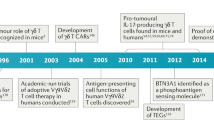
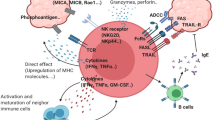
Abstract
Current cancer immunotherapies are primarily predicated on αβ T cells, with a stringent dependence on MHC-mediated presentation of tumour-enriched peptides or unique neoantigens that can limit their efficacy and applicability in various contexts. After two decades of preclinical research and preliminary clinical studies involving very small numbers of patients, γδ T cells are now being explored as a viable and promising approach for cancer immunotherapy. The unique features of γδ T cells, including their tissue tropisms, antitumour activity that is independent of neoantigen burden and conventional MHC-dependent antigen presentation, and combination of typical properties of T cells and natural killer cells, make them very appealing effectors in multiple cancer settings. Herein, we review the main functions of γδ T cells in antitumour immunity, focusing on human γδ T cell subsets, with a particular emphasis on the differences between Vδ1+ and Vδ2+ γδ T cells, to discuss their prognostic value in patients with cancer and the key therapeutic strategies that are being developed in an attempt to improve the outcomes of these patients.
Key points
-
Human γδ T cells comprise subsets with distinct tissue tropisms: Vδ1+ cells are enriched in mucosal tissues (and often predominate within carcinomas), whereas Vδ2+ cells are most abundant in the blood and lymphoid organs.
-
Most human γδ T cells have antitumour functions, but have also been reported to have pro-tumour properties in specific contexts.
-
γδ T cells have prognostic value in patients with cancer, being associated with favourable outcomes for most tumour types, especially when focusing on Vδ1+ cells.
-
γδ T cells are endowed with antitumour mechanisms of both αβ T cells and natural killer (NK) cells, mediated not only by T cell receptor (TCR) and co-stimulatory signals but also by activating NK cell receptors.
-
γδ T cells mostly act in a manner independent of MHC class I-mediated antigen presentation, making them suitable for treatment of MHC class I-deficient tumours, as well as for application in allogeneic settings.
-
Emerging therapeutic approaches based on γδ T cells comprise cell engagers, adoptive transfer of expanded Vδ1+ or Vδ2+ T cell subsets, and genetic engineering of γδ T cells to express chimeric antigen receptors (CARs) or αβ T cells to express specific γδTCRs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hayday, A. C. et al. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell 40, 259–269 (1985).
Bank, I. et al. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature 322, 179–181 (1986).
Brenner, M. B. et al. Identification of a putative second T-cell receptor. Nature 322, 145–149 (1986).
Born, W. et al. Peptide sequences of T-cell receptor δ and γ chains are identical to predicted X and γ proteins. Nature 330, 572–574 (1987).
Vantourout, P. & Hayday, A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013).
Ribot, J. C., Lopes, N. & Silva-Santos, B. γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 21, 221–232 (2021).
Silva-Santos, B., Mensurado, S. & Coffelt, S. B. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 19, 392–404 (2019).
Sebestyen, Z., Prinz, I., Déchanet-Merville, J., Silva-Santos, B. & Kuball, J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 19, 169–184 (2020).
Dolgin, E. Unconventional γδ T cells ‘the new black’ in cancer therapy. Nat. Biotechnol. 40, 805–808 (2022).
Saura-Esteller, J. et al. Gamma delta T-cell based cancer immunotherapy: past-present-future. Front. Immunol. 13, 915837 (2022).
Kabelitz, D., Serrano, R., Kouakanou, L., Peters, C. & Kalyan, S. Cancer immunotherapy with γδ T cells: many paths ahead of us. Cell. Mol. Immunol. 17, 925–939 (2020).
Bigby, M. et al. Most gamma delta T cells develop normally in the absence of MHC class II molecules. J. Immunol. 151, 4465–4475 (1993).
Deseke, M. & Prinz, I. Ligand recognition by the γδ TCR and discrimination between homeostasis and stress conditions. Cell. Mol. Immunol. 17, 914–924 (2020).
Silva-Santos, B., Serre, K. & Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 15, 683–691 (2015).
Mokuno, Y. et al. Expression of toll-like receptor 2 on γδ T cells bearing invariant Vγ6/Vδ1 induced by Escherichia coli infection in mice. J. Immunol. 165, 931–940 (2000).
Sheridan, B. S. et al. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity 39, 184–195 (2013).
Lefranc, M. P. IMGT, the international ImMunoGeneTics information system. Cold Spring Harb. Protoc. 6, 595–603 (2011).
Kabelitz, D., Kalyan, S., Oberg, H. H. & Wesch, D. Human Vδ2 versus non-Vδ2 γδ T cells in antitumor immunity. Oncoimmunology 2, 37–41 (2013).
Holtmeier, W. et al. The TCR δ repertoire in normal human skin is restricted and distinct from the TCR δ repertoire in the peripheral blood. J. Invest. Dermatol. 116, 275–280 (2001).
Kalyan, S. & Kabelitz, D. Defining the nature of human γδ T cells: a biographical sketch of the highly empathetic. Cell. Mol. Immunol. 10, 21–29 (2013).
Krangel, M. S., Yssel, H., Brocklehurst, C. & Spits, H. A distinct wave of human T cell receptor γ/δ lymphocytes in the early fetal thymus: evidence for controlled gene rearrangement and cytokine production. J. Exp. Med. 172, 847–859 (1990).
Willcox, C. R., Davey, M. S. & Willcox, B. E. Development and selection of the human Vγ9Vδ2+ T-cell repertoire. Front. Immunol. 9, 1501 (2018).
McVay, L. D. & Carding, S. R. Extrathymic origin of human gamma delta T cells during fetal development. J. Immunol. 157, 2873–2882 (1996).
Sherwood, A. M. et al. Deep sequencing of the human TCRγ and TCRβ repertoires suggests that TCRβ rearranges after αβ and γδ T cell commitment. Sci. Transl. Med. 3, 90ra61 (2011).
Wang, H., Fang, Z. & Morita, C. T. Vγ2Vδ2 T cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J. Immunol. 184, 6209–6222 (2010).
Hintz, M. et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 509, 317–322 (2001).
Tanaka, Y., Morita, C. T., Nieves, E., Brenner, M. B. & Bloom, B. R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 375, 155–158 (1995).
Gober, H. J. et al. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 197, 163–168 (2003).
Benzaïd, I. et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vγ9Vδ2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 71, 4562–4572 (2011).
Ashihara, E. et al. Isopentenyl pyrophosphate secreted from zoledronate-stimulated myeloma cells, activates the chemotaxis of γδT cells. Biochem. Biophys. Res. Commun. 463, 650–655 (2015).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 120, 2269–2279 (2012).
Sandstrom, A. et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2T cells. Immunity 40, 490–500 (2014).
Peigné, C.-M. et al. The juxtamembrane domain of butyrophilin BTN3A1 controls phosphoantigen-mediated activation of human Vγ9Vδ2 T cells. J. Immunol. 198, 4228–4234 (2017).
Gu, S. et al. Phosphoantigen-induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vγ9Vδ2 T cell activation. Proc. Natl Acad. Sci. USA 114, E7311–E7320 (2017).
Yang, Y. et al. A structural change in butyrophilin upon phosphoantigen binding underlies phosphoantigen-mediated Vγ9Vδ2 T cell activation. Immunity 50, 1043–1053.e5 (2019).
Hsiao, C.-H. C., Nguyen, K., Jin, Y., Vinogradova, O. & Wiemer, A. J. Ligand-induced interactions between butyrophilin 2A1 and 3A1 internal domains in the HMBPP receptor complex. Cell Chem. Biol. 29, 985–995.e5 (2022).
Rigau, M. et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367, eaay5516 (2020).
Tanaka, Y. et al. Synthesis of pyrophosphate-containing compounds that stimulate Vγ2Vδ2 T cells: application to cancer immunotherapy. Med. Chem. 3, 85–99 (2007).
Wang, R. N. et al. Optimized protocols for γδ T cell expansion and lentiviral transduction. Mol. Med. Rep. 19, 1471–1480 (2019).
Burjanadzé, M. et al. In vitro expansion of gamma delta T cells with anti-myeloma cell activity by phosphostim and IL-2 in patients with multiple myeloma. Br. J. Haematol. 139, 206–216 (2007).
Hoeres, T., Smetak, M., Pretscher, D. & Wilhelm, M. Improving the efficiency of Vγ9Vδ2 T-cell immunotherapy in cancer. Front. Immunol. 9, 800 (2018).
Starick, L. et al. Butyrophilin 3A (BTN3A, CD277)-specific antibody 20.1 differentially activates Vγ9Vδ2 TCR clonotypes and interferes with phosphoantigen activation. Eur. J. Immunol. 47, 982–992 (2017).
Payne, K. K. et al. BTN3A1 governs antitumor responses by coordinating αβ and γδ T cells. Science 369, 942–949 (2020).
De Gassart, A. et al. Development of ICT01, a first-in-class, anti-BTN3A antibody for activating Vγ9Vδ2 T cell-mediated antitumor immune response. Sci. Transl. Med. 13, eabj0835 (2021).
Melandri, D. et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 19, 1352–1365 (2018).
Rincon-Orozco, B. et al. Activation of Vγ9Vδ2 T cells by NKG2D. J. Immunol. 175, 2144–2151 (2005).
Wrobel, P. et al. Lysis of a broad range of epithelial tumour cells by human γδ T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand. J. Immunol. 66, 320–328 (2007).
Toutirais, O. et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vγ9Vδ2 T cells. Eur. J. Immunol. 39, 1361–1368 (2009).
Angelini, D. F. et al. FcγRIII discriminates between 2 subsets of Vγ9Vδ2 effector cells with different responses and activation pathways. Blood 104, 1801–1807 (2004).
Gertner-Dardenne, J. et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood 113, 4875–4884 (2009).
Tokuyama, H. et al. Vγ9Vδ2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs–rituximab and trastuzumab. Int. J. Cancer 122, 2526–2534 (2008).
Brandes, M. et al. Cross-presenting human γδ T cells induce robust CD8+ αβ T cell responses. Proc. Natl Acad. Sci. USA 106, 2307–2312 (2009).
Carding, S. R. & Egan, P. J. γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345 (2002).
Pitard, V. et al. Long-term expansion of effector/memory Vδ2− γδ T cells is a specific blood signature of CMV infection. Blood 112, 1317–1324 (2008).
Khairallah, C., Déchanet-Merville, J. & Capone, M. γδ T cell-mediated immunity to cytomegalovirus infection. Front. Immunol. 8, 105 (2017).
Farnault, L. et al. Clinical evidence implicating gamma-delta T cells in EBV control following cord blood transplantation. Bone Marrow Transpl. 48, 1478–1479 (2013).
Fujishima, N. et al. Skewed T cell receptor repertoire of Vδ1+ γδ T lymphocytes after human allogeneic haematopoietic stem cell transplantation and the potential role for Epstein-Barr virus-infected B cells in clonal restriction. Clin. Exp. Immunol. 149, 70–79 (2007).
Ravens, S. et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 18, 393–401 (2017).
Davey, M. S. et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 8, 14760 (2017).
Di Lorenzo, B., Ravens, S. & Silva-Santos, B. High-throughput analysis of the human thymic Vδ1+ T cell receptor repertoire. Sci. Data 6, 115 (2019).
Spada, F. M. et al. Self-recognition of CD1 by γ/δ T cells: implications for innate immunity. J. Exp. Med. 191, 937–948 (2000).
Uldrich, A. P. et al. CD1d-lipid antigen recognition by the γδ TCR. Nat. Immunol. 14, 1137–1145 (2013).
Luoma, A. M. et al. Crystal structure of Vδ1T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity 39, 1032–1042 (2013).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-lnducible MICA. Science 285, 727–729 (1999).
Poggi, A. et al. Vδ1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 64, 9172–9179 (2004).
Knight, A., MacKinnon, S. & Lowdell, M. W. Human Vδ1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy 14, 1110–1118 (2012).
Almeida, A. R. et al. Delta one T cells for immunotherapy of chronic lymphocytic leukemia: clinical-grade expansion/differentiation and preclinical proof-of-concept. Clin. Cancer Res. 22, 5795–5804 (2016).
Mikulak, J. et al. NKp46-expressing human gut-resident intraepithelial Vδ1 T cell subpopulation exhibits high antitumor activity against colorectal cancer. J. Clin. Invest. 4, e125884 (2019).
Correia, D. V. et al. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 118, 992–1001 (2011).
De Vries, N. L. et al. γδ T cells are effectors of immune checkpoint blockade in mismatch repair-deficient colon cancers with antigen presentation defects. Preprint at https://doi.org/10.1101/2021.10.14.464229 (2021).
Dunne, M. R. et al. Persistent changes in circulating and intestinal γδ T cell subsets, invariant natural killer T cells and mucosal-associated invariant T cells in children and adults with coeliac disease. PLoS ONE 8, 2–11 (2013).
Kenna, T. et al. Distinct subpopulations of γδ T cells are present in normal and tumor-bearing human liver. Clin. Immunol. 113, 56–63 (2004).
Déchanet, J. et al. Implication of γδ T cells in the human immune response to cytomegalovirus. J. Clin. Invest. 103, 1437–1449 (1999).
Bartkowiak, J., Kulczyck-Wojdala, D., Blonski, J. Z. & Robak, T. Molecular diversity of gammadelta T cells in peripheral blood from patients with B-cell chronic lymphocytic leukaemia. Neoplasma 49, 86–90 (2002).
Mangan, B. A. et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3 T cells. J. Immunol. 191, 30–34 (2013).
Marlin, R. et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl Acad. Sci. USA 114, 3163–3168 (2017).
Le Nours, J. et al. A class of γδ T cell receptors recognize the underside of the antigen-presenting molecule MR1. Science 366, 1522–1527 (2019).
Rice, M. T. et al. Recognition of the antigen-presenting molecule MR1 by a Vδ3+ γδ T cell receptor. Proc. Natl Acad. Sci. USA 118, e2110288118 (2021).
Chitadze, G., Oberg, H. H., Wesch, D. & Kabelitz, D. The ambiguous role of γδ T lymphocytes in antitumor immunity. Trends Immunol. 38, 668–678 (2017).
Foulkes, W., Smith, I. & Reis-Filho, J. Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948 (2010).
Reis, B. S. et al. TCR-Vγδ usage distinguishes protumor from antitumor intestinal γδ T cell subsets. Science 377, 276–284 (2022).
Mensurado, S. & Silva-Santos, B. Battle of the γδ T cell subsets in the gut. Trends Cancer 8, 881–883 (2022).
Papotto, P. H., Ribot, J. C. & Silva-Santos, B. IL-17+ γδ T cells as kick-starters of inflammation. Nat. Immunol. 18, 604–611 (2017).
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Rei, M., Pennington, D. J. & Silva-Santos, B. The emerging protumor role of γδ T lymphocytes: implications for cancer immunotherapy. Cancer Res 75, 798–802 (2015).
Ribot, J. C., Silva-Santos, B., Correia, D. V., Sousa, A. E. & Ribeiro, S. T. Human γδ thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J. Immunol. 192, 2237–2243 (2014).
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001).
Gao, Y. et al. γδ T cells provide an early source of interferon γ in tumor immunity. J. Exp. Med. 198, 433–442 (2003).
Street, S. E. A. et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and γδ T cells. J. Exp. Med. 199, 879–884 (2004).
Liu, Z. et al. Protective immunosurveillance and therapeutic antitumor activity of γδ T cells demonstrated in a mouse model of prostate cancer. J. Immunol. 180, 6044–6053 (2008).
Kunzmann, V. et al. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Hematology 96, 384–392 (2000).
Viey, E. et al. Phosphostim-activated γδ T cells kill autologous metastatic renal cell carcinoma. J. Immunol. 174, 1338–1347 (2005).
Mattarollo, S. R., Kenna, T., Nieda, M. & Nicol, A. J. Chemotherapy and zoledronate sensitize solid tumour cells to Vγ9Vδ2 T cell cytotoxicity. Cancer Immunol. Immunother. 56, 1285–1297 (2007).
D’Asaro, M. et al. Vγ9Vδ2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J. Immunol. 184, 3260–3268 (2010).
Simões, A. E., Di Lorenzo, B. & Silva-Santos, B. Molecular determinants of target cell recognition by human γδ T cells. Front. Immunol. 9, 929 (2018).
Alexander, A. A. Z. et al. Isopentenyl pyrophosphate-activated CD56+ γδ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin. Cancer Res. 14, 4232–4240 (2008).
Todaro, M. et al. Efficient killing of human colon cancer stem cells by γδ T lymphocytes. J. Immunol. 182, 7287–7296 (2009).
Dokouhaki, P. et al. NKG2D regulates production of soluble TRAIL by ex vivo expanded human γδ T cells. Eur. J. Immunol. 43, 3175–3182 (2013).
Capietto, A.-H., Martinet, L. & Fournie, J.-J. Stimulated γδ T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J. Immunol. 187, 1031–1038 (2011).
Fisher, J. P. H. et al. Neuroblastoma killing properties of Vδ2 and Vδ2-negative γδT cells following expansion by artificial antigen presenting cells. Clin. Cancer Res. 20, 5720–5732 (2014).
Riond, J., Rodriguez, S., Nicolau, M., Saati, T. & Gairin, J. E. In vivo major histocompatibility complex class I (MHCI) expression on MHCIlow tumor cells is regulated by γδ T and NK cells during the early steps of tumor growth. Cancer Immun. 9, 10 (2009).
Maniar, A. et al. Human γδ T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood 116, 1726–1733 (2010).
Brandes, M., Willimann, K. & Moser, B. Professional antigen-presentation function by human γδ T cells. Science 309, 264–268 (2005).
Altvater, B., Pscherer, S., Landmeier, S. & Kailayangiri, S. Activated human γδ T cells induce peptide-specific CD8+ T-cell responses to tumor-associated self-antigens. Cancer Immunol. Immunother. 61, 385–396 (2012).
Holmen Olofsson, G. et al. Vγ9Vδ2 T cells concurrently kill cancer cells and cross-present tumor antigens. Front. Immunol. 12, 645131 (2021).
Himoudi, N. et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J. Immunol. 188, 1708–1716 (2012).
Rampoldi, F., Ullrich, L. & Prinz, I. Revisiting the interaction of γδ T-cells and B-cells. Cells 9, 743 (2020).
Wen, L., Peng, Q. & Kelsoe, G. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non α/β” T cells. J. Exp. Med. 183, 2271–2282 (1996).
Rezende, R. M. et al. γδ T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat. Commun. 9, 3151 (2018).
Caccamo, N. et al. CXCR5 identifies a subset of Vγ9Vδ2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J. Immunol. 177, 5290–5295 (2006).
Bansal, R. R., Mackay, C. R., Moser, B. & Eberl, M. IL-21 enhances the potential of human γδ T cells to provide B-cell help. Eur. J. Immunol. 42, 110–119 (2012).
Rei, M. et al. Murine CD27(−) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc. Natl Acad. Sci. USA 111, E3562–E3570 (2014).
Gentles, A. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Wu, P. et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 40, 785–800 (2014).
Meraviglia, S. et al. Distinctive features of tumor-infiltrating γδ T lymphocytes in human colorectal cancer. Oncoimmunology 6, e1347742 (2017).
Lo Presti, E. et al. Squamous cell tumors recruit γδ T cells producing either IL17 or IFNγ depending on the tumor stage. Cancer Immunol. Res. 5, 397–407 (2017).
Chen, X. et al. Distribution and functions of γδ T cells infiltrated in the ovarian cancer microenvironment. J. Transl. Med. 17, 144 (2019).
Patil, R. S. et al. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int. J. Cancer 139, 869–881 (2016).
Van Hede, D. et al. Human papillomavirus oncoproteins induce a reorganization of epithelial-associated γδ T cells promoting tumor formation. Proc. Natl Acad. Sci. USA 114, E9056–E9065 (2017).
Hu, Z. et al. IL-17 activates the IL-6/STAT3 signal pathway in the proliferation of hepatitis B virus-related hepatocellular carcinoma. Cell. Physiol. Biochem. 43, 2379–2390 (2017).
Guo, N. et al. Interleukin-17 promotes migration and invasion of human cancer cells through upregulation of MTA1 expression. Front. Oncol. 9, 546 (2019).
Benevides, L. et al. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 75, 3788–3799 (2015).
Wakita, D. et al. Tumor-infiltrating IL-17-producing γδ T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 40, 1927–1937 (2010).
Kulig, P. et al. IL17A-mediated endothelial breach promotes metastasis formation. Cancer Immunol. Res. 4, 26–32 (2016).
Ma, S. et al. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 74, 1969–1982 (2014).
Sacchi, A. et al. Myeloid-derived suppressor cells specifically suppress IFN-γ production and antitumor cytotoxic activity of Vδ2 T cells. Front. Immunol. 9, 1271 (2018).
Papotto, P. H., Yilmaz, B. & Silva-Santos, B. Crosstalk between γδ T cells and the microbiota. Nat. Microbiol. 6, 1110–1117 (2021).
Jin, C. et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell 176, 998–1013.e16 (2019).
Kalyan, S. & Kabelitz, D. When neutrophils meet T cells: beginnings of a tumultuous relationship with underappreciated potential. Eur. J. Immunol. 44, 627–633 (2014).
Mensurado, S. et al. Tumor-associated neutrophils suppress pro-tumoral IL-17+ γδ T cells through induction of oxidative stress. PLoS Biol. 16, e2004990 (2018).
Zhang, Z. et al. “γδT cell-IL17A-neutrophil” axis drives immunosuppression and confers breast cancer resistance to high-dose anti-VEGFR2 therapy. Front. Immunol. 12, 699478 (2021).
Rutkowski, M. R. et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 27, 27–40 (2015).
Daley, D. et al. γδ T cells support pancreatic oncogenesis by restraining αβ T cell activation. Cell 166, 1485–1499 (2016).
Hao, J. et al. Regulatory role of Vγ1 γδ T cells in tumor immunity through IL-4 production. J. Immunol. 187, 4979–4986 (2011).
Mao, Y. et al. A new effect of IL-4 on human γδ T cells: promoting regulatory Vδ1 T cells via IL-10 production and inhibiting function of Vδ2 T cells. Cell. Mol. Immunol. 13, 217–228 (2016).
Peng, G. et al. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 27, 334–348 (2007).
Ye, J. et al. Tumor-derived γδ regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J. Immunol. 190, 2403–2414 (2013).
Chabab, G. et al. Identification of a regulatory Vδ1 gamma delta T cell subpopulation expressing CD73 in human breast cancer. J. Leukoc. Biol. 107, 1057–1067 (2020).
Wu, Y. et al. An innate-like Vδ1+ γδ T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci. Transl. Med. 11, eaax9364 (2019).
Ma, C. et al. Tumor-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. J. Immunol. 189, 5029–5036 (2012).
Bruni, E. et al. Intrahepatic CD69+Vδ1 T cells re-circulate in the blood of patients with metastatic colorectal cancer and limit tumor progression. J. Immunother. Cancer 10, e004579 (2022).
Zakeri, N. et al. Characterisation and induction of tissue-resident gamma delta T-cells to target hepatocellular carcinoma. Nat. Commun. 13, 1372 (2022).
Wang, J. et al. Tumor-infiltrating γδT cells predict prognosis and adjuvant chemotherapeutic benefit in patients with gastric cancer. Oncoimmunology 6, e1353858 (2017).
Lu, H. et al. High abundance of intratumoral γδ T cells favors a better prognosis in head and neck squamous cell carcinoma: a bioinformatic analysis. Front. Immunol. 11, 573920 (2020).
Nguyen, S. et al. Vδ2 T cells are associated with favorable clinical outcomes in patients with bladder cancer and their tumor reactivity can be boosted by BCG and zoledronate treatments. J. Immunother. Cancer 10, e004880 (2022).
Gherardin, N. A. et al. γδ T cells in Merkel cell carcinomas have a proinflammatory profile prognostic of patient survival. Cancer Immunol. Res. 9, 612–623 (2021).
Wu, Y. et al. A local human Vδ1 T cell population is associated with survival in nonsmall-cell lung cancer. Nat. Cancer 3, 696–709 (2022).
Buccheri, S., Guggino, G., Caccamo, N., Li Donni, P. & Dieli, F. Efficacy and safety of γδT cell-based tumor immunotherapy: a meta-analysis. J. Biol. Regul. Homeost. Agents 28, 81–90 (2014).
Wilhelm, M. et al. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood 102, 200–206 (2003).
Dieli, F. et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 67, 7450–7457 (2007).
Kobayashi, H., Tanaka, Y., Yagi, J., Minato, N. & Tanabe, K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol. Immunother. 60, 1075–1084 (2011).
Dong, T. et al. CD277 agonist enhances the immunogenicity of relapsed/refractory acute myeloid leukemia towards Vδ2+ T cell cytotoxicity. Ann. Hematol. 101, 2195–2208 (2022).
Oberg, H. H. et al. Novel bispecific antibodies increase γδ T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 74, 1349–1360 (2014).
de Weerdt, I. et al. A bispecific antibody antagonizes prosurvival CD40 signaling and promotes Vγ9Vδ2 T cell-mediated antitumor responses in human B-cell malignancies. Cancer Immunol. Res. 9, 50–61 (2021).
de Weerdt, I. et al. A bispecific single-domain antibody boosts autologous Vγ9Vδ2-T cell responses toward CD1d in chronic lymphocytic leukemia. Clin. Cancer Res. 27, 1744–1755 (2021).
Ganesan, R. et al. Selective recruitment of γδ T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia 35, 2274–2284 (2021).
de Bruin, R. C. G. et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology 7, e1375641 (2017).
Van Diest, E. et al. Gamma delta TCR anti-CD3 bispecific molecules (GABs) as novel immunotherapeutic compounds. J. Immunother. Cancer 9, e003850 (2021).
Schiller, C. B. et al. CD19-specific triplebody SPM-1 engages NK and γδ T cells for rapid and efficient lysis of malignant B-lymphoid cells. Oncotarget 7, 83392–83408 (2016).
Xu, Y. et al. Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell. Mol. Immunol. 18, 427–439 (2021).
Di Lorenzo, B. et al. Broad cytotoxic targeting of acute myeloid leukemia by polyclonal delta one T cells. Cancer Immunol. Res. 7, 552–558 (2019).
Sánchez Martínez, D. et al. Generation and proof-of-concept for allogeneic CD123 CAR-delta one T (DOT) cells in acute myeloid leukemia. J. Immunother. Cancer 10, e005400 (2022).
Lamb, L. S. et al. A combined treatment regimen of MGMT-modified γδ T cells and temozolomide chemotherapy is effective against primary high grade gliomas. Sci. Rep. 11, 21133 (2021).
Benjamin, R. et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): a phase 1, dose-escalation trial. Lancet Haematol. 9, e833–e843 (2022).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Ying, Z. et al. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 25, 947–953 (2019).
Shah, B. D. et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood 138, 11–22 (2021).
Rischer, M. et al. Human γδ T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br. J. Haematol. 126, 583–592 (2004).
Deniger, D. C. et al. Bispecific T-cells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol. Ther. 21, 638–647 (2013).
Capsomidis, A. et al. Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol. Ther. 26, 354–365 (2018).
Zhai, X. et al. MUC1-Tn-targeting chimeric antigen receptor-modified Vγ9Vδ2 T cells with enhanced antigen-specific anti-tumor activity. Am. J. Cancer Res. 11, 79–91 (2021).
Ang, W. X. et al. Electroporation of NKG2D RNA CAR improves Vγ9Vδ2 T cell responses against human solid tumor xenografts. Mol. Ther. Oncolytics 17, 421–430 (2020).
Ferry, G. M. et al. A simple and robust single-step method for CAR-Vδ1 γδT cell expansion and transduction for cancer immunotherapy. Front. Immunol. 13, 863155 (2022).
Nishimoto, K. P. et al. Allogeneic CD20-targeted γδ T cells exhibit innate and adaptive antitumor activities in preclinical B-cell lymphoma models. Clin. Transl. Immunol. 11, e1373 (2022).
Neelapu, S. S. et al. A phase 1 study of ADI-001: anti-CD20 CAR-engineered allogeneic gamma delta (γδ) T cells in adults with B-cell malignancies [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 7509 (2022).
Makkouk, A. et al. Off-the-shelf Vδ 1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J. Immunother. Cancer 9, e003441 (2021).
Marcu-Malina, V. et al. Redirecting αβT cells against cancer cells by transfer of a broadly tumor-reactive γδT-cell receptor. Blood 118, 50–59 (2011).
Straetemans, T. et al. GMP-grade manufacturing of T cells engineered to express a defined γδTCR. Front. Immunol. 9, 1062 (2018).
Johanna, I. et al. Evaluating in vivo efficacy–toxicity profile of TEG001 in humanized mice xenografts against primary human AML disease and healthy hematopoietic cells. J. Immunother. Cancer 7, 69 (2019).
Xu, Y. et al. A novel antibody-TCR (AbTCR) platform combines Fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov. 4, 62 (2018).
Nicolas, L. et al. Human γδ T cells express a higher TCR/CD3 complex density than αβ T cells. Clin. Immunol. 98, 358–363 (2001).
Liu, C. et al. Validation and promise of a TCR mimic antibody for cancer immunotherapy of hepatocellular carcinoma. Sci. Rep. 12, 12068 (2022).
Acknowledgements
The authors thank A. Hayday (King’s College London) for insightful discussions on this topic, and acknowledge funding from the Fundação para a Ciência e Tecnologia of the Portuguese Ministério da Ciência, Tecnologia e Ensino Superior (PTDC/MED-ONC/6829/2020 to B.S.-S. and 2021.01953.CEECIND to S.M.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interest
The authors receive funding from a sponsored research agreement with Takeda Development Center Americas, Inc, Lexington, MA.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks S. Coffelt, R. Lopez, Y. Tanaka and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mensurado, S., Blanco-Domínguez, R. & Silva-Santos, B. The emerging roles of γδ T cells in cancer immunotherapy. Nat Rev Clin Oncol 20, 178–191 (2023). https://doi.org/10.1038/s41571-022-00722-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-022-00722-1
This article is cited by
-
Unlocking γδ T cell power: pathways that boost cancer defense
Molecular Biomedicine (2024)
-
Unsynchronized butyrophilin molecules dictate cancer cell evasion of Vγ9Vδ2 T-cell killing
Cellular & Molecular Immunology (2024)
-
Computational immunogenomic approaches to predict response to cancer immunotherapies
Nature Reviews Clinical Oncology (2024)
-
PD-1 defines a distinct, functional, tissue-adapted state in Vδ1+ T cells with implications for cancer immunotherapy
Nature Cancer (2024)
-
Evolution of T cells in the cancer-resistant naked mole-rat
Nature Communications (2024)