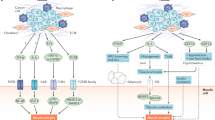
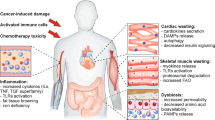
Abstract
Cachexia is a devastating, multifactorial and often irreversible systemic syndrome characterized by substantial weight loss (mainly of skeletal muscle and adipose tissue) that occurs in around 50–80% of patients with cancer. Although this condition mainly affects skeletal muscle (which accounts for approximately 40% of total body weight), cachexia is a multi-organ syndrome that also involves white and brown adipose tissue, and organs including the bones, brain, liver, gut and heart. Notably, cachexia accounts for up to 20% of cancer-related deaths. Cancer-associated cachexia is invariably associated with systemic inflammation, anorexia and increased energy expenditure. Understanding these mechanisms is essential, and the progress achieved in this area over the past decade could help to develop new therapeutic approaches. In this Review, we examine the currently available evidence on the roles of both the tumour macroenvironment and microenvironment in cancer-associated cachexia, and provide an overview of the novel therapeutic strategies developed to manage this syndrome.
Key points
-
Cachexia is a wasting syndrome characterized by weight loss (both skeletal muscle and adipose tissue), anorexia and inflammation that commonly occurs in patients with cancer and contributes substantially to cancer-related mortality.
-
Both the macroenvironment and the microenvironment contribute to the inflammatory process that characterizes cancer-associated cachexia; new therapeutic approaches must effectively target these mechanisms.
-
International guidelines for the management of patients with cancer-associated cachexia strongly recommend promoting anabolism and reducing catabolism through nutritional, physical and pharmacological interventions.
-
The development of new therapeutic approaches to the prevention of low skeletal muscle mass in patients with cancer-associated cachexia is an unmet need that can be addressed through multidisciplinary collaborations involving, among others, oncologists, radiologists, surgeons, nutritionists, dietitians and physiotherapists.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
European Commission. European Cancer Information System: 21% increase in new cancer cases by 2040. EU Science Hub https://joint-research-centre.ec.europa.eu/jrc-news/european-cancer-information-system-21-increase-new-cancer-cases-2040-2022-03-16_en (2022).
Argilés, J. M., Busquets, S., Stemmler, B. & López-Soriano, F. J. Cancer cachexia: understanding the molecular basis. Nat. Rev. Cancer 14, 754–762 (2014).
Bossi, P., Delrio, P., Mascheroni, A. & Zanetti, M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients 13, 1980 (2021).
Dewys, W. D. et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am. J. Med. 69, 491–497 (1980).
Teunissen, S. C. C. M. et al. Symptom prevalence in patients with incurable cancer: a systematic review. J. Pain. Symptom Manag. 34, 94–104 (2007).
Evans, W. J. et al. Cachexia: a new definition. Clin. Nutr. 27, 793–799 (2008).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Argilés, J. M. et al. Consensus on cachexia definitions. J. Am. Med. Dir. Assoc. 11, 229–230 (2010).
Argilés, J. M., Stemmler, B., López-Soriano, F. J. & Busquets, S. Inter-tissue communication in cancer cachexia. Nat. Rev. Endocrinol. 15, 9–20 (2018).
Strasser, F. & Bruera, E. D. Update on anorexia and cachexia. Hematol. Oncol. Clin. North. Am. 16, 589–617 (2002).
Maltoni, M. et al. Successful validation of the palliative prognostic score in terminally ill cancer patients. J. Pain. Symptom Manag. 17, 240–247 (1999).
Poisson, J. et al. Prevalence and prognostic impact of cachexia among older patients with cancer: a nationwide cross-sectional survey (NutriAgeCancer). J. Cachexia Sarcopenia Muscle 12, 1477–1488 (2021).
von Haehling, S., Anker, M. S. & Anker, S. D. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J. Cachexia Sarcopenia Muscle 7, 507–509 (2016).
Martin, L. et al. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 33, 90–99 (2014).
Zhou, T. et al. Development and validation of a clinically applicable score to classify cachexia stages in advanced cancer patients. J. Cachexia Sarcopenia Muscle 9, 306–314 (2018).
Fu, X. et al. Comparing SARC-F with SARC-CalF for screening sarcopenia in advanced cancer patients. Clin. Nutr. 39, 3337–3345 (2020).
Arends, J. et al. Cancer cachexia in adult patients: ESMO clinical practice guidelines. ESMO Open. 6, i00092 (2021).
Cederholm, T. et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin. Nutr. 38, 1–9 (2019).
Molfino, A., Imbimbo, G. & Laviano, A. Current screening methods for the risk or presence of malnutrition in cancer patients. Cancer Manag. Res. 14, 561–567 (2022).
Argilés, J. M. et al. The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J. Cachexia Sarcopenia Muscle 2, 87–93 (2011).
Argilés, J. M. et al. Validation of the CAchexia SCOre (CASCO). Staging cancer patients: the use of miniCASCO as a simplified tool. Front. Physiol. 8, 92 (2017).
Argilés, J. M. The 2015 ESPEN Sir David Cuthbertson lecture: Inflammation as the driving force of muscle wasting in cancer. Clin. Nutr. 36, 798–803 (2017).
Zhang, Q. et al. Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study. J. Cachexia Sarcopenia Muscle 12, 1466–1476 (2021).
Maurel, D. B. et al. Muscle-bone crosstalk: emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicines 5, 62 (2017).
Argilés, J. M., Busquets, S. & López-Soriano, F. J. Cancer cachexia, a clinical challenge. Curr. Opin. Oncol. 31, 286–290 (2019).
Petruzzelli, M. & Wagner, E. F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes. Dev. 30, 489–501 (2016).
Yeom, E. & Yu, K. Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp. Mol. Med. 54, 426–432 (2022).
Li, W. G. et al. Ghrelin inhibits proinflammatory responses and nuclear factor-κB activation in human endothelial cells. Circulation 109, 2221–2226 (2004).
Nagaya, N. et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 110, 3674–3679 (2004).
Tschöp, M., Smiley, D. L. & Heiman, M. L. Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000).
Mano-Otagiri, A. et al. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regul. Pept. 160, 81–90 (2010).
Argilés, J. M. & Stemmler, B. The potential of ghrelin in the treatment of cancer cachexia. Expert. Opin. Biol. Ther. 13, 67–76 (2013).
Joshi, M. & Patel, B. M. The burning furnace: alteration in lipid metabolism in cancer-associated cachexia. Mol. Cell Biochem. 477, 1709–1723 (2022).
Kir, S. et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513, 100–104 (2014).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Xiao, J. et al. The association of medical and demographic characteristics with sarcopenia and low muscle radiodensity in patients with nonmetastatic colorectal cancer. Am. J. Clin. Nutr. 109, 626–634 (2019).
van Amsterdam, W. A. C. et al. The association between muscle quantity and overall survival depends on muscle radiodensity: a cohort study in non-small-cell lung cancer patients. J. Pers. Med. 12, 1191 (2022).
Argilés, J. M., López-Soriano, F. J. & Busquets, S. Muscle wasting in cancer: the role of mitochondria. Curr. Opin. Clin. Nutr. Metab. Care 18, 221–225 (2015).
Barreto, R. et al. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front. Physiol. 7, 472 (2016).
van der Ende, M. et al. Mitochondrial dynamics in cancer-induced cachexia. Biochim. Biophys. Acta Rev. Cancer 1870, 137–150 (2018).
Penna, F. et al. Autophagy exacerbates muscle wasting in cancer cachexia and impairs mitochondrial function. J. Mol. Biol. 431, 2674–2686 (2019).
Molinari, F. et al. The mitochondrial metabolic reprogramming agent trimetazidine as an ‘exercise mimetic’ in cachectic C26-bearing mice. J. Cachexia Sarcopenia Muscle 8, 954–973 (2017).
Ballarò, R. et al. Targeting mitochondria by SS-31 ameliorates the whole body energy status in cancer- and chemotherapy-induced cachexia. Cancers 13, 850 (2021).
Pin, F., Huot, J. R. & Bonetto, A. The mitochondria-targeting agent MitoQ improves muscle atrophy, weakness and oxidative metabolism in C26 tumor-bearing mice. Front. Cell Dev. Biol. 10, 861622 (2022).
Dudek, J. Role of cardiolipin in mitochondrial signaling pathways. Front. Cell Dev. Biol. 5, 90 (2017).
Montero-Bullon, J. F. et al. Exercise training counteracts urothelial carcinoma-induced alterations in skeletal muscle mitochondria phospholipidome in an animal model. Sci. Rep. 9, 13423 (2019).
de Castro, G. S. et al. Human cachexia induces changes in mitochondria, autophagy and apoptosis in the skeletal muscle. Cancers 11, 1264 (2019).
Lee, C. M. & Kang, J. Prognostic impact of myosteatosis in patients with colorectal cancer: a systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 11, 1270–1282 (2020).
Aleixo, G. F. P. et al. Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 145, 1028390 (2020).
Pötgens, S. A. et al. Multi-compartment metabolomics and metagenomics reveal major hepatic and intestinal disturbances in cancer cachectic mice. J. Cachexia Sarcopenia Muscle 12, 456–475 (2021).
Pötgens, S. A. et al. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci. Rep. 8, 12321 (2018).
Ubachs, J. et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J. Cachexia Sarcopenia Muscle 12, 2007–2021 (2021).
Bindels, L. B. et al. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 10, 1456–1470 (2016).
Argilés, J. M., Fontes-Oliveira, C. C., Toledo, M., López-Soriano, F. J. & Busquets, S. Cachexia: a problem of energetic inefficiency. J. Cachexia Sarcopenia Muscle 5, 279–286 (2014).
Beck, S. A. & Tisdale, M. J. Effect of cancer cachexia on triacylglycerol/fatty acid substrate cycling in white adipose tissue. Lipids 39, 1187–1189 (2004).
Fontes-Oliveira, C. C. et al. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim. Biophys. Acta Gen. Subj. 1830, 2770–2778 (2013).
Jouinot, A. et al. Hypermetabolism is an independent prognostic factor of survival in metastatic non-small cell lung cancer patients. Clin. Nutr. 39, 1893–1899 (2020).
Vazeille, C. et al. Relation between hypermetabolism, cachexia, and survival in cancer patients: a prospective study in 390 cancer patients before initiation of anticancer therapy. Am. J. Clin. Nutr. 105, 1139–1147 (2017).
Barcellos, P. S., Borges, N. & Torres, D. P. M. Resting energy expenditure in cancer patients: agreement between predictive equations and indirect calorimetry. Clin. Nutr. ESPEN 42, 286–291 (2021).
Argilés, J. M., López-Soriano, F. J. & Busquets, S. Mediators of cachexia in cancer patients. Nutrition 66, 11–15 (2019).
Devine, R. D., Bicer, S., Reiser, P. J., Velten, M. & Wold, L. E. Metalloproteinase expression is altered in cardiac and skeletal muscle in cancer cachexia. Am. J. Physiol. Heart Circ. Physiol. 309, H685–H691 (2015).
Olson, B. et al. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat. Commun. 12, 2057 (2021).
Suzuki, H. et al. Clinical and tumor characteristics of patients with high serum levels of growth differentiation factor 15 in advanced pancreatic cancer. Cancers 13, 4842 (2021).
Prado, B. L. & Qian, Y. Anti-cytokines in the treatment of cancer cachexia. Ann. Palliat. Med. 8, 67–79 (2019).
Ando, K. et al. Tocilizumab, a proposed therapy for the cachexia of interleukin6-expressing lung cancer. PLoS ONE 9, e102436 (2014).
Jatoi, A. et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 68, 234–239 (2010).
Chasen, M., Hirschman, S. Z. & Bhargava, R. Phase II study of the novel peptide-nucleic acid OHR118 in the management of cancer-related anorexia/cachexia. J. Am. Med. Dir. Assoc. 12, 62–67 (2011).
Todorov, P. T., Wyke, S. M. & Tisdale, M. J. Identification and characterization of a membrane receptor for proteolysis-inducing factor on skeletal muscle. Cancer Res. 67, 11419–11427 (2007).
Russell, S. T., Zimmerman, T. P., Domin, B. A. & Tisdale, M. J. Induction of lipolysis in vitro and loss of body fat in vivo by zinc-α2-glycoprotein. Biochim. Biophys. Acta 1636, 59–68 (2004).
Kandarian, S. C. et al. Tumour-derived leukaemia inhibitory factor is a major driver of cancer cachexia and morbidity in C26 tumour-bearing mice. J. Cachexia Sarcopenia Muscle 9, 1109–1120 (2018).
Song, W. et al. Tumor-derived ligands trigger tumor growth and host wasting via differential MEK activation. Dev. Cell 48, 277–286.e6 (2019).
Ding, G. et al. Coordination of tumor growth and host wasting by tumor-derived Upd3. Cell Rep. 36, 109553 (2021).
de Castro, G. S. et al. Myokines in treatment-naïve patients with cancer-associated cachexia. Clin. Nutr. 40, 2443–2455 (2021).
Pritt, M. I. et al. Fabp3 as a biomarker of skeletal muscle toxicity in the rat: comparison with conventional biomarkers. Toxicol. Sci. 103, 382–396 (2008).
Freire, P. P. et al. The expression landscape of cachexia-inducing factors in human cancers. J. Cachexia Sarcopenia Muscle 11, 947–961 (2020).
Chitti, S. V., Fonseka, P. & Mathivanan, S. Emerging role of extracellular vesicles in mediating cancer cachexia. Biochem. Soc. Trans. 46, 1129–1136 (2018).
Pin, F. et al. Extracellular vesicles derived from tumour cells as a trigger of energy crisis in the skeletal muscle. J. Cachexia Sarcopenia Muscle 13, 481–494 (2022).
Zhang, G. et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 8, 589 (2017).
Vu, L. T., Gong, J., Pham, T. T., Kim, Y. & Le, M. T. N. microRNA exchange via extracellular vesicles in cancer. Cell Prolif. 53, e12877 (2020).
Wu, Q. et al. Tumour-originated exosomal miR-155 triggers cancer-associated cachexia to promote tumour progression. Mol. Cancer 17, 155 (2018).
Fan, M. et al. The critical role of STAT3 in biogenesis of tumor-derived exosomes with potency of inducing cancer cachexia in vitro and in vivo. Oncogene 41, 1050–1062 (2022).
Narasinhan, A. et al. Small RNAome profiling from human skeletal muscle: novel miRNAs and their targets associated with cancer cachexia. J. Cachexia Sarcopenia Muscle 8, 405–416 (2017).
He, W. A. et al. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl Acad. Sci. USA 111, 4525–4529 (2014).
Wu, Q. et al. Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumour progression. Adipocyte 8, 31–45 (2018).
Gao, X. et al. Extracellular vesicles derived from oesophageal cancer containing P4HB promote muscle wasting via regulating PHGDH/Bcl-2/caspase-3 pathway. J. Extracell. Vesicles 10, e12060 (2021).
Hu, W. et al. Lung cancer-derived extracellular vesicles induced myotube atrophy and adipocyte lipolysis via the extracellular IL-6-mediated STAT3 pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 1091–1102 (2019).
Zhou, L. et al. Amiloride ameliorates muscle wasting in cancer cachexia through inhibiting tumor-derived exosome release. Skelet. Muscle 11, 17 (2021).
Kasprzak, A. The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int. J. Mol. Sci. 22, 1565 (2021).
VanderVeen, B. N., Murphy, E. A. & Carson, J. A. The impact of immune cells on the skeletal muscle microenvironment during cancer cachexia. Front. Physiol. 11, 1037 (2020).
Yuan, C. et al. Prediagnostic inflammation and pancreatic cancer survival. J. Natl Cancer Inst. 113, 1186–1193 (2021).
Dolan, R. D., Lim, J., McSorley, S. T., Horgan, P. G. & McMillan, D. C. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci. Rep. 7, 16717 (2017).
Xiong, Y. et al. Hematopoietic stem cell-derived adipocytes and fibroblasts in the tumor microenvironment. World J. Stem Cell 7, 253–265 (2015).
Masucci, M. T., Minopoli, M. & Carriero, M. V. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front. Oncol. 9, 1146 (2019).
Cuenca, A. G. et al. Novel role for tumor-induced expansion of myeloid-derived cells in cancer cachexia. J. Immunol. 192, 6111–6119 (2014).
Arends, J. et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 36, 1187–1196 (2017).
Mavropalias, G. et al. Exercise medicine for cancer cachexia: targeted exercise to counteract mechanisms and treatment side effects. J. Cancer Res. Clin. Oncol. 148, 1389–1406 (2022).
Allan, J., Buss, L. A., Draper, N. & Currie, M. J. Exercise in people with cancer: a spotlight on energy regulation and cachexia. Front. Physiol. 13, 836804 (2022).
Mangano, G. D., Fouani, M., D’amico, D., di Felice, V. & Barone, R. Cancer-related cachexia: the vicious circle between inflammatory cytokines, skeletal muscle, lipid metabolism and the possible role of physical training. Int. J. Mol. Sci. 23, 3004 (2022).
Daou, H. Exercise as an anti-inflammatory therapy for cancer cachexia: a focus on interleukin-6 regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R296–R310 (2020).
Cereda, E. et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother. Oncol. 126, 81–88 (2018).
Gomes, F. et al. Association of nutritional support with clinical outcomes among medical inpatients who are malnourished or at nutritional risk: an updated systematic review and meta-analysis. JAMA Netw. Open. 2, e1915138 (2019).
Gioulbasanis, I. et al. Nutritional assessment in overweight and obese patients with metastatic cancer: does it make sense? Ann. Oncol. 26, 217–221 (2015).
Blackwood, H. A. et al. A systematic review examining nutrition support interventions in patients with incurable cancer. Support. Care Cancer 28, 1877–1889 (2020).
Ravasco, P., Monteiro-Grillo, I., Vidal, P. M. & Camilo, M. E. Dietary counseling improves patient outcomes: a prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 23, 1431–1438 (2005).
van der Werf, A. et al. The effect of nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: a randomized controlled trial. Clin. Nutr. 39, 3005–3013 (2020).
Ravasco, P. Nutrition in cancer patients. J. Clin. Med. 8, 1211 (2019).
Orsso, C. E. et al. Mapping ongoing nutrition intervention trials in muscle, sarcopenia, and cachexia: a scoping review of future research. J. Cachexia Sarcopenia Muscle 13, 1442–1459 (2022).
Muscaritoli, M. et al. ESPEN practical guideline: clinical nutrition in cancer. Clin. Nutr. 40, 2898–2913 (2021).
Murphy, R. A. et al. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 117, 1775–1782 (2011).
Wei, L., Wu, Z. & Chen, Y. Q. Multi-targeted therapy of cancer by omega-3 fatty acids–an update. Cancer Lett. 526, 193–204 (2022).
de van der Schueren, M. A. E. et al. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann. Oncol. 29, 1141–1153 (2018).
Aredes, M. A. et al. Efficacy of ω-3 supplementation on nutritional status, skeletal muscle, and chemoradiotherapy toxicity in cervical cancer patients: a randomized, triple-blind, clinical trial conducted in a middle-income country. Nutrition 67–68, 110528 (2019).
Busquets, S. et al. L-Carnitine: an adequate supplement for a multi-targeted anti-wasting therapy in cancer. Clin. Nutr. 31, 889–895 (2012).
Wu, C. et al. L-carnitine ameliorates the muscle wasting of cancer cachexia through the AKT/FOXO3a/MaFbx axis. Nutr. Metab. 18, 98 (2021).
Esfahani, M., Sahafi, S., Derakhshandeh, A. & Moghaddas, A. The anti-wasting effects of L-carnitine supplementation on cancer: experimental data and clinical studies. Asia Pac. J. Clin. Nutr. 27, 503–511 (2018).
Kraft, M. et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)–a randomized multicentre trial. Nutr. J. 11, 52 (2012).
Pascoe, J. et al. Beta-hydroxy beta-methylbutyrate/arginine/glutamine (HMB/Arg/Gln) supplementation to improve the management of cachexia in patients with advanced lung cancer: an open-label, multicentre, randomised, controlled phase II trial (NOURISH). BMC Cancer 21, 800 (2021).
Prado, C. M., Orsso, C. E., Pereira, S. L., Atherton, P. J. & Deutz, N. E. P. Effects of β-hydroxy β-methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: a systematic review. J. Cachexia Sarcopenia Muscle 13, 1623–1641 (2022).
Wyart, E. et al. Iron supplementation is sufficient to rescue skeletal muscle mass and function in cancer cachexia. EMBO Rep. 23, e53746 (2022).
van Dijk, D. P. J. et al. Effects of oral meal feeding on whole body protein breakdown and protein synthesis in cachectic pancreatic cancer patients. J. Cachexia Sarcopenia Muscle 6, 212–221 (2015).
Engelen, M. P. K. J., Safar, A. M., Bartter, T., Koeman, F. & Deutz, N. E. P. High anabolic potential of essential amino acid mixtures in advanced nonsmall cell lung cancer. Ann. Oncol. 26, 1960–1966 (2015).
Lira, F. S., Antunes, B., de, M. M., Seelaender, M. & Neto, J. C. R. The therapeutic potential of exercise to treat cachexia. Curr. Opin. Support. Palliat. Care 9, 317–324 (2015).
Wood, L. J. et al. Does muscle-derived interleukin-6 mediate some of the beneficial effects of exercise on cancer treatment-related fatigue? Oncol. Nurs. Forum 36, 519–524 (2009).
Alves, C. R. R., da Cunha, T. F., da Paixão, N. A. & Brum, P. C. Aerobic exercise training as therapy for cardiac and cancer cachexia. Life Sci. 125, 9–14 (2015).
Lønbro, S. et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy – results from the randomized DAHANCA 25B trial. Radiother. Oncol. 108, 314–319 (2013).
Peddle-McIntyre, C. J., Bell, G., Fenton, D., McCargar, L. & Courneya, K. S. Feasibility and preliminary efficacy of progressive resistance exercise training in lung cancer survivors. Lung Cancer 75, 126–132 (2012).
Segura, A. et al. An epidemiological evaluation of the prevalence of malnutrition in Spanish patients with locally advanced or metastatic cancer. Clin. Nutr. 24, 801–814 (2005).
Argilés, J. M., Anguera, A. & Stemmler, B. A new look at an old drug for the treatment of cancer cachexia: megestrol acetate. Clin. Nutr. 32, 319–324 (2013).
Bruera, E., Macmillan, K., Kuehn, N., Hanson, J. & MacDonald, R. N. A controlled trial of megestrol acetate on appetite, caloric intake, nutritional status, and other symptoms in patients with advanced cancer. Cancer 66, 1279–1282 (1990).
Busquets, S. et al. Megestrol acetate: its impact on muscle protein metabolism supports its use in cancer cachexia. Clin. Nutr. 29, 733–737 (2010).
Simon, L., Baldwin, C., Kalea, A. Z. & Slee, A. Cannabinoid interventions for improving cachexia outcomes in cancer: a systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 13, 23–41 (2022).
Garcia, J. M. et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 16, 108–116 (2015).
Temel, J. S. et al. Anamorelin in patients with advanced non-small cell lung cancer and cachexia: results from the phase III studies ROMANA 1 and 2. J. Clin. Oncol. 33, 175–175 (2015).
Temel, J. S. et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 17, 519–531 (2016).
Hamauchi, S. et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 125, 4294–4302 (2019).
Wakabayashi, H., Arai, H. & Inui, A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J. Cachexia Sarcopenia Muscle 12, 14–16 (2021).
Naito, T. et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in patients with cancer cachexia and low body mass index. Cancer 128, 2025–2035 (2022).
Yennurajalingam, S. et al. Anamorelin combined with physical activity, and nutritional counseling for cancer-related fatigue: a preliminary study. Support. Care Cancer 30, 497–509 (2022).
Argilés, J. M., López-Soriano, F. J., Stemmler, B. & Busquets, S. Therapeutic strategies against cancer cachexia. Eur. J. Transl. Myol. 29, 4–13 (2019).
Bayliss, T. J., Smith, J. T., Schuster, M., Dragnev, K. H. & Rigas, J. R. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert. Opin. Biol. Ther. 11, 1663–1668 (2011).
Mesa, R. A. et al. Effects of ruxolitinib treatment on metabolic and nutritional parameters in patients with myelofibrosis from COMFORT-I. Clin. Lymphoma Myeloma Leuk. 15, 214–221.e1 (2015).
Figueras, M. et al. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett. 569, 201–206 (2004).
Argilés, J. M., López-Soriano, F. J. & Busquets, S. Therapeutic potential of interleukin-15: a myokine involved in muscle wasting and adiposity. Drug. Discov. Today 14, 208–213 (2009).
Argilés, J. M., Busquets, S. & López-Soriano, F. J. The role of uncoupling proteins in pathophysiological states. Biochem. Biophys. Res. Commun. 293, 1145–1152 (2002).
Busquets, S. et al. Interleukin-15 decreases proteolysis in skeletal muscle: a direct effect. Int. J. Mol. Med. 16, 471–476 (2005).
Carbó, N. et al. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br. J. Cancer 83, 526–531 (2020).
Martínez-Hernández, P. L. et al. Serum interleukin-15 levels in cancer patients with cachexia. Oncol. Rep. 28, 1443–1452 (2012).
Reid, J., Hughes, C. M., Murray, L. J., Parsons, C. & Cantwell, M. M. Non-steroidal anti-inflammatory drugs for the treatment of cancer cachexia: a systematic review. Palliat. Med 27, 295–303 (2013).
Lainscak, M. & Laviano, A. ACT-ONE - ACTION at last on cancer cachexia by adapting a novel action beta-blocker. J. Cachexia Sarcopenia Muscle 7, 400–402 (2016).
Stewart Coats, A. J. et al. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non-small cell lung cancer or colorectal cancer: a randomized, double-blind, placebo-controlled, international multicentre phase II study (the ACT-ONE trial). J. Cachexia Sarcopenia Muscle 7, 355–365 (2016).
Toledo, M. et al. Formoterol in the treatment of experimental cancer cachexia: effects on heart function. J. Cachexia Sarcopenia Muscle 5, 315–320 (2014).
Busquets, S. et al. Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res. 64, 6725–6731 (2004).
Wang, Q. & McPherron, A. C. Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cell activation. J. Physiol. 590, 2151–2165 (2012).
Smith, R. C. & Lin, B. K. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr. Opin. Support. Palliat. Care 7, 352–360 (2013).
Toledo, M. et al. Complete reversal of muscle wasting in experimental cancer cachexia: additive effects of activin type II receptor inhibition and β-2 agonist. Int. J. Cancer 138, 2021–2029 (2016).
Greig, C. A. et al. Phase I/II trial of formoterol fumarate combined with megestrol acetate in cachectic patients with advanced malignancy. Support. Care Cancer 22, 1269–1275 (2014).
Morimoto, M., Aikawa, K., Hara, T. & Yamaoka, M. Prevention of body weight loss and sarcopenia by a novel selective androgen receptor modulator in cancer cachexia models. Oncol. Lett. 14, 8066–8071 (2017).
Dobs, A. S. et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 14, 335–345 (2013).
Crawford, J. et al. Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER trials). Curr. Oncol. Rep. 18, 37 (2016).
Crawford, J., Johnston, M. A., Taylor, R. P., Dalton, J. T. & Steiner, M. S. Enobosarm and lean body mass in patients with non-small cell lung cancer [abstract]. J. Clin. Oncol. 32 (Suppl. 15), 9618 (2014).
Amato, A. A. et al. Efficacy and safety of bimagrumab in sporadic inclusion body myositis: long-term extension of RESILIENT. Neurology 96, E1595–E1607 (2021).
Lach-Trifilieff, E. et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol. Cell Biol. 34, 606–618 (2014).
Golan, T. et al. LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J. Cachexia Sarcopenia Muscle 9, 871–879 (2018).
Prado, C. M. M. et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br. J. Cancer 106, 1583–1586 (2012).
Finn, R. S. et al. Phase 1b investigation of the MEK inhibitor binimetinib in patients with advanced or metastatic biliary tract cancer. Invest. New Drugs 36, 1037–1043 (2018).
Wang, Q. et al. Combined treatment of carfilzomib and z-VAD-fmk inhibits skeletal proteolysis and apoptosis and ameliorates cancer cachexia. Med. Oncol. 32, 100 (2015).
Penna, F. et al. Effect of the specific proteasome inhibitor bortezomib on cancer-related muscle wasting. J. Cachexia Sarcopenia Muscle 7, 345–354 (2016).
Murphy, K. T., Chee, A., Trieu, J., Naim, T. & Lynch, G. S. Inhibition of the renin-angiotensin system improves physiological outcomes in mice with mild or severe cancer cachexia. Int. J. Cancer 133, 1234–1246 (2013).
di Felice, V. et al. Physiactisome: a new nanovesicle drug containing heat shock protein 60 for treating muscle wasting and cachexia. Cells 11, 1406 (2022).
Palus, S. et al. The erythropoietin-derived peptide ARA 284 reduces tissue wasting and improves survival in a rat model of cancer cachexia. J. Cachexia Sarcopenia Muscle 13, 2202–2210 (2022).
Pin, F. et al. Combination of exercise training and erythropoietin prevents cancer-induced muscle alterations. Oncotarget 6, 43202–43215 (2014).
Johnen, H. et al. Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nat. Med. 13, 1333–1340 (2007).
Rohm, M. & Herzig, S. An antibody attack against body wasting in cancer. Cell Metab. 32, 331–333 (2020).
Mullican, S. E. et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 23, 1150–1157 (2017).
Suriben, R. et al. Antibody-mediated inhibition of GDF15–GFRAL activity reverses cancer cachexia in mice. Nat. Med. 26, 1264–1270 (2020).
Hong, D. S. et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 15, 656–666 (2014).
Muscaritoli, M., Molfino, A., Lucia, S. & Rossi Fanelli, F. Cachexia: a preventable comorbidity of cancer. A T.A.R.G.E.T. approach. Crit. Rev. Oncol. Hematol. 94, 251–259 (2015).
Maeng, C. H. et al. Effect of multimodal intervention care on cachexia in patients with advanced cancer compared to conventional management (MIRACLE): an open-label, parallel, randomized, phase 2 trial. Trials 23, 281 (2022).
Khorasanchi, A., Nemani, S., Pandey, S. & del Fabbro, E. Managing nutrition impact symptoms in cancer cachexia: a case series and mini review. Front. Nutr. 9, 831934 (2022).
Cavaliere, R. & Schiff, D. Neurologic toxicities of cancer therapies. Curr. Neurol. Neurosci. Rep. 6, 218–226 (2006).
Newton, H. B. Neurological complications of chemotherapy to the central nervous system. Handb. Clin. Neurol. 105, 903–916 (2012).
Hain, B. A., Xu, H. & Waning, D. L. Loss of REDD1 prevents chemotherapy-induced muscle atrophy and weakness in mice. J. Cachexia Sarcopenia Muscle 12, 1597–1612 (2021).
Zhidkova, E. M. et al. Nutritional sensor REDD1 in cancer and inflammation: friend or foe? Int. J. Mol. Sci. 23, 9686 (2022).
Huot, J. R., Pin, F. & Bonetto, A. Muscle weakness caused by cancer and chemotherapy is associated with loss of motor unit connectivity. Am. J. Cancer Res. 11, 2990–3001 (2021).
Mora, S. & Adegoke, O. A. J. The effect of a chemotherapy drug cocktail on myotube morphology, myofibrillar protein abundance, and substrate availability. Physiol. Rep. 9, e14927 (2021).
Gilliam, L. A. A. et al. Doxorubicin acts via mitochondrial ROS to stimulate catabolism in C2C12 myotubes. Am. J. Physiol. Cell Physiol. 302, C195–C202 (2012).
Rybalka, E. et al. Chemotherapeutic agents induce mitochondrial superoxide production and toxicity but do not alter respiration in skeletal muscle in vitro. Mitochondrion 42, 33–49 (2018).
Beltrà, M., Pin, F., Ballarò, R., Costelli, P. & Penna, F. Mitochondrial dysfunction in cancer cachexia: impact on muscle health and regeneration. Cells 10, 3150 (2021).
Guigni, B. A. et al. Skeletal muscle atrophy and dysfunction in breast cancer patients: role for chemotherapy-derived oxidant stress. Am. J. Physiol. Cell Physiol. 315, C744–C756 (2018).
Mallard, J. et al. Chemotherapy impairs skeletal muscle mitochondrial homeostasis in early breast cancer patients. J. Cachexia Sarcopenia Muscle 13, 1896–1907 (2022).
Sorensen, J. C. et al. Mitochondria: inadvertent targets in chemotherapy-induced skeletal muscle toxicity and wasting? Cancer Chemother. Pharmacol. 78, 673–683 (2016).
D’Lugos, A. C. et al. Chronic doxorubicin administration impacts satellite cell and capillary abundance in a muscle-specific manner. Physiol. Rep. 7, e14052 (2019).
Amrute-Nayak, M. et al. Chemotherapy triggers cachexia by deregulating synergetic function of histone-modifying enzymes. J. Cachexia Sarcopenia Muscle 12, 159–176 (2021).
Courneya, K. S. et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J. Clin. Oncol. 25, 4396–4404 (2007).
Battaglini, C. et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med. J. 125, 22–28 (2007).
Campbell, K. L. et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51, 2375–2390 (2019).
Ichiki, T., Tian, Q., Imayama, I. & Sunagawa, K. Telmisartan manifests powerful anti-inflammatory effects beyond class effects of angiotensin II type 1 blocker by inhibiting tumor necrosis factor α-induced interleukin 6 expressions through peroxisome proliferator activated receptorγ activation [abstract 5249]. Circulation 118 (Suppl. 18), 513 (2008).
Sukumaran, S., Patel, H. J. & Patel, B. M. Evaluation of role of telmisartan in combination with 5-fluorouracil in gastric cancer cachexia. Life Sci. 154, 15–23 (2016).
Patel, B. M. & Damle, D. Combination of telmisartan with cisplatin controls oral cancer cachexia in rats. Biomed. Res. Int. 2013, 642848 (2013).
Kim, G. T. et al. PLAG alleviates cisplatin-induced cachexia in lung cancer implanted mice. Transl. Oncol. 20, 10398 (2022).
Choi, S. et al. 1-Palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol ameliorates chemoradiation-induced oral mucositis. Oral. Dis. 26, 111–121 (2020).
Go, S., Park, M. J. & Lee, G. W. Clinical significance of the cachexia index in patients with small cell lung cancer. BMC Cancer 21, 563 (2021).
Go, S. et al. Cachexia index as a potential biomarker for cancer cachexia and a prognostic indicator in diffuse large B-cell lymphoma. J. Cachexia Sarcopenia Muscle 12, 2211–2219 (2021).
Yang, Q. J. et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J. Cachexia Sarcopenia Muscle 9, 71–85 (2018).
Roma-Rodrigues, C., Mendes, R., Baptista, P. V. & Fernandes, A. R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 20, 840 (2019).
Author information
Authors and Affiliations
Contributions
J.M.A. researched data and wrote the article. All the other authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks B. Laird, T. Naito, C. Prado, who co-reviewed with C. Orsso, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Argilés, J.M., López-Soriano, F.J., Stemmler, B. et al. Cancer-associated cachexia — understanding the tumour macroenvironment and microenvironment to improve management. Nat Rev Clin Oncol 20, 250–264 (2023). https://doi.org/10.1038/s41571-023-00734-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00734-5
This article is cited by
-
The vasculogenic mimicry related signature predicts the prognosis and immunotherapy response in renal clear cell carcinoma
BMC Cancer (2024)
-
Assessment of lipolysis biomarkers in adipose tissue of patients with gastrointestinal cancer
Cancer & Metabolism (2024)
-
Identification of PANoptosis-related signature reveals immune infiltration characteristics and immunotherapy responses for renal cell carcinoma
BMC Cancer (2024)
-
Extracting value from total-body PET/CT image data - the emerging role of artificial intelligence
Cancer Imaging (2024)
-
Effect of preoperative immunonutrition on postoperative short-term clinical outcomes in patients with gastric cancer cachexia: a prospective randomized controlled trial
World Journal of Surgical Oncology (2024)