activity determines proliferation/migration choices in Ewing sarcoma cells. Oncogene 36, 3505–3514 (2017). This study shows that transient reduction of EWSR1–FLI1 expression is a major factor in Ewing sarcoma metastasis.
Pedersen, E. A. et al. Activation of Wnt/β-catenin in Ewing sarcoma cells antagonizes EWS/ETS function and promotes phenotypic transition to more metastatic cell states. Cancer Res. 76, 5040–5053 (2016).
Wiles, E. T., Bell, R., Thomas, D., Beckerle, M. & Lessnick, S. L. ZEB2 represses the epithelial phenotype and facilitates metastasis in Ewing sarcoma. Genes Cancer 4, 486–500 (2013).
Pizzo, P. A. & Poplack, D. G. (eds) Principles and Practice of Pediatric Oncology 7th edn (Lippincott Wililiams & Wilkins, Philadelphia, 2015).
Widhe, B. & Widhe, T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J. Bone Joint Surg. Am. 82, 667–674 (2000).
Alonso, L., Navarro-Perez, V., Sanchez-Muñoz, A. & Alba, E. Time to diagnosis of ewing tumors in children and adolescents is not associated with metastasis or survival. J. Clin. Oncol. 32, 4020 (2014).
Worch, J. et al. Age dependency of primary tumor sites and metastases in patients with Ewing sarcoma. Pediatr. Blood Cancer https://doi.org/10.1002/pbc.27251 (2018).
Rochefort, P. et al. A retrospective multicentric study of ewing sarcoma family of tumors in patients older than 50: management and outcome. Sci. Rep. 7, 17917 (2017).
Zhang, J. et al. Germline mutations in predisposition genes in pediatric cancer. N. Engl. J. Med. 373, 2336–2346 (2015).
Kuleta-Bosak, E. et al. Suitability of imaging methods (X-ray, CT, MRI) in the diagnostics of Ewing’s sarcoma in children - analysis of own material. Pol. J. Radiol. 75, 18–28 (2010).
Franzius, C. et al. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann. Oncol. 12, 479–486 (2001).
Völker, T. et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J. Clin. Oncol. 25, 5435–5441 (2007).
Gerth, H. U. et al. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. J. Nucl. Med. 48, 1932–1939 (2007).
Kasalak, Ö., Glaudemans, A. W. J. M., Overbosch, J., Jutte, P. C. & Kwee, T. C. Can FDG-PET/CT replace blind bone marrow biopsy of the posterior iliac crest in Ewing sarcoma? Skeletal Radiol. 47, 363–367 (2018).
Newman, E. N., Jones, R. L. & Hawkins, D. S. An evaluation of [F-18]-fluorodeoxy-d-glucose positron emission tomography, bone scan, and bone marrow aspiration/biopsy as staging investigations in Ewing sarcoma. Pediatr. Blood Cancer 60, 1113–1117 (2013).
Kopp, L. M. et al. Utility of bone marrow aspiration and biopsy in initial staging of Ewing sarcoma. Pediatr. Blood Cancer 62, 12–15 (2015).
Baldauf, M. C. et al. Robust diagnosis of Ewing sarcoma by immunohistochemical detection of super-enhancer-driven EWSR1-ETS targets. Oncotarget 9, 1587–1601 (2018).
ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 25 (Suppl 3), iii113–iii123 (2014).
Nascimento, A. G., Unii, K. K., Pritchard, D. J., Cooper, K. L. & Dahlin, D. C. A clinicopathologic study of 20 cases of large-cell (atypical) Ewing’s sarcoma of bone. Am. J. Surg. Pathol. 4, 29–36 (1980).
Ambros, I. M. et al. MIC2 is a specific marker for Ewing’s sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing’s sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer 67, 1886–1893 (1991).
Hornick, J. L. Novel uses of immunohistochemistry in the diagnosis and classification of soft tissue tumors. Mod. Pathol. 27 (Suppl. 1), S47–S63 (2014).
Llombart-Bosch, A. et al. Histological heterogeneity of Ewing’s sarcoma/PNET: an immunohistochemical analysis of 415 genetically confirmed cases with clinical support. Virchows Arch. Int. J. Pathol. 455, 397–411 (2009).
Machado, I. et al. Review with novel markers facilitates precise categorization of 41 cases of diagnostically challenging, ‘undifferentiated small round cell tumors’. A clinicopathologic, immunophenotypic and molecular analysis. Ann. Diagn. Pathol. 34, 1–12 (2017).
Sorensen, P. H. et al. Reverse transcriptase PCR amplification of EWS/FLI-1 fusion transcripts as a diagnostic test for peripheral primitive neuroectodermal tumors of childhood. Diagn. Mol. Pathol. Am. J. Surg. Pathol. Part B 2, 147–157 (1993).
Machado, I. et al. Molecular diagnosis of Ewing sarcoma family of tumors: a comparative analysis of 560 cases with FISH and RT-PCR. Diagn. Mol. Pathol. Am. J. Surg. Pathol. Part B 18, 189–199 (2009).
Chen, S. et al. Ewing sarcoma with ERG gene rearrangements: a molecular study focusing on the prevalence of FUS-ERG and common pitfalls in detecting EWSR1-ERG fusions by FISH. Genes Chromosomes Cancer 55, 340–349 (2016).
Paulussen, M. et al. Second malignancies after ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. Ann. Oncol. 12, 1619–1630 (2001).
Ladenstein, R. et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J. Clin. Oncol. 28, 3284–3291 (2010).
Juergens, C. et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr. Blood Cancer 47, 22–29 (2006).
Whelan, J. et al. Efficacy of busulfan-melphalan high dose chemotherapy consolidation (BuMel) in localized high-risk Ewing sarcoma (ES): results of EURO-EWING 99-R2 randomized trial (EE99R2Loc). J. Clin. Oncol. 34, (15 Suppl.), Abstr. 11000 (2016).
Ladenstein, R. et al. Impact of megatherapy in children with high-risk Ewing’s tumours in complete remission: a report from the EBMT Solid Tumour Registry. Bone Marrow Transplant. 15, 697–705 (1995).
Luksch, R. et al. Primary metastatic Ewing’s family tumors: results of the Italian Sarcoma Group and Scandinavian Sarcoma Group ISG/SSG IV Study including myeloablative chemotherapy and total-lung irradiation. Ann. Oncol. 23, 2970–2976 (2012).
Womer, R. B. et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J. Clin. Oncol. 30, 4148–4154 (2012).
Odri, G. A. et al. Zoledronic acid as a new adjuvant therapeutic strategy for Ewing’s sarcoma patients. Cancer Res. 70, 7610–7619 (2010).
ISRCTN Registry. iscrtcn.com https://doi.org/10.1186/ISRCTN92192408 (2014).
Paulussen, M. et al. Ewing’s tumors with primary lung metastases: survival analysis of 114 (European Intergroup) Cooperative Ewing’s Sarcoma Studies patients. J. Clin. Oncol. 16, 3044–3052 (1998).
Oberlin, O. et al. Impact of high-dose busulfan plus melphalan as consolidation in metastatic Ewing tumors: a study by the Société Française des Cancers de l’Enfant. J. Clin. Oncol. 24, 3997–4002 (2006).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00987636 (2016).
Brunetto, A. L. et al. Carboplatin in the treatment of Ewing sarcoma: results of the first Brazilian collaborative study group for Ewing sarcoma family tumors-EWING1. Pediatr. Blood Cancer 62, 1747–1753 (2015).
DuBois, S. G. et al. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: a report from the Children’s Oncology Group. Cancer 121, 467–475 (2015).
Schuck, A. et al. Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int. J. Radiat. Oncol. Biol. Phys. 55, 168–177 (2003).
Foulon, S. et al. Can postoperative radiotherapy be omitted in localised standard-risk Ewing sarcoma? An observational study of the Euro-E.W.I.N.G. Group. Eur. J. Cancer 61, 128–136 (2016).
Andreou, D. et al. Prognostic factors for local control in Ewing sarcoma (ES) in the Euro-EWING99 trial. J. Clin. Oncol. 34 (15 suppl.), Abstr. 11026 (2016).
Shamberger, R. C. et al. Ewing sarcoma/primitive neuroectodermal tumor of the chest wall: impact of initial versus delayed resection on tumor margins, survival, and use of radiation therapy. Ann. Surg. 238, 563–568 (2003).
Bedetti, B. et al. Local control in Ewing sarcoma of the chest wall: results of the EURO-EWING 99 trial. Ann. Surg. Oncol. 22, 2853–2859 (2015).
Meyers, P. A. et al. High-dose melphalan, etoposide, total-body irradiation, and autologous stem-cell reconstitution as consolidation therapy for high-risk Ewing’s sarcoma does not improve prognosis. J. Clin. Oncol. 19, 2812–2820 (2001).
Burdach, S. et al. High-dose therapy for patients with primary multifocal and early relapsed Ewing’s tumors: results of two consecutive regimens assessing the role of total-body irradiation. J. Clin. Oncol. 21, 3072–3078 (2003).
Rasper, M. et al. The value of high-dose chemotherapy in patients with first relapsed Ewing sarcoma. Pediatr. Blood Cancer 61, 1382–1386 (2014).
Thiel, U. et al. No improvement of survival with reduced- versus high-intensity conditioning for allogeneic stem cell transplants in Ewing tumor patients. Ann. Oncol. 22, 1614–1621 (2011).
Berghuis, D. et al. Reduced human leukocyte antigen expression in advanced-stage Ewing sarcoma: implications for immune recognition. J. Pathol. 218, 222–231 (2009).
Kailayangiri, S. et al. The ganglioside antigen G(D2) is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br. J. Cancer 106, 1123–1133 (2012).
Roth, M. et al. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer 120, 548–554 (2014).
Navid, F. et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J. Clin. Oncol. 32, 1445–1452 (2014).
Thiel, U. et al. Ewing sarcoma partial regression without GvHD by chondromodulin-I/HLA-A*02:01-specific allorestricted T cell receptor transgenic T cells. Oncoimmunology 6, e1312239 (2017).
Senzer, N. et al. Phase I trial of ‘bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell’ vaccine (FANG) in advanced cancer. Mol. Ther. 20, 679–686 (2012).
Machado, I., López-Guerrero, J. A., Scotlandi, K., Picci, P. & Llombart-Bosch, A. Immunohistochemical analysis and prognostic significance of PD-L1, PD-1, and CD8+ tumor-infiltrating lymphocytes in Ewing’s sarcoma family of tumors (ESFT). Virchows Arch. Int. J. Pathol. 472, 815–824 (2018).
Spurny, C. et al. Programmed cell death ligand 1 (PD-L1) expression is not a predominant feature in Ewing sarcomas. Pediatr. Blood Cancer 65, e26719 (2018).
Erkizan, H. V. et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma. Nat. Med. 15, 750–756 (2009). This paper describes a novel approach of targeting EWSR1–FLI1 by blocking its interaction with factors of its transcriptional complex.
Ferrari, S. et al. Ewing’s sarcoma of bone: relation between clinical characteristics and staging. Oncol. Rep. 8, 553–556 (2001).
Heinemann, M. et al. Recurrence of Ewing sarcoma: is detection by imaging follow-up protocol associated with survival advantage? Pediatr. Blood Cancer 65, e27011 (2018).
Raciborska, A. et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr. Blood Cancer 60, 1621–1625 (2013).
ISRCTN Registry. isrctn.com https://doi.org/10.1186/ISRCTN36453794 (2015).
Wagner, L. M. et al. Fractures in pediatric Ewing sarcoma. J. Pediatr. Hematol. Oncol. 23, 568–571 (2001).
Mirzaei, L., Kaal, S. E. J., Schreuder, H. W. B. & Bartels, R. H. M. A. The neurological compromised spine due to Ewing sarcoma. What first: surgery or chemotherapy? therapy, survival, and neurological outcome of 15 cases with primary Ewing sarcoma of the vertebral column. Neurosurgery 77, 718–725 (2015).
Ginsberg, J. P. et al. Long-term survivors of childhood Ewing sarcoma: report from the childhood cancer survivor study. J. Natl Cancer Inst. 102, 1272–1283 (2010).
Longhi, A. et al. Late effects of chemotherapy and radiotherapy in osteosarcoma and Ewing sarcoma patients: the Italian Sarcoma Group experience Cancer 118, 5050–5059 (2012). (1983–2006).
Marina, N. M. et al. Longitudinal follow-up of adult survivors of Ewing sarcoma: a report from the Childhood Cancer Survivor Study. Cancer 123, 2551–2560 (2017).
Ranft, A. et al. Quality of survivorship in a rare disease: clinicofunctional outcome and physical activity in an observational cohort study of 618 long-term survivors of Ewing sarcoma. J. Clin. Oncol. 35, 1704–1712 (2017).
Geier, B., Kurmashev, D., Kurmasheva, R. T. & Houghton, P. J. Preclinical childhood sarcoma models: drug efficacy biomarker identification and validation. Front. Oncol. 5, 193 (2015).
Stewart, E. et al. Targeting the DNA repair pathway in Ewing sarcoma. Cell Rep. 9, 829–841 (2014).
Wang, Y. X. et al. Inhibiting platelet-derived growth factor beta reduces Ewing’s sarcoma growth and metastasis in a novel orthotopic human xenograft model. In Vivo 23, 903–909 (2009).
Minas, T. Z. et al. Combined experience of six independent laboratories attempting to create an Ewing sarcoma mouse model. Oncotarget 8, 34141–34163 (2017).
Javaheri, T. et al. Increased survival and cell cycle progression pathways are required for EWS/FLI1-induced malignant transformation. Cell Death Dis. 7, e2419 (2016).
Leacock, S. W. et al. A zebrafish transgenic model of Ewing’s sarcoma reveals conserved mediators of EWS-FLI1 tumorigenesis. Dis. Model. Mech. 5, 95–106 (2012).
Lin, P. P. et al. EWS-FLI1 induces developmental abnormalities and accelerates sarcoma formation in a transgenic mouse model. Cancer Res. 68, 8968–8975 (2008).
Stewart, E. et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature 549, 96–100 (2017).
Ordóñez, J. L. et al. The PARP inhibitor olaparib enhances the sensitivity of Ewing sarcoma to trabectedin. Oncotarget 6, 18875–18890 (2015).
Berger, M. et al. Genomic EWS-FLI1 fusion sequences in Ewing sarcoma resemble breakpoint characteristics of immature lymphoid malignancies. PLOS ONE 8, e56408 (2013).
Krumbholz, M. et al. Genomic EWSR1 Fusion sequence as highly sensitive and dynamic plasma tumor marker in Ewing sarcoma. Clin. Cancer Res. 22, 4356–4365 (2016).
Schleiermacher, G. et al. Increased risk of systemic relapses associated with bone marrow micrometastasis and circulating tumor cells in localized ewing tumor. J. Clin. Oncol. 21, 85–91 (2003).
Tsugita, M. et al. Ewing sarcoma cells secrete EWS/Fli-1 fusion mRNA via microvesicles. PLOS ONE 8, e77416 (2013).
Dubois, S. G., Epling, C. L., Teague, J., Matthay, K. K. & Sinclair, E. Flow cytometric detection of Ewing sarcoma cells in peripheral blood and bone marrow. Pediatr. Blood Cancer 54, 13–18 (2010).
Medizinische Fakultât WWU Muenster. PROVABES Network https://www.medizin.uni-muenster.de/provabes/network/ (2018).
Arnaldez, F. I. & Helman, L. J. New strategies in ewing sarcoma: lost in translation? Clin. Cancer Res. 20, 3050–3056 (2014).
Zöllner, S. K. et al. Inhibition of the oncogenic fusion protein EWS-FLI1 causes G2-M cell cycle arrest and enhanced vincristine sensitivity in Ewing’s sarcoma. Sci. Signal. 10, eaam8429 (2017).
Theisen, E. R., Pishas, K. I., Saund, R. S. & Lessnick, S. L. Therapeutic opportunities in Ewing sarcoma: EWS-FLI inhibition via LSD1 targeting. Oncotarget 7, 17616–17630 (2016).
Sonnemann, J. et al. Histone deacetylase inhibitors induce cell death and enhance the apoptosis-inducing activity of TRAIL in Ewing’s sarcoma cells. J. Cancer Res. Clin. Oncol. 133, 847–858 (2007).
Hurtubise, A., Bernstein, M. L. & Momparler, R. L. Preclinical evaluation of the antineoplastic action of 5-aza-2′-deoxycytidine and different histone deacetylase inhibitors on human Ewing’s sarcoma cells. Cancer Cell. Int. 8, 16 (2008).
Engert, F., Schneider, C., Weiβ, L. M., Probst, M. & Fulda, S. PARP inhibitors sensitize Ewing sarcoma cells to temozolomide-induced apoptosis via the mitochondrial pathway. Mol. Cancer Ther. 14, 2818–2830 (2015).
Anderson, P. M. et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 63, 1761–1770 (2016).
Evans, C. H. et al. EWS-FLI-1-targeted cytotoxic T cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors. Clin. Cancer Res. 18, 5341–5351 (2012).
Antonescu, C. R. et al. Sarcomas with CIC-rearrangements are a distinct pathologic entity with aggressive outcome: a clinicopathologic and molecular study of 115 cases. Am. J. Surg. Pathol. 41, 941–949 (2017).
Gambarotti, M. et al. CIC-DUX4 fusion-positive round-cell sarcomas of soft tissue and bone: a single-institution morphological and molecular analysis of seven cases. Histopathology 69, 624–634 (2016).
Kao, Y.-C. et al. BCOR-CCNB3 fusion positive sarcomas: a clinicopathologic and molecular analysis of 36 cases with comparison to morphologic spectrum and clinical behavior of other round cell sarcomas. Am. J. Surg. Pathol. 42, 604–615 (2017).
Szuhai, K. et al. The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clin. Cancer Res. 15, 2259–2268 (2009).
Berghuis, D. et al. Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8+ T-lymphocyte infiltration and affect tumour progression. J. Pathol. 223, 347–357 (2011).
Volchenboum, S. L. et al. Gene expression profiling of ewing sarcoma tumors reveals the prognostic importance of tumor-stromal interactions: a report from the Children’s Oncology Group. J. Pathol. Clin. Res. 1, 83–94 (2015).
Fujiwara, T. et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am. J. Pathol. 179, 1157–1170 (2011).
Haeusler, J. et al. The value of local treatment in patients with primary, disseminated, multifocal Ewing sarcoma (PDMES). Cancer 116, 443–450 (2010).
Ferrari, S. et al. Nonmetastatic Ewing family tumors: high-dose chemotherapy with stem cell rescue in poor responder patients. Results of the Italian Sarcoma Group/Scandinavian Sarcoma Group III protocol. Ann. Oncol. 22, 1221–1227 (2011).
Jürgens, H. et al. [The German Society of Pediatric Oncology Cooperative Ewing Sarcoma Studies CESS 81/86: report after 6 1/2 years]. Klin. Padiatr. 200, 243–252 (1988).
Bacci, G. et al. Predictive factors of histological response to primary chemotherapy in Ewing’s sarcoma. Acta Oncol. Stockh. Swed. 37, 671–676 (1998).
Dirksen, U. et al. Efficacy of busulfan-melphalan high dose chemotherapy consolidation (BuMel) compared to conventional chemotherapy combined with lung irradiation in ewing sarcoma (ES) with primary lung metastases: results of EURO-EWING 99-R2pulm randomized trial (EE99R2pul). J. Clin. Oncol. 34, 11001–11001 (2016).
Bilke, S. et al. Oncogenic ETS fusions deregulate E2F3 target genes in Ewing sarcoma and prostate cancer. Genome Res. 23, 1797–1809 (2013).
Girnita, L. et al. A link between basic fibroblast growth factor (bFGF) and EWS/FLI-1 in Ewing’s sarcoma cells. Oncogene 19, 4298–4301 (2000).
Kadoch, C. & Crabtree, G. R. Mammalian SWI/SNF chromatin remodeling complexes and cancer: mechanistic insights gained from human genomics. Sci. Adv. 1, e1500447 (2015).
Huynh, K. D., Fischle, W., Verdin, E. & Bardwell, V. J. BCoR, a novel corepressor involved in BCL-6 repression. Genes Dev. 14, 1810–1823 (2000).
Pierron, G. et al. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat. Genet. 44, 461–466 (2012).
Pagan, J. K. et al. A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J. Biol. Chem. 282, 15248–15257 (2007).
Shi, J. & Vakoc, C. R. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol. Cell 54, 728–736 (2014).
Loganathan, S. N. et al. BET bromodomain inhibitors suppress EWS-FLI1-dependent transcription and the IGF1 autocrine mechanism in Ewing sarcoma. Oncotarget 7, 43504–43517 (2016).
Hensel, T. et al. Targeting the EWS-ETS transcriptional program by BET bromodomain inhibition in Ewing sarcoma. Oncotarget 7, 1451–1463 (2016).
Wood, A. J., Severson, A. F. & Meyer, B. J. Condensin and cohesin complexity: the expanding repertoire of functions. Nat. Rev. Genet. 11, 391–404 (2010).
Kim, K. H. & Roberts, C. W. M. Targeting EZH2 in cancer. Nat. Med. 22, 128–134 (2016).
Riggi, N. et al. EWS-FLI-1 expression triggers a Ewing’s sarcoma initiation program in primary human mesenchymal stem cells. Cancer Res. 68, 2176–2185 (2008).
Marques Howarth, M. et al. Long noncoding RNA EWSAT1-mediated gene repression facilitates Ewing sarcoma oncogenesis. J. Clin. Invest. 124, 5275–5290 (2014).
Bhan, A. & Mandal, S. S. LncRNA HOTAIR: a master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta 1856, 151–164 (2015).
Siddiqui, H. et al. HOTAIR primes the Ewing sarcoma family of tumors for tumorigenesis via epigenetic dysregulation involving LSD1. Preprint at bioRxiv 244558 https://doi.org/10.1101/244558 (2018).
Greer, E. L. & Shi, Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 13, 343–357 (2012).
Paigen, K. & Petkov, P. M. PRDM9 and its role in genetic recombination. Trends Genet. 34, 291–300 (2018).
Edmunds, J. W., Mahadevan, L. C. & Clayton, A. L. Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. EMBO J. 27, 406–420 (2008).
Yamane, K. et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 125, 483–495 (2006).
Sechler, M., Parrish, J. K., Birks, D. K. & Jedlicka, P. The histone demethylase KDM3A, and its downstream target MCAM, promote Ewing Sarcoma cell migration and metastasis. Oncogene 36, 4150–4160 (2017).
Lai, A. Y. & Wade, P. A. Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat. Rev. Cancer 11, 588–596 (2011).
Sankar, S. et al. Reversible LSD1 inhibition interferes with global EWS/ETS transcriptional activity and impedes Ewing sarcoma tumor growth. Clin. Cancer Res. 20, 4584–4597 (2014).
Li, X. J., Ren, Z. J. & Tang, J. H. MicroRNA-34a: a potential therapeutic target in human cancer. Cell Death Dis. 5, e1327 (2014).
Nakatani, F. et al. miR-34a predicts survival of Ewing’s sarcoma patients and directly influences cell chemo-sensitivity and malignancy. J. Pathol. 226, 796–805 (2012).
Xu, N., Papagiannakopoulos, T., Pan, G., Thomson, J. A. & Kosik, K. S. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 137, 647–658 (2009).
Ban, J. et al. Hsa-mir-145 is the top EWS-FLI1-repressed microRNA involved in a positive feedback loop in Ewing’s sarcoma. Oncogene 30, 2173–2180 (2011).
De Vito, C. et al. A TARBP2-dependent miRNA expression profile underlies cancer stem cell properties and provides candidate therapeutic reagents in Ewing sarcoma. Cancer Cell 21, 807–821 (2012).
Zhang, T. & Kraus, W. L. SIRT1-dependent regulation of chromatin and transcription: linking NAD(+) metabolism and signaling to the control of cellular functions. Biochim. Biophys. Acta 1804, 1666–1675 (2010).
Aranda, S., Mas, G. & Di Croce, L. Regulation of gene transcription by Polycomb proteins. Sci. Adv. 1, e1500737 (2015).
Douglas, D. et al. BMI-1 promotes ewing sarcoma tumorigenicity independent of CDKN2A repression. Cancer Res. 68, 6507–6515 (2008).
Ooi, L. & Wood, I. C. Chromatin crosstalk in development and disease: lessons from REST. Nat. Rev. Genet. 8, 544–554 (2007).
Zhou, Z., Yu, L. & Kleinerman, E. S. EWS-FLI-1 regulates the neuronal repressor gene REST, which controls Ewing sarcoma growth and vascular morphology. Cancer 120, 579–588 (2014).
Leung, J. W. C. et al. ZMYM3 regulates BRCA1 localization at damaged chromatin to promote DNA repair. Genes Dev. 31, 260–274 (2017).
Hu, X. et al. Gene knockout of Zmym3 in mice arrests spermatogenesis at meiotic metaphase with defects in spindle assembly checkpoint. Cell Death Dis. 8, e2910 (2017).
Le Loarer, F., Pissaloux, D., Coindre, J. M., Tirode, F. & Vince, D. R. Update on families of round cell sarcomas other than classical Ewing sarcomas. Surg. Pathol. Clin. 10, 587–620 (2017).
Indelicato, D. J. et al. Definitive radiotherapy for ewing tumors of extremities and pelvis: long-term disease control, limb function, and treatment toxicity. Int. J. Radiat. Oncol. Biol. Phys. 72, 871–877 (2008).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02063022 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03011528 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02306161 (2018).
Pappo, A. S. et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a Sarcoma Alliance for Research Through Collaboration study. Cancer 120, 2448–2456 (2014).
Becker, R. G. et al. What is the impact of local control in Ewing sarcoma: analysis of the first Brazilian collaborative study group - EWING1. BMC Cancer 17, 420 (2017).
Juergens, H. et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J. Clin. Oncol. 29, 4534–4540 (2011).
Ghisoli, M. et al. Pilot trial of FANG immunotherapy in Ewing’s sarcoma. Mol. Ther. 23, 1103–1109 (2015).
Merchant, M. S. et al. Adjuvant immunotherapy to improve outcome in high-risk pediatric sarcomas. Clin. Cancer Res. 22, 3182–3191 (2016).
Norris, R. E. et al. Phase 1 trial of ontuxizumab (MORAb-004) in children with relapsed or refractory solid tumors: A report from the Children’s Oncology Group Phase 1 Pilot Consortium (ADVL1213). Pediatr. Blood Cancer https://doi.org/10.1002/pbc.26944 (2018).
Bagatell, R. et al. Phase 1 trial of temsirolimus in combination with irinotecan and temozolomide in children, adolescents and young adults with relapsed or refractory solid tumors: a Children’s Oncology Group Study. Pediatr. Blood Cancer 61, 833–839 (2014).
Acknowledgements
T.G.P.G. is supported by grants from the Wilhelm Sander Foundation (2016.167.1), German Cancer Aid (DKH-111886 and DKH-70112257) and the Deutsche Forschungsgemeinschaft (DFG 391665916). F.C.-A. is supported by a grant from the Barbara and Hubertus Trettner Foundation. E.M.T. is supported by a fellowship from the Austrian Science Fund (FWF; Elise Richter Fellowship V506-B28). E.d.A. is supported by grants from the Spanish Ministry of Economy-FEDER (PI17/00464, CB16/12/00361), the Asociación Pablo Ugarte and the María García-Estrada Foundation. U.D. is supported by grants from the German Cancer Aid (DKH108128 and DKH 70112018); ERA-Net-TRANSCAN (01KT1310), EEC, EU-FP7 602856, Trettner Foundation and Stiftung für krebskranke Kinder in Essen. The authors express their apologies to all authors whose valuable work could not be cited owing to restrictions in the number of references.
Reviewer information
Nature Reviews Disease Primers thanks S. DuBois, A. Llombart-Bosch, K. Scotlandi and other anonymous referee(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (T.G.P.G. and F.C.-A.); Epidemiology (T.G.P.G., F.C.-A. and O.D.); Mechanisms/pathophysiology (T.G.P.G., F.C.-A., D.S., E.M.T., E.d.A., H.K., O.D. and P.H.S.); Diagnosis, screening and prevention (T.G.P.G., F.C.-A., E.d.A., P.H.S. and U.D.); Management (T.G.P.G., F.C.-A. and U.D.); Quality of life (T.G.P.G., F.C.-A. and U.D.); Outlook (T.G.P.G., F.C.-A., D.S., E.M.T. and U.D.); Overview of the Primer (T.G.P.G. and F.C.-A.). T.G.P.G. and F.C.-A. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NCI Pediatric Preclinical Testing Consortium: http://www.ncipptc.org/
SARC Clinical trials: https://www.clinicaltrials.gov/
Rights and permissions
About this article
Cite this article
Grünewald, T.G.P., Cidre-Aranaz, F., Surdez, D. et al. Ewing sarcoma. Nat Rev Dis Primers 4, 5 (2018). https://doi.org/10.1038/s41572-018-0003-x
Published:
DOI: https://doi.org/10.1038/s41572-018-0003-x
This article is cited by
-
AURKA inhibition induces Ewing’s sarcoma apoptosis and ferroptosis through NPM1/YAP1 axis
Cell Death & Disease (2024)
-
Effective combination of cold physical plasma and chemotherapy against Ewing sarcoma cells in vitro
Scientific Reports (2024)
-
The DNA/RNA helicase DHX9 orchestrates the KDM2B-mediated transcriptional regulation of YAP1 in Ewing sarcoma
Oncogene (2024)
-
DisP-seq reveals the genome-wide functional organization of DNA-associated disordered proteins
Nature Biotechnology (2024)
-
Hypoxia and HIFs in Ewing sarcoma: new perspectives on a multi-facetted relationship
Molecular Cancer (2023)