Abstract
During the past 40 years, cytokines and cytokine receptors have been extensively investigated as either cancer targets or cancer treatments. A strong preclinical rationale supports therapeutic strategies to enhance the growth inhibitory and immunostimulatory effects of interferons and interleukins, including IL-2, IL-7, IL-12 and IL-15, or to inhibit the inflammatory and tumour-promoting actions of cytokines such as TNF, IL-1β and IL-6. This rationale is underscored by the discovery of altered and dysregulated cytokine expression in all human cancers. These findings prompted clinical trials of several cytokines or cytokine antagonists, revealing relevant biological activity but limited therapeutic efficacy. However, most trials involved patients with advanced-stage disease, which might not be the optimal setting for cytokine-based therapy. The advent of more effective immunotherapies and an increased understanding of the tumour microenvironment have presented new approaches to harnessing cytokine networks in the treatment of cancer, which include using cytokine-based therapies to enhance the activity or alleviate the immune-related toxicities of other treatments as well as to target early stage cancers. Many challenges remain, especially concerning delivery methods, context dependencies, and the pleiotropic, redundant and often conflicting actions of many cytokines. Herein, we discuss the lessons learnt from the initial trials of single-agent cytokine-based therapies and subsequent efforts to better exploit such agents for the treatment of solid tumours.
Key points
-
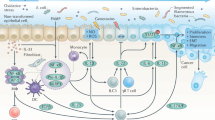
Cytokines are key mediators of cell communication in the tumour microenvironment.
-
Some cytokines contribute to host antitumour responses, but the production and function of many cytokines is dysregulated in cancer.
-
A robust rationale supports the use of both cytokines and cytokine antagonists in cancer therapy.
-
Despite strong preclinical evidence, neither cytokines nor cytokine antagonists have been effective as monotherapies in patients with advanced-stage cancers.
-
New approaches in this area include exploiting cytokines to enhance the actions or reduce the adverse effects of other cancer treatments, treatment at earlier disease stages and using the cytokine profile of each tumour type to define how best to leverage cytokines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lin, J. X. & Leonard, W. J. Fine-tuning cytokine signals. Annu. Rev. Immunol. 37, 295–324 (2019).
Aggarwal, B. B., Gupta, S. C. & Kim, J. H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 119, 651–665 (2012).
Alspach, E., Lussier, D. M. & Schreiber, R. D. Interferon γ and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb. Perspect. Biol. 11, a028480 (2019).
Lazear, H. M., Schoggins, J. W. & Diamond, M. S. Shared and distinct functions of type I and type III Interferons. Immunity 50, 907–923 (2019).
Rochman, Y., Spolski, R. & Leonard, W. J. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 9, 480–490 (2009).
Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 10, a028415 (2018).
Zhang, Y., Alexander, P. B. & Wang, X. F. TGF-β family signaling in the control of cell proliferation and survival. Cold Spring Harb. Perspect. Biol. 9, a022145 (2017).
Briukhovetska, D. et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer 21, 481–499 (2021).
Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 9, 361–371 (2009).
Graham, G. J., Handel, T. M. & Proudfoot, A. E. I. Leukocyte adhesion: reconceptualizing chemokine presentation by glycosaminoglycans. Trends Immunol. 40, 472–481 (2019).
Shalapour, S. & Karin, M. Pas de deux: control of anti-tumor immunity by cancer-associated inflammation. Immunity 51, 15–26 (2019).
Waldmann, T. A. Cytokines in cancer immunotherapy. Cold Spring Harb. Perspect. Biol. 10, a028472 (2018).
Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019).
Borrello, M. G. et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc. Natl Acad. Sci. USA 102, 14825–14830 (2005).
Sodir, N. M. et al. MYC instructs and maintains pancreatic adenocarcinoma phenotype. Cancer Discov. 10, 588–607 (2020).
Balkwill, F. R. & Mantovani, A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin. Cancer Biol. 22, 33–40 (2012).
Diakos, C. I., Charles, K. A., McMillan, D. C. & Clarke, S. J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 15, e493–e503 (2014).
Mouasni, S. & Tourneur, L. FADD at the crossroads between cancer and inflammation. Trends Immunol. 39, 1036–1053 (2018).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e14 (2018).
Yang, Y. & Lundqvist, A. Immunomodulatory effects of IL-2 and IL-15; implications for cancer immunotherapy. Cancers 12, 3586 (2020).
Rosenberg, S. A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 192, 5451–5458 (2014).
Payne, R. et al. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a community hospital biotherapy program. J. Immunother. Cancer 2, 13 (2014).
McDermott, D. F. et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 23, 133–141 (2004).
Dutcher, J. P. et al. High dose interleukin-2 (Aldesleukin) - expert consensus on best management practices-2014. J. Immunother. Cancer 2, 26 (2014).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Boyman, O. & Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 12, 180–190 (2012).
Doberstein, S. K. Bempegaldesleukin (NKTR-214): a CD-122-biased IL-2 receptor agonist for cancer immunotherapy. Expert Opin. Biol. Ther. 19, 1223–1228 (2019).
Bentebibel, S. E. et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 9, 711–721 (2019).
Diab, A. et al. NKTR-214 (CD122-biased agonist) plus nivolumab in patients with advanced solid tumors: preliminary phase 1/2 results of PIVOT. J. Clin. Oncol. 36, 3006–3006 (2018).
Diab, A. et al. Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: phase I dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov. 10, 1158–1173 (2020).
Lopes, J. E. et al. ALKS 4230: a novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. J. Immunother. Cancer 8, e000673 (2020).
Hamid, O. et al. Selection of the recommended phase 2 dose (RP2D) for subcutaneous nemvaleukin alfa: ARTISTRY-2. J. Clin. Oncol. 39, 2552–2552 (2021).
Boni, V. et al. ARTISTRY-1: Nemvaleukin alfa monotherapy and in combination with pembrolizumab in patients (pts) with advanced solid tumors. J. Clin. Oncol. 39, 2513–2513 (2021).
Sampson, J. H. et al. A pilot study of IL-2Rα blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS ONE 7, e31046 (2012).
Jacobs, J. F. et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin. Cancer Res. 16, 5067–5078 (2010).
Rech, A. J. & Vonderheide, R. H. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann. N. Y. Acad. Sci. 1174, 99–106 (2009).
Kolben, T. Abstract ND08: anti-CD25 Mab: selective depletion of T-regulatory cells. Cancer Res. 81, ND08 (2021).
Barata, J. T., Durum, S. K. & Seddon, B. Flip the coin: IL-7 and IL-7R in health and disease. Nat. Immunol. 20, 1584–1593 (2019).
Dwyer, C. J. et al. Fueling cancer immunotherapy with common gamma chain cytokines. Front. Immunol. 10, 263 (2019).
Kim, J. H., Lee, K. J. & Lee, S. W. Cancer immunotherapy with T-cell targeting cytokines: IL-2 and IL-7. BMB Rep. 54, 21–30 (2021).
Waldmann, T. A., Miljkovic, M. D. & Conlon, K. C. Interleukin-15 (dys)regulation of lymphoid homeostasis: implications for therapy of autoimmunity and cancer. J. Exp. Med. 217, e20191062 (2020).
Waldmann, T. A., Dubois, S., Miljkovic, M. D. & Conlon, K. C. IL-15 in the combination immunotherapy of cancer. Front. Immunol. 11, 868 (2020).
Conlon, K. C. et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 33, 74–82 (2015).
Miller, J. S. et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin. Cancer Res. 24, 1525–1535 (2018).
Knudson, K. M., Hodge, J. W., Schlom, J. & Gameiro, S. R. Rationale for IL-15 superagonists in cancer immunotherapy. Expert Opin. Biol. Ther. 20, 705–709 (2020).
Chamie, K. et al. Phase II/III clinical results of IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ (CIS) patients. J. Clin. Oncol. 39, 510–510 (2021).
Wrangle, J. M. et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 19, 694–704 (2018).
Wrangle, J. M. et al. Preliminary data from QUILT 3.055: a phase 2 multi-cohort study of N803 (IL-15 superagonist) in combination with checkpoint inhibitors (CPI). J. Clin. Oncol. 39, 2596–2596 (2021).
Tait Wojno, E. D., Hunter, C. A. & Stumhofer, J. S. The immunobiology of the interleukin-12 family: room for discovery. Immunity 50, 851–870 (2019).
Voest, E. E. et al. Inhibition of angiogenesis in vivo by interleukin 12. J. Natl Cancer Inst. 87, 581–586 (1995).
Del Vecchio, M. et al. Interleukin-12: biological properties and clinical application. Clin. Cancer Res. 13, 4677–4685 (2007).
Lasek, W., Zagożdżon, R. & Jakobisiak, M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 63, 419–435 (2014).
Duvic, M. et al. A phase II open-label study of recombinant human interleukin-12 in patients with stage IA, IB, or IIA mycosis fungoides. J. Am. Acad. Dermatol. 55, 807–813 (2006).
Rook, A. H. et al. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood 94, 902–908 (1999).
Little, R. F. et al. Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood 107, 4650–4657 (2006).
Younes, A. et al. Phase II clinical trial of interleukin-12 in patients with relapsed and refractory non-Hodgkin’s lymphoma and Hodgkin’s disease. Clin. Cancer Res. 10, 5432–5438 (2004).
Gollob, J. A. et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-γ induction is associated with clinical response. Clin. Cancer Res. 6, 1678–1692 (2000).
Mortarini, R. et al. Peripheral burst of tumor-specific cytotoxic T lymphocytes and infiltration of metastatic lesions by memory CD8+ T cells in melanoma patients receiving interleukin 12. Cancer Res. 60, 3559–3568 (2000).
Fallon, J. et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget 5, 1869–1884 (2014).
Greiner, J. W., Morillon, Y. M. II & Schlom, J. NHS-IL12, a tumor-targeting immunocytokine. Immunotargets Ther. 10, 155–169 (2021).
Strauss, J. et al. First-in-human phase I trial of a tumor-targeted cytokine (NHS-IL12) in subjects with metastatic solid tumors. Clin. Cancer Res. 25, 99–109 (2019).
Ongaro, T. et al. A novel anti-cancer L19-interleukin-12 fusion protein with an optimized peptide linker efficiently localizes in vivo at the site of tumors. J. Biotechnol. 291, 17–25 (2019).
Daud, A. I. et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 26, 5896–5903 (2008).
Algazi, A. P. et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin. Cancer Res. 26, 2827–2837 (2020).
Telli, M. L. et al. Intratumoral plasmid IL12 expands CD8+ T cells and induces a CXCR3 gene signature in triple-negative breast tumors that sensitizes patients to anti-PD-1 Therapy. Clin. Cancer Res. 27, 2481–2493 (2021).
Nguyen, K. G. et al. Localized interleukin-12 for cancer immunotherapy. Front. Immunol. 11, 575597 (2020).
Saraiva, M., Vieira, P. & O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 217, e20190418 (2020).
Emmerich, J. et al. IL-10 directly activates and expands tumor-resident CD8+ T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 72, 3570–3581 (2012).
Mumm, J. B. et al. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell 20, 781–796 (2011).
Oft, M. Immune regulation and cytotoxic T cell activation of IL-10 agonists - preclinical and clinical experience. Semin. Immunol. 44, 101325 (2019).
Autio, K. & Oft, M. Pegylated interleukin-10: clinical development of an immunoregulatory cytokine for use in cancer therapeutics. Curr. Oncol. Rep. 21, 19 (2019).
Naing, A. et al. PEGylated IL-10 (Pegilodecakin) induces systemic immune activation, CD8+ T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell 34, 775–791.e3 (2018).
Tannir, N. M. et al. Pegilodecakin as monotherapy or in combination with anti-PD-1 or tyrosine kinase inhibitor in heavily pretreated patients with advanced renal cell carcinoma: final results of cohorts A, G, H and I of IVY phase I study. Int. J. Cancer 149, 403–408 (2021).
Naing, A. et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J. Clin. Oncol. 34, 3562–3569 (2016).
Hecht, J. R. et al. Overall survival of PEGylated human IL-10 (AM0010) with 5-FU/LV and oxaliplatin (FOLFOX) in metastatic pancreatic adenocarcinoma (PDAC). J. Clin. Oncol. 36, 374–374 (2018).
Hecht, J. R. et al. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J. Clin. Oncol. 39, 1108–1118 (2021).
Naing, A. et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol. 20, 1544–1555 (2019).
Pal, S., Hu-Lieskovan, S. & Agarwal, N. Can pegylated IL-10 add to a backbone of PD-1 inhibition for solid tumours? Lancet Oncol. 20, 1473–1474 (2019).
Spigel, D. et al. Randomized Phase 2 studies of checkpoint inhibitors alone or in combination with pegilodecakin in patients with metastatic NSCLC (CYPRESS 1 and CYPRESS 2). J. Thorac. Oncol. 16, 327–333 (2021).
Chapman, P. B. Targeting tumor-rejection antigens in melanoma with tumor-infiltrating lymphocytes. J. Clin. Oncol. 39, 2640–2642 (2021).
Dafni, U. et al. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann. Oncol. 30, 1902–1913 (2019).
Cao, G., Lei, L. & Zhu, X. Efficiency and safety of autologous chimeric antigen receptor T-cells therapy used for patients with lymphoma: a systematic review and meta-analysis. Medicine 98, e17506 (2019).
Koneru, M., O’Cearbhaill, R., Pendharkar, S., Spriggs, D. R. & Brentjens, R. J. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J. Transl. Med. 13, 102 (2015).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36, 346–351 (2018).
Zhang, X. et al. Double-edged effects of interferons on the regulation of cancer-immunity cycle. Oncoimmunology 10, 1929005 (2021).
Génin, P., Vaccaro, A. & Civas, A. The role of differential expression of human interferon-A genes in antiviral immunity. Cytokine Growth Factor Rev. 20, 283–295 (2009).
Turinetto, M., Scotto, G., Tuninetti, V., Giannone, G. & Valabrega, G. The role of PARP inhibitors in the ovarian cancer microenvironment: moving forward from synthetic lethality. Front. Oncol. 11, 689829 (2021).
Borden, E. C. et al. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 6, 975–990 (2007).
Kirkwood, J. M. et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 14, 7–17 (1996).
Lens, M. B. & Dawes, M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J. Clin. Oncol. 20, 1818–1825 (2002).
Ives, N. J. et al. Adjuvant interferon-α for the treatment of high-risk melanoma: an individual patient data meta-analysis. Eur. J. Cancer 82, 171–183 (2017).
Motzer, R. J., Bacik, J., Murphy, B. A., Russo, P. & Mazumdar, M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J. Clin. Oncol. 20, 289–296 (2002).
No authors listed. Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators.Lancet 353, 14–17 (1999).
Koneru, R. & Hotte, S. J. Role of cytokine therapy for renal cell carcinoma in the era of targeted agents. Curr. Oncol. 16 (Suppl. 1), S40–S44 (2009).
Chou, R. et al. Intravesical therapy for the treatment of nonmuscle invasive bladder cancer: a systematic review and meta-analysis. J. Urol. 197, 1189–1199 (2017).
Lu, J. L. et al. Efficacy of intravesical therapies on the prevention of recurrence and progression of non-muscle-invasive bladder cancer: a systematic review and network meta-analysis. Cancer Med. 9, 7800–7809 (2020).
Tur, E. & Brenner, S. Classic Kaposi’s sarcoma: low-dose interferon alfa treatment. Dermatology 197, 37–42 (1998).
Lebbe, C. et al. Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Eur. J. Cancer 114, 117–127 (2019).
Sleijfer, S., Bannink, M., Van Gool, A. R., Kruit, W. H. & Stoter, G. Side effects of interferon-α therapy. Pharm. World Sci. 27, 423–431 (2005).
How, J. & Hobbs, G. Use of interferon alfa in the treatment of myeloproliferative neoplasms: perspectives and review of the literature. Cancers 12, 1954 (2020).
Hasselbalch, H. C. & Holmström, M. O. Perspectives on interferon-alpha in the treatment of polycythemia vera and related myeloproliferative neoplasms: minimal residual disease and cure? Semin. Immunopathol. 41, 5–19 (2019).
Tarhini, A. et al. Neoadjuvant ipilimumab (3mg/kg or 10mg/kg) and high dose IFN-α2b in locally/regionally advanced melanoma: safety, efficacy and impact on T-cell repertoire. J. Immunother. Cancer 6, 112 (2018).
Tarhini, A. A. et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J. Clin. Oncol. 30, 322–328 (2012).
Brohl, A. S. et al. A phase IB study of ipilimumab with peginterferon alfa-2b in patients with unresectable melanoma. J. Immunother. Cancer 4, 85 (2016).
Atkins, M. B. et al. Pembrolizumab (pembro) plus ipilimumab (ipi) or pegylated interferon alfa-2b (PEG-IFN) for advanced melanoma or renal cell carcinoma (RCC). J. Clin. Oncol. 34, 3013–3013 (2016).
Duggan, M. C. et al. A phase I study of recombinant (r) vaccinia-CEA(6D)-TRICOM and rFowlpox-CEA(6D)-TRICOM vaccines with GM-CSF and IFN-α-2b in patients with CEA-expressing carcinomas. Cancer Immunol. Immunother. 65, 1353–1364 (2016).
Sheng, L., Chen, X., Wang, Q., Lyu, S. & Li, P. Interferon-α2b enhances survival and modulates transcriptional profiles and the immune response in melanoma patients treated with dendritic cell vaccines. Biomed. Pharmacother. 125, 109966 (2020).
Pujade-Lauraine, E. et al. Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second-look laparotomy. J. Clin. Oncol. 14, 343–350 (1996).
Windbichler, G. H. et al. Interferon-gamma in the first-line therapy of ovarian cancer: a randomized phase III trial. Br. J. Cancer 82, 1138–1144 (2000).
Alberts, D. S. et al. Randomized phase 3 trial of interferon gamma-1b plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel alone for first-line treatment of advanced ovarian and primary peritoneal carcinomas: results from a prospectively designed analysis of progression-free survival. Gynecol. Oncol. 109, 174–181 (2008).
Cole, C. B. & Annunziata, C. M. First-in-human phase I study of intraperitoneally administered interferon-activated autologous monocytes in platinum-resistant or refractory ovarian cancer. J. Clin. Oncol. 38, 1–1 (2020).
Gleave, M. E. et al. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. N. Engl. J. Med. 338, 1265–1271 (1998).
Wiesenfeld, M. et al. Controlled clinical trial of interferon-gamma as postoperative surgical adjuvant therapy for colon cancer. J. Clin. Oncol. 13, 2324–2329 (1995).
Schiller, J. H. et al. Eastern cooperative group trial of interferon gamma in metastatic melanoma: an innovative study design. Clin. Cancer Res. 2, 29–36 (1996).
Meyskens, F. L. Jr. et al. Randomized trial of adjuvant human interferon gamma versus observation in high-risk cutaneous melanoma: a Southwest Oncology Group study. J. Natl Cancer Inst. 87, 1710–1713 (1995).
Jett, J. R. et al. Phase III trial of recombinant interferon gamma in complete responders with small-cell lung cancer. J. Clin. Oncol. 12, 2321–2326 (1994).
Von Hoff, D. D. et al. Phase II evaluation of recombinant gamma-interferon in patients with advanced pancreatic carcinoma: a Southwest Oncology Group study. J. Biol. Response Mod. 9, 584–587 (1990).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Higgs, B. W. et al. Interferon gamma messenger RNA signature in tumor biopsies predicts outcomes in patients with non-small cell lung carcinoma or urothelial cancer treated with durvalumab. Clin. Cancer Res. 24, 3857–3866 (2018).
Grasso, C. S. et al. Conserved interferon-γ signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell 38, 500–515.e3 (2020).
Gao, J. et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167, 397–404.e9 (2016).
Propper, D. J. et al. Low-dose IFN-γ induces tumor MHC expression in metastatic malignant melanoma. Clin. Cancer Res. 9, 84–92 (2003).
Zhang, S. et al. Systemic interferon-γ increases MHC class I expression and T-cell infiltration in cold tumors: results of a phase 0 clinical trial. Cancer Immunol. Res. 7, 1237–1243 (2019).
Moore, R. J. et al. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat. Med. 5, 828–831 (1999).
Greten, F. R. et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Taniguchi, K. & Karin, M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18, 309–324 (2018).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Feldmann, M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2, 364–371 (2002).
D’Haens, G. R. & van Deventer, S. 25 years of anti-TNF treatment for inflammatory bowel disease: lessons from the past and a look to the future. Gut 70, 1396–1405 (2021).
Brown, E. R. et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-α inhibitor, in patients with advanced cancer. Ann. Oncol. 19, 1340–1346 (2008).
Harrison, M. L. et al. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J. Clin. Oncol. 25, 4542–4549 (2007).
Bertrand, F. et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 8, 2256 (2017).
Perez-Ruiz, E. et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569, 428–432 (2019).
Montfort, A. et al. Combining nivolumab and ipilimumab with infliximab or certolizumab in patients with advanced melanoma: first results of a phase Ib clinical trial. Clin. Cancer Res. 27, 1037–1047 (2021).
Choy, E. H. et al. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 16, 335–345 (2020).
Taniguchi, K. & Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 26, 54–74 (2014).
van Rhee, F. et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 15, 966–974 (2014).
Nishimoto, N. et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 106, 2627–2632 (2005).
Rossi, J. F., Lu, Z. Y., Jourdan, M. & Klein, B. Interleukin-6 as a therapeutic target. Clin. Cancer Res. 21, 1248–1257 (2015).
Coward, J. et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin. Cancer Res. 17, 6083–6096 (2011).
Stone, R. L. et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 366, 610–618 (2012).
Culig, Z. & Puhr, M. Interleukin-6 and prostate cancer: current developments and unsolved questions. Mol. Cell Endocrinol. 462, 25–30 (2018).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Morris, E. C., Neelapu, S. S., Giavridis, T. & Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-021-00547-6 (2021).
Voronov, E., Dinarello, C. A. & Apte, R. N. Interleukin-1α as an intracellular alarmin in cancer biology. Semin. Immunol. 38, 3–14 (2018).
Voronov, E. & Apte, R. N. Targeting the tumor microenvironment by intervention in interleukin-1 biology. Curr. Pharm. Des. 23, 4893–4905 (2017).
Ershaid, N. et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat. Commun. 10, 4375 (2019).
Heath, O. et al. Chemotherapy induces tumor-associated macrophages that aid adaptive immune responses in ovarian cancer. Cancer Immunol. Res. 9, 665–681 (2021).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Gottschlich, A., Endres, S. & Kobold, S. Therapeutic strategies for targeting IL-1 in Cancer. Cancers https://doi.org/10.3390/cancers13030477 (2021).
Novartis. Novartis provides update on Phase III Study Evaluating Canakinumab (ACZ885) as second or third-line Treatment in Combination with Chemotherapy in Non-Small Cell Lung Cancer https://www.novartis.com/news/media-releases/novartis-provides-update-phase-iii-study-evaluating-canakinumab-acz885-second-or-third-line-treatment-combination-chemotherapy-non-small-cell-lung-cancer (2021).
Cavalli, G. & Dinarello, C. A. Anakinra therapy for non-cancer inflammatory diseases. Front. Pharmacol. 9, 1157 (2018).
Missiroli, S. et al. Targeting the NLRP3 inflammasome as a new therapeutic option for overcoming cancer. Cancers 13, 2297 (2021).
Wu, T. C. et al. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. 78, 5243–5258 (2018).
Isambert, N. et al. Fluorouracil and bevacizumab plus anakinra for patients with metastatic colorectal cancer refractory to standard therapies (IRAFU): a single-arm phase 2 study. Oncoimmunology 7, e1474319 (2018).
Strati, P. et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 4, 3123–3127 (2020).
Derynck, R., Turley, S. J. & Akhurst, R. J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 18, 9–34 (2021).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Morris, J. C. et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE 9, e90353 (2014).
Necchi, A. et al. PF-03446962, a fully-human monoclonal antibody against transforming growth-factor β (TGFβ) receptor ALK1, in pre-treated patients with urothelial cancer: an open label, single-group, phase 2 trial. Invest. N. Drugs 32, 555–560 (2014).
Goff, L. W. et al. A phase I study of the anti-activin receptor-like kinase 1 (ALK-1) monoclonal antibody PF-03446962 in patients with advanced solid tumors. Clin. Cancer Res. 22, 2146–2154 (2016).
Simonelli, M. et al. Phase I study of PF-03446962, a fully human monoclonal antibody against activin receptor-like kinase-1, in patients with hepatocellular carcinoma. Ann. Oncol. 27, 1782–1787 (2016).
Clarke, J. M. et al. A phase Ib study of the combination regorafenib with PF-03446962 in patients with refractory metastatic colorectal cancer (REGAL-1 trial). Cancer Chemother. Pharmacol. 84, 909–917 (2019).
Lan, Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aan5488 (2018).
Lind, H. et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J. Immunother. Cancer https://doi.org/10.1136/jitc-2019-000433 (2020).
Yoo, C. et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-000564 (2020).
Kang, Y. K. et al. Safety and tolerability of bintrafusp alfa, a bifunctional fusion protein targeting TGFβ and PD-L1, in Asian patients with pretreated recurrent or refractory gastric cancer. Clin. Cancer Res. 26, 3202–3210 (2020).
Liu, S., Ren, J. & ten Dijke, P. Targeting TGFβ signal transduction for cancer therapy. Signal. Transduct. Target. Ther. 6, 8 (2021).
Korbecki, J. et al. CC chemokines in a tumor: a review of pro-cancer and anti-cancer properties of the ligands of receptors CCR1, CCR2, CCR3, and CCR4. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21218412 (2020).
Bule, P., Aguiar, S. I., Aires-Da-Silva, F. & Dias, J. N. R. Chemokine-directed tumor microenvironment modulation in cancer immunotherapy. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22189804 (2021).
Kohli, K., Pillarisetty, V. G. & Kim, T. S. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. https://doi.org/10.1038/s41417-021-00303-x (2021).
Lim, S. Y., Yuzhalin, A. E., Gordon-Weeks, A. N. & Muschel, R. J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 7, 28697–28710 (2016).
Jahchan, N. S. et al. Tuning the tumor myeloid microenvironment to fight cancer. Front. Immunol. 10, 1611 (2019).
Pienta, K. J. et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest. N. Drugs 31, 760–768 (2013).
Brana, I. et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target. Oncol. 10, 111–123 (2015).
Nywening, T. M. et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 17, 651–662 (2016).
Noel, M. et al. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Invest. N. Drugs 38, 800–811 (2020).
Linehan, D. et al. Overall survival in a trial of orally administered CCR2 inhibitor CCX872 in locally advanced/metastatic pancreatic cancer: correlation with blood monocyte counts. J. Clin. Oncol. 36, 92–92 (2018).
Le, D. et al. Abstract CT124: A phase Ib/II study of BMS-813160, a CC chemokine receptor (CCR) 2/5 dual antagonist, in combination with chemotherapy or nivolumab in patients (pts) with advanced pancreatic or colorectal cancer. Cancer Res. 78, CT124–CT124 (2018).
Berlato, C. et al. A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer. J. Clin. Invest. 127, 801–813 (2017).
Marshall, L. A. et al. Tumors establish resistance to immunotherapy by regulating T(reg) recruitment via CCR4. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-000764 (2020).
Kim, Y. H. et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 19, 1192–1204 (2018).
Zamarin, D. et al. Mogamulizumab in combination with durvalumab or tremelimumab in patients with advanced solid tumors: a phase I study. Clin. Cancer Res. 26, 4531–4541 (2020).
Doi, T. et al. A Phase I Study of the Anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin. Cancer Res. 25, 6614–6622 (2019).
Cohen, E. E. W. et al. A phase Ib study of utomilumab (PF-05082566) in combination with mogamulizumab in patients with advanced solid tumors. J. Immunother. Cancer 7, 342 (2019).
Ho, W., Nasrah, N. & Johnson, D. Phase I/II dose escalation and expansion study of FLX475 alone and in combination with pembrolizumab in advanced cancer. J. Clin. Oncol. 37, TPS24–TPS24 (2019).
RAPT Therapeutics. RAPT therapeutics reports positive initial data from ongoing phase 1/2 clinical trial of FLX475 in multiple cancer indications. https://www.globenewswire.com/en/news-release/2020/11/16/2127181/0/en/RAPT-Therapeutics-Reports-Positive-Initial-Data-from-Ongoing-Phase-1-2-Clinical-Trial-of-FLX475-in-Multiple-Cancer-Indications.html (16 November 2020).
Aldinucci, D., Borghese, C. & Casagrande, N. The CCL5/CCR5 axis in cancer progression. Cancers https://doi.org/10.3390/cancers12071765 (2020).
Miao, M., De Clercq, E. & Li, G. Clinical significance of chemokine receptor antagonists. Expert Opin. Drug. Metab. Toxicol. 16, 11–30 (2020).
Qi, B. et al. Advances of CCR5 antagonists: From small molecules to macromolecules. Eur. J. Med. Chem. 208, 112819 (2020).
Haag, G. M. et al. Combined PD-1 inhibition (Pembrolizumab) and CCR5 inhibition (Maraviroc) for the treatment of refractory microsatellite stable (MSS) metastatic colorectal cancer (mCRC): first results of the PICCASSO phase I trial. J. Clin. Oncol. 38, 3010–3010 (2020).
Alfaro, C. et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 60, 24–31 (2017).
Schalper, K. A. et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 26, 688–692 (2020).
Fousek, K., Horn, L. A. & Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. https://doi.org/10.1016/j.pharmthera.2020.107692 (2020).
Bilusic, M. et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 7, 240 (2019).
Davar, D. et al. 394 Interleukin-8–neutralizing monoclonal antibody BMS-986253 plus nivolumab (NIVO) in biomarker-enriched, primarily anti–PD-(L)1–experienced patients with advanced cancer: initial phase 1 results. J. Immunother. Cancer 8, A239–A240 (2020).
Schott, A. F. et al. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin. Cancer Res. 23, 5358–5365 (2017).
Goldstein, L. J. et al. A randomized phase II trial of reparixin, a CXCR1 inhibitor, in combination with paclitaxel in the treatment of mTNBC [abstract]. Cancer Res. 80 (Suppl. 4), Abstr. P3-11-07 (2020).
Balkwill, F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin. Cancer Biol. 14, 171–179 (2004).
Domanska, U. M. et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur. J. Cancer 49, 219–230 (2013).
Sacco, A. et al. Cancer cell dissemination and homing to the bone marrow in a zebrafish model. Cancer Res. 76, 463–471 (2016).
Pernas, S. et al. Balixafortide plus eribulin in HER2-negative metastatic breast cancer: a phase 1, single-arm, dose-escalation trial. Lancet Oncol. 19, 812–824 (2018).
Lee, E. Q. et al. Phase I and biomarker study of plerixafor and bevacizumab in recurrent high-grade glioma. Clin. Cancer Res. 24, 4643–4649 (2018).
Adlere, I. et al. Modulators of CXCR4 and CXCR7/ACKR3 function. Mol. Pharmacol. 96, 737–752 (2019).
Daniel, S. K., Seo, Y. D. & Pillarisetty, V. G. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin. Cancer Biol. 65, 176–188 (2020).
Galsky, M. D. et al. A phase I trial of LY2510924, a CXCR4 peptide antagonist, in patients with advanced cancer. Clin. Cancer Res. 20, 3581–3588 (2014).
Hainsworth, J. D. et al. A randomized, open-label phase 2 study of the CXCR4 inhibitor LY2510924 in combination with sunitinib versus sunitinib alone in patients with metastatic renal cell carcinoma (RCC). Target. Oncol. 11, 643–653 (2016).
Salgia, R. et al. A randomized phase II study of LY2510924 and carboplatin/etoposide versus carboplatin/etoposide in extensive-disease small cell lung cancer. Lung Cancer 105, 7–13 (2017).
O’Hara, M. H. et al. Safety and Pharmacokinetics of CXCR4 Peptide Antagonist, LY2510924, in combination with durvalumab in advanced refractory solid tumors. J. Pancreat. Cancer 6, 21–31 (2020).
Bockorny, B. et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat. Med. 26, 878–885 (2020).
Biasci, D. et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl Acad. Sci. USA 117, 28960–28970 (2020).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Anfray, C., Ummarino, A., Andón, F. T. & Allavena, P. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells https://doi.org/10.3390/cells9010046 (2019).
Cassetta, L. & Pollard, J. W. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 17, 887–904 (2018).
Cannarile, M. A. et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 5, 53 (2017).
Tap, W. D. et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet 394, 478–487 (2019).
Benner, B. et al. Pexidartinib, a novel small molecule CSF-1R inhibitor in use for tenosynovial giant cell tumor: a systematic review of pre-clinical and clinical development. Drug Des. Devel Ther. 14, 1693–1704 (2020).
Butowski, N. et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 18, 557–564 (2016).
Gomez-Roca, C. A. et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann. Oncol. 30, 1381–1392 (2019).
Papadopoulos, K. P. et al. First-in-Human Study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin. Cancer Res. 23, 5703–5710 (2017).
Autio, K. A. et al. Immunomodulatory activity of a colony-stimulating factor-1 receptor inhibitor in patients with advanced refractory breast or prostate cancer: a phase I study. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.Ccr-20-0855 (2020).
Ries, C. H. et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25, 846–859 (2014).
Cassier, P. A. et al. MEDIPLEX: a phase 1 study of durvalumab (D) combined with pexidartinib (P) in patients (pts) with advanced pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer (CRC). J. Clin. Oncol. 37, 2579–2579 (2019).
Wainberg, Z. et al. First-in-human phase 1 dose escalation and expansion of a novel combination, anti–CSF-1 Receptor (cabiralizumab) Plus Anti–PD-1 (nivolumab), in patients with advanced solid tumors. J. Immunother. Cancer 8, e000530 (2018).
Wainberg, Z. et al. 32nd Annual Meeting and Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2017): Late-Breaking Abstracts. National Harbor, MD, USA. 8-12 November 2017. J. Immunother. Cancer 5 (Suppl. 3), 89 (2017). abstract 042.
Carleton, M. et al. Pharmacodynamics (PD) and genomic profiling of pts treated with cabiralizumab (cabira)+nivolumab (NIVO) provide evidence of on-target tumor immune modulations and support future clinical applications. J. Clin. Oncol. 36, 3020–3020 (2018).
Businesswire. Five Prime Therapeutics provides update on phase 2 trial of cabiralizumab Combined with Opdivo® in pancreatic cancer. https://www.businesswire.com/news/home/20200218005144/en/ (18 February 2020).
Micic, D., Komaki, Y., Alavanja, A., Rubin, D. T. & Sakuraba, A. Risk of cancer recurrence among individuals exposed to antitumor necrosis factor therapy: a systematic review and meta-analysis of observational studies. J. Clin. Gastroenterol. 53, e1–e11 (2019).
Mercer, L. K. et al. Risk of solid cancer in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 74, 1087–1093 (2015).
Böhm, S. et al. Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo-ovarian high-grade serous carcinoma. Clin. Cancer Res. 22, 3025–3036 (2016).
Maniati, E. et al. Mouse ovarian cancer models recapitulate the human tumor microenvironment and patient response to treatment. Cell Rep. 30, 525–540.e527 (2020).
Rodrigues, J., Heinrich, M. A., Teixeira, L. M. & Prakash, J. 3D in vitro model (R)evolution: unveiling tumor-stroma interactions. Trends Cancer 7, 249–264 (2021).
Malacrida, B. et al. A human multi-cellular model shows how platelets drive production of diseased extracellular matrix and tissue invasion. iScience 24, 102676 (2021).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
Pease, D. F. & Kratzke, R. A. Oncolytic viral therapy for mesothelioma. Front. Oncol. 7, 179 (2017).
Cho, B. C. et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in advanced squamous cell carcinoma of the head and neck: results from a phase I cohort. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-000664 (2020).
Acknowledgements
The work of F.R.B. is supported by Cancer Research UK programme grant A25714.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
F.R.B. is on the scientific advisory board of Verseau Therapeutics and is an adviser to iOmix Therapeutics. D.J.P. declares no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks K. C. Conlon, F. Greten, C. Krieg and I. Melero for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Propper, D.J., Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol 19, 237–253 (2022). https://doi.org/10.1038/s41571-021-00588-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00588-9
This article is cited by
-
The application of nanoparticles-based ferroptosis, pyroptosis and autophagy in cancer immunotherapy
Journal of Nanobiotechnology (2024)
-
Targeting inflammation as cancer therapy
Journal of Hematology & Oncology (2024)
-
Small extracellular vesicles in plasma carry luminal cytokines that remain undetectable by antibody-based assays in cancer patients and healthy donors
BJC Reports (2024)
-
Lyophilized lymph nodes for improved delivery of chimeric antigen receptor T cells
Nature Materials (2024)
-
Roles of exosomes in immunotherapy for solid cancers
Cell Death & Disease (2024)