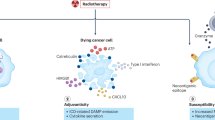
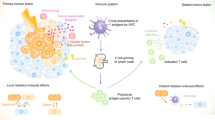
Abstract
A variety of targeted anticancer agents have been successfully introduced into clinical practice, largely reflecting their ability to inhibit specific molecular alterations that are required for disease progression. However, not all malignant cells rely on such alterations to survive, proliferate, disseminate and/or evade anticancer immunity, implying that many tumours are intrinsically resistant to targeted therapies. Radiotherapy is well known for its ability to activate cytotoxic signalling pathways that ultimately promote the death of cancer cells, as well as numerous cytoprotective mechanisms that are elicited by cellular damage. Importantly, many cytoprotective mechanisms elicited by radiotherapy can be abrogated by targeted anticancer agents, suggesting that radiotherapy could be harnessed to enhance the clinical efficacy of these drugs. In this Review, we discuss preclinical and clinical data that introduce radiotherapy as a tool to elicit or amplify clinically actionable signalling pathways in patients with cancer.
Key points
-
Targeted anticancer agents are commonly used in the treatment of various solid and haematological malignancies.
-
Not all tumours are sensitive to these agents, largely reflecting the lack of or inactivity of the targetable alteration.
-
Radiotherapy is also frequently used for the treatment of cancer, owing to its prominent cytostatic and cytotoxic effects on malignant cells.
-
A wide panel of cytoprotective pathways can be activated by radiotherapy, thus limiting therapeutic efficacy.
-
However, these signal transduction cascades can be effectively inhibited with targeted anticancer agents, potentially supporting superior treatment efficacy.
-
Radiotherapy stands out as a promising tool to elicit clinically actionable signalling pathways in cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
18 February 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41571-022-00611-7
References
Bedard, P. L., Hyman, D. M., Davids, M. S. & Siu, L. L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 395, 1078–1088 (2020).
Boumahdi, S. & de Sauvage, F. J. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 19, 39–56 (2020).
Sotorasib edges closer to approval. Cancer Discov. 11, OF2 (2021).
Doroshow, D. B. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 18, 345–362 (2021).
Luo, J., Solimini, N. L. & Elledge, S. J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136, 823–837 (2009).
Harmenberg, U., Hamdy, F. C., Widmark, A., Lennernäs, B. & Nilsson, S. Curative radiation therapy in prostate cancer. Acta Oncol. 50, 98–103 (2011).
Nakano, T., Ohno, T., Ishikawa, H., Suzuki, Y. & Takahashi, T. Current advancement in radiation therapy for uterine cervical cancer. J. Radiat. Res. 51, 1–8 (2010).
Spencer, K., Parrish, R., Barton, R. & Henry, A. Palliative radiotherapy. BMJ 360, k821 (2018).
Riet, F. G. et al. Preoperative radiotherapy in breast cancer patients: 32 years of follow-up. Eur. J. Cancer 76, 45–51 (2017).
Calvo, F. A. et al. ESTRO/ACROP IORT recommendations for intraoperative radiation therapy in primary locally advanced rectal cancer. Clin. Transl. Radiat. Oncol. 25, 29–36 (2020).
Zaorsky, N. G. et al. The evolution of brachytherapy for prostate cancer. Nat. Rev. Urol. 14, 415–439 (2017).
Pilié, P. G., Tang, C., Mills, G. B. & Yap, T. A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 16, 81–104 (2019).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Formenti, S. C. et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 24, 1845–1851 (2018).
Rodriguez-Ruiz, M. E., Vitale, I., Harrington, K. J., Melero, I. & Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 21, 120–134 (2020).
Dalwadi, S. M., Herman, J. M., Das, P. & Holliday, E. B. Novel radiotherapy technologies in the treatment of gastrointestinal malignancies. Hematol. Oncol. Clin. North. Am. 34, 29–43 (2020).
Oh, D. Y. & Bang, Y. J. HER2-targeted therapies–a role beyond breast cancer. Nat. Rev. Clin. Oncol. 17, 33–48 (2020).
Guo, R. et al. MET-dependent solid tumours–molecular diagnosis and targeted therapy. Nat. Rev. Clin. Oncol. 17, 569–587 (2020).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Rybstein, M. D., Bravo-San Pedro, J. M., Kroemer, G. & Galluzzi, L. The autophagic network and cancer. Nat. Cell Biol. 20, 243–251 (2018).
McLaughlin, M. et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 20, 203–217 (2020).
Barker, H. E., Paget, J. T., Khan, A. A. & Harrington, K. J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 15, 409–425 (2015).
Vanneste, B. G. L. et al. Immunotherapy as sensitizer for local radiotherapy. Oncoimmunology 9, 1832760 (2020).
Moding, E. J., Kastan, M. B. & Kirsch, D. G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 12, 526–542 (2013).
Vitale, I., Shema, E., Loi, S. & Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 27, 212–224 (2021).
Petroni, G. & Galluzzi, L. Impact of treatment schedule on the efficacy of cytostatic and immunostimulatory agents. Oncoimmunology 10, 1889101 (2021).
Altorki, N. K. et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 22, 824–835 (2021).
Coleman, C. N. et al. Radiation-induced adaptive response: new potential for cancer treatment. Clin. Cancer Res. 26, 5781–5790 (2020).
Huang, R. X. & Zhou, P. K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal. Transduct. Target. Ther. 5, 60 (2020).
Galluzzi, L. et al. Molecular mechanisms of cisplatin resistance. Oncogene 31, 1869–1883 (2012).
Sansregret, L., Vanhaesebroeck, B. & Swanton, C. Determinants and clinical implications of chromosomal instability in cancer. Nat. Rev. Clin. Oncol. 15, 139–150 (2018).
Tubbs, A. & Nussenzweig, A. Endogenous DNA damage as a source of genomic instability in cancer. Cell 168, 644–656 (2017).
Ashworth, A. & Lord, C. J. Synthetic lethal therapies for cancer: what’s next after PARP inhibitors? Nat. Rev. Clin. Oncol. 15, 564–576 (2018).
Michels, J. et al. Cisplatin resistance associated with PARP hyperactivation. Cancer Res. 73, 2271–2280 (2013).
Shiloh, Y. & Ziv, Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 14, 197–210 (2013).
Taylor, A. M. et al. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature 258, 427–429 (1975).
Imray, F. P. & Kidson, C. Perturbations of cell-cycle progression in ɣ-irradiated ataxia telangiectasia and Huntington’s disease cells detected by DNA flow cytometric analysis. Mutat. Res. 112, 369–382 (1983).
Carruthers, R. et al. Abrogation of radioresistance in glioblastoma stem-like cells by inhibition of ATM kinase. Mol. Oncol. 9, 192–203 (2015).
Golding, S. E. et al. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol. Cancer Ther. 8, 2894–2902 (2009).
Vecchio, D. et al. Predictability, efficacy and safety of radiosensitization of glioblastoma-initiating cells by the ATM inhibitor KU-60019. Int. J. Cancer 135, 479–491 (2014).
Tang, S., Li, Z., Yang, L., Shen, L. & Wang, Y. A potential new role of ATM inhibitor in radiotherapy: suppressing ionizing radiation-activated EGFR. Int. J. Radiat. Biol. 96, 461–468 (2020).
Takeuchi, M. et al. Anti-tumor effect of inhibition of DNA damage response proteins, ATM and ATR, in endometrial cancer cells. Cancers 11, 1913 (2019).
Durant, S. T. et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci. Adv. 4, eaat1719 (2018).
Karlin, J. et al. Orally bioavailable and blood-brain barrier-penetrating ATM inhibitor (AZ32) radiosensitizes intracranial gliomas in mice. Mol. Cancer Ther. 17, 1637–1647 (2018).
Biddlestone-Thorpe, L. et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin. Cancer Res. 19, 3189–3200 (2013).
Fokas, E. et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 3, e441 (2012).
Foote, K. M. et al. Discovery of 4-{4-[(3R)-3-methylmorpholin-4-yl]-6-[1-(methylsulfonyl)cyclopropyl]pyrimidin-2-yl}-1H-indole (AZ20): a potent and selective inhibitor of ATR protein kinase with monotherapy in vivo antitumor activity. J. Med. Chem.56, 2125–2138 (2013).
Dunne, V. et al. Inhibition of ataxia telangiectasia related-3 (ATR) improves therapeutic index in preclinical models of non-small cell lung cancer (NSCLC) radiotherapy. Radiother. Oncol. 124, 475–481 (2017).
Wengner, A. M. et al. The novel ATR inhibitor BAY 1895344 is efficacious as monotherapy and combined with DNA damage-inducing or repair-compromising therapies in preclinical cancer models. Mol. Cancer Ther. 19, 26–38 (2020).
Pires, I. M. et al. Targeting radiation-resistant hypoxic tumour cells through ATR inhibition. Br. J. Cancer 107, 291–299 (2012).
Tu, X. et al. ATR inhibition is a promising radiosensitizing strategy for triple-negative breast cancer. Mol. Cancer Ther. 17, 2462–2472 (2018).
Zenke, F. T. et al. Pharmacologic inhibitor of DNA-PK, M3814, potentiates radiotherapy and regresses human tumors in mouse models. Mol. Cancer Ther. 19, 1091–1101 (2020).
Fok, J. H. L. et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nat. Commun. 10, 5065 (2019).
Timme, C. R., Rath, B. H., O’Neill, J. W., Camphausen, K. & Tofilon, P. J. The DNA-PK inhibitor VX-984 enhances the radiosensitivity of glioblastoma cells grown in vitro and as orthotopic xenografts. Mol. Cancer Ther. 17, 1207–1216 (2018).
Willoughby, C. E. et al. Selective DNA-PKcs inhibition extends the therapeutic index of localized radiotherapy and chemotherapy. J. Clin. Invest. 130, 258–271 (2020).
Yamazaki, T. et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 21, 1160–1171 (2020).
Feng, X. et al. ATR inhibition potentiates ionizing radiation-induced interferon response via cytosolic nucleic acid-sensing pathways. EMBO J. 39, e104036 (2020).
Dillon, M. T. et al. ATR inhibition potentiates the radiation-induced inflammatory tumor microenvironment. Clin. Cancer Res. 25, 3392–3403 (2019).
Zhang, Q. et al. Inhibition of ATM increases interferon signaling and sensitizes pancreatic cancer to immune checkpoint blockade therapy. Cancer Res. 79, 3940–3951 (2019).
Sheng, H. et al. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J. Immunother. Cancer 8, e000340 (2020).
Vendetti, F. P. et al. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J. Clin. Invest. 128, 3926–3940 (2018).
He, H., Chang, R., Zhang, T., Yang, C. & Kong, Z. ATM mediates DAB2IP-deficient bladder cancer cell resistance to ionizing radiation through the p38MAPK and NF-κB signaling pathway. Mol. Med. Rep. 16, 1216–1222 (2017).
Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018).
Bian, L., Meng, Y., Zhang, M. & Li, D. MRE11-RAD50-NBS1 complex alterations and DNA damage response: implications for cancer treatment. Mol. Cancer 18, 169 (2019).
Fagan-Solis, K. D. et al. A P53-independent dna damage response suppresses oncogenic proliferation and genome instability. Cell Rep. 30, 1385–1399 e7 (2020).
Mattiello, L. et al. The targeting of MRE11 or RAD51 sensitizes colorectal cancer stem cells to CHK1 inhibition. Cancers (Basel) 13, 1957 (2021).
Manic, G. et al. Control of replication stress and mitosis in colorectal cancer stem cells through the interplay of PARP1, MRE11 and RAD51. Cell Death Differ. 28, 2060–2082 (2021).
Ho, V. et al. Overexpression of the MRE11-RAD50-NBS1 (MRN) complex in rectal cancer correlates with poor response to neoadjuvant radiotherapy and prognosis. BMC Cancer 18, 869 (2018).
Chang, L. et al. Targeting Rad50 sensitizes human nasopharyngeal carcinoma cells to radiotherapy. BMC Cancer 16, 190 (2016).
Choudhury, A. et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 70, 7017–7026 (2010).
Kondo, T. et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc. Natl Acad. Sci. USA 110, 2969–2974 (2013).
Nicholson, J. et al. E3 ligase cIAP2 mediates downregulation of MRE11 and radiosensitization in response to HDAC inhibition in bladder cancer. Cancer Res. 77, 3027–3039 (2017).
Groselj, B. et al. Radiosensitization in vivo by histone deacetylase inhibition with no increase in early normal tissue radiation toxicity. Mol. Cancer Ther. 17, 381–392 (2018).
Paillas, S. et al. The histone deacetylase inhibitor romidepsin spares normal tissues while acting as an effective radiosensitizer in bladder tumors in vivo. Int. J. Radiat. Oncol. Biol. Phys. 107, 212–221 (2020).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Caron, M. C. et al. Poly(ADP-ribose) polymerase-1 antagonizes DNA resection at double-strand breaks. Nat. Commun. 10, 2954 (2019).
Strickfaden, H. et al. Poly(ADP-ribosyl)ation-dependent transient chromatin decondensation and histone displacement following laser microirradiation. J. Biol. Chem. 291, 1789–1802 (2016).
Liu, C. et al. PARP inhibitor olaparib increases the sensitization to radiotherapy in FaDu cells. J. Cell Mol. Med. 24, 2444–2450 (2020).
Bi, Y. et al. Radiosensitization by the PARP inhibitor olaparib in BRCA1-proficient and deficient high-grade serous ovarian carcinomas. Gynecol. Oncol. 150, 534–544 (2018).
Michmerhuizen, A. R. et al. PARP1 inhibition radiosensitizes models of inflammatory breast cancer to ionizing radiation. Mol. Cancer Ther. 18, 2063–2073 (2019).
Cho, E. J. et al. Preclinical evaluation of radiation therapy of BRCA1-associated mammary tumors using a mouse model. Int. J. Biol. Sci. 17, 689–701 (2021).
Soni, A. et al. Inhibition of Parp1 by BMN673 effectively sensitizes cells to radiotherapy by upsetting the balance of repair pathways processing DNA double-strand breaks. Mol. Cancer Ther. 17, 2206–2216 (2018).
Tuli, R. et al. Radiosensitization of pancreatic cancer cells in vitro and in vivo through poly (ADP-ribose) polymerase inhibition with ABT-888. Transl. Oncol. 7, 439–445 (2014).
Luo, J. et al. Fluzoparib increases radiation sensitivity of non-small cell lung cancer (NSCLC) cells without BRCA1/2 mutation, a novel PARP1 inhibitor undergoing clinical trials. J. Cancer Res. Clin. Oncol. 146, 721–737 (2020).
Ahmed, S. U. et al. Selective inhibition of parallel DNA damage response pathways optimizes radiosensitization of glioblastoma stem-like cells. Cancer Res. 75, 4416–4428 (2015).
Chabanon, R. M. et al. PBRM1 deficiency confers synthetic lethality to DNA repair inhibitors in cancer. Cancer Res. 81, 2888–2902 (2021).
Lord, C. J. & Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 16, 110–120 (2016).
Zhao, B., Rothenberg, E., Ramsden, D. A. & Lieber, M. R. The molecular basis and disease relevance of non-homologous DNA end joining. Nat. Rev. Mol. Cell Biol. 21, 765–781 (2020).
Chabanon, R. M. et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J. Clin. Invest. 129, 1211–1228 (2019).
Zhang, N. et al. PARP inhibitor niraparib as a radiosensitizer promotes antitumor immunity of radiotherapy in EGFR-mutated non-small cell lung cancer. Clin. Transl. Oncol. 23, 1827–1837 (2021).
Petroni, G., Buqué, A., Zitvogel, L., Kroemer, G. & Galluzzi, L. Immunomodulation by targeted anticancer agents. Cancer Cell 39, 310–345 (2021).
Wang, W. J. et al. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 73, 1219–1231 (2013).
Zhang, P. et al. ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1. Nat. Cell Biol. 16, 864–875 (2014).
Yan, Y., Black, C. P. & Cowan, K. H. Irradiation-induced G2/M checkpoint response requires ERK1/2 activation. Oncogene 26, 4689–4698 (2007).
Barker, H. E. et al. CHK1 inhibition radiosensitizes head and neck cancers to paclitaxel-based chemoradiotherapy. Mol. Cancer Ther. 15, 2042–2054 (2016).
Richer, A. L. et al. WEE1 kinase inhibitor AZD1775 has preclinical efficacy in LKB1-deficient non-small cell lung cancer. Cancer Res. 77, 4663–4672 (2017).
Lee, Y. Y. et al. Anti-tumor effects of Wee1 kinase inhibitor with radiotherapy in human cervical cancer. Sci. Rep. 9, 15394 (2019).
Yang, L. et al. Wee1 kinase inhibitor AZD1775 effectively sensitizes esophageal cancer to radiotherapy. Clin. Cancer Res. 26, 3740–3750 (2020).
Mitchell, J. B. et al. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762. Clin. Cancer Res. 16, 2076–2084 (2010).
Morgan, M. A. et al. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 70, 4972–4981 (2010).
Patties, I. et al. The Chk1 inhibitor SAR-020106 sensitizes human glioblastoma cells to irradiation, to temozolomide, and to decitabine treatment. J. Exp. Clin. Cancer Res. 38, 420 (2019).
Zeng, L., Nikolaev, A., Xing, C., Della Manna, D. L. & Yang, E. S. CHK1/2 inhibitor prexasertib suppresses NOTCH signaling and enhances cytotoxicity of cisplatin and radiation in head and neck squamous cell carcinoma. Mol. Cancer Ther. 19, 1279–1288 (2020).
Karnak, D. et al. Combined inhibition of Wee1 and PARP1/2 for radiosensitization in pancreatic cancer. Clin. Cancer Res. 20, 5085–5096 (2014).
Parsels, L. A. et al. PARP1 trapping and DNA replication stress enhance radiosensitization with combined WEE1 and PARP inhibitors. Mol. Cancer Res. 16, 222–232 (2018).
Vance, S. et al. Selective radiosensitization of p53 mutant pancreatic cancer cells by combined inhibition of Chk1 and PARP1. Cell Cycle 10, 4321–4329 (2011).
Parmar, K. et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin. Cancer Res. 25, 6127–6140 (2019).
Choi, C. et al. Checkpoint kinase 1 (CHK1) inhibition enhances the sensitivity of triple-negative breast cancer cells to proton irradiation via Rad51 downregulation. Int. J. Mol. Sci. 21, 2691 (2020).
Raghavan, P. et al. AZD5438, an inhibitor of Cdk1, 2, and 9, enhances the radiosensitivity of non-small cell lung carcinoma cells. Int. J. Radiat. Oncol. Biol. Phys. 84, e507–e514 (2012).
O’Leary, B., Finn, R. S. & Turner, N. C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 13, 417–430 (2016).
Bosacki, C. et al. CDK 4/6 inhibitors combined with radiotherapy: a review of literature. Clin. Transl. Radiat. Oncol. 26, 79–85 (2021).
Göttgens, E. L. et al. Inhibition of CDK4/CDK6 enhances radiosensitivity of HPV negative head and neck squamous cell carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 105, 548–558 (2019).
Naz, S. et al. Abemaciclib, a selective CDK4/6 inhibitor, enhances the radiosensitivity of non-small cell lung cancer in vitro and in vivo. Clin. Cancer Res. 24, 3994–4005 (2018).
Huang, C. Y. et al. Palbociclib enhances radiosensitivity of hepatocellular carcinoma and cholangiocarcinoma via inhibiting ataxia telangiectasia-mutated kinase-mediated DNA damage response. Eur. J. Cancer 102, 10–22 (2018).
Petroni, G. et al. Radiotherapy delivered before CDK4/6 inhibitors mediates superior therapeutic effects in ER(+) breast cancer. Clin. Cancer Res. 27, 1855–1863 (2021).
Xie, X. et al. CDK4/6 inhibitor palbociclib amplifies the radiosensitivity to nasopharyngeal carcinoma cells via mediating apoptosis and suppressing dna damage repair. Onco Targets Ther. 12, 11107–11117 (2019).
Fernández-Aroca, D. M. et al. P53 pathway is a major determinant in the radiosensitizing effect of palbociclib: implication in cancer therapy. Cancer Lett. 451, 23–33 (2019).
Hashizume, R. et al. Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol. 18, 1519–1528 (2016).
Patel, P. et al. Enhancing direct cytotoxicity and response to immune checkpoint blockade following ionizing radiation with Wee1 kinase inhibition. Oncoimmunology 8, e1638207 (2019).
Wang, B., Sun, L., Yuan, Z. & Tao, Z. Wee1 kinase inhibitor AZD1775 potentiates CD8+ T cell-dependent antitumour activity via dendritic cell activation following a single high dose of irradiation. Med. Oncol. 37, 66 (2020).
Chao, H. H. et al. Combination of CHEK1/2 inhibition and ionizing radiation results in abscopal tumor response through increased micronuclei formation. Oncogene 39, 4344–4357 (2020).
Petroni, G., Formenti, S. C., Chen-Kiang, S. & Galluzzi, L. Immunomodulation by anticancer cell cycle inhibitors. Nat. Rev. Immunol. 20, 669–679 (2020).
Huang, A., Garraway, L. A., Ashworth, A. & Weber, B. Synthetic lethality as an engine for cancer drug target discovery. Nat. Rev. Drug Discov. 19, 23–38 (2020).
Paluch-Shimon, S. & Cardoso, F. PARP inhibitors coming of age. Nat. Rev. Clin. Oncol. 18, 69–70 (2021).
Li, N. et al. An open-label, multicenter, single-arm, phase II study of fluzoparib in patients with germline BRCA1/2 mutation and platinum-sensitive recurrent ovarian cancer. Clin. Cancer Res. 27, 2452–2458 (2021).
Sonnenblick, A., de Azambuja, E., Azim, H. A. Jr. & Piccart, M. An update on PARP inhibitors–moving to the adjuvant setting. Nat. Rev. Clin. Oncol. 12, 27–41 (2015).
Karam, S. D. et al. Final report of a phase I trial of olaparib with cetuximab and radiation for heavy smoker patients with locally advanced head and neck cancer. Clin. Cancer Res. 24, 4949–4959 (2018).
Loap, P. et al. Combination of olaparib and radiation therapy for triple negative breast cancer: preliminary results of the RADIOPARP phase 1 trial. Int. J. Radiat. Oncol. Biol. Phys. 109, 436–440 (2021).
de Haan, R. et al. Phase I and pharmacologic study of olaparib in combination with high-dose radiotherapy with and without concurrent cisplatin for non-small cell lung cancer. Clin. Cancer Res. 27, 1256–1266 (2021).
Konstantinopoulos, P. A. et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 20, 570–580 (2019).
Matulonis, U. A. et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann. Oncol. 28, 512–518 (2017).
Yap, T. A. et al. Phase I trial of first-in-class ATR inhibitor M6620 (VX-970) as monotherapy or in combination with carboplatin in patients with advanced solid tumors. J. Clin. Oncol. 38, 3195–3204 (2020).
Thomas, A. et al. Phase I study of ATR inhibitor M6620 in combination with topotecan in patients with advanced solid tumors. J. Clin. Oncol. 36, 1594–1602 (2018).
Dillon, M. T. et al. PATRIOT: a phase I study to assess the tolerability, safety and biological effects of a specific ataxia telangiectasia and Rad3-related (ATR) inhibitor (AZD6738) as a single agent and in combination with palliative radiation therapy in patients with solid tumours. Clin. Transl. Radiat. Oncol. 12, 16–20 (2018).
Kim, S. T. et al. Phase I study of ceralasertib (AZD6738), a novel DNA damage repair agent, in combination with weekly paclitaxel in refractory cancer. Clin. Cancer Res. 27, 4700–4709 (2021).
van Bussel, M. T. J. et al. A first-in-man phase 1 study of the DNA-dependent protein kinase inhibitor peposertib (formerly M3814) in patients with advanced solid tumours. Br. J. Cancer 124, 728–735 (2021).
Cuneo, K. C. et al. Dose escalation trial of the Wee1 inhibitor adavosertib (AZD1775) in combination with gemcitabine and radiation for patients with locally advanced pancreatic cancer. J. Clin. Oncol. 37, 2643–2650 (2019).
Yang, E. S. et al. A phase 1b trial of prexasertib in combination with chemoradiation in patients with locally advanced head and neck squamous cell carcinoma. Radiother. Oncol. 157, 203–209 (2021).
Boss, D. S. et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral cyclin-dependent kinase inhibitor AZD5438 when administered at intermittent and continuous dosing schedules in patients with advanced solid tumours. Ann. Oncol. 21, 884–894 (2010).
Sausville, E. et al. Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother. Pharmacol. 73, 539–549 (2014).
Beddok, A. et al. Concurrent use of palbociclib and radiation therapy: single-centre experience and review of the literature. Br. J. Cancer 123, 905–908 (2020).
Ratosa, I. et al. Cyclin-dependent kinase 4/6 inhibitors combined with radiotherapy for patients with metastatic breast cancer. Clin. Breast Cancer 20, 495–502 (2020).
Ippolito, E. et al. Concurrent radiotherapy with palbociclib or ribociclib for metastatic breast cancer patients: preliminary assessment of toxicity. Breast 46, 70–74 (2019).
Meattini, I., Desideri, I., Scotti, V., Simontacchi, G. & Livi, L. Ribociclib plus letrozole and concomitant palliative radiotherapy for metastatic breast cancer. Breast 42, 1–2 (2018).
Chowdhary, M. et al. Safety and efficacy of palbociclib and radiation therapy in patients with metastatic breast cancer: initial results of a novel combination. Adv. Radiat. Oncol. 4, 453–457 (2019).
Guerini, A. E. et al. A single-center retrospective safety analysis of cyclin-dependent kinase 4/6 inhibitors concurrent with radiation therapy in metastatic breast cancer patients. Sci. Rep. 10, 13589 (2020).
DeWire, M. et al. A phase I/II study of ribociclib following radiation therapy in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). J. Neurooncol 149, 511–522 (2020).
Hoxhaj, G. & Manning, B. D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020).
Thorpe, L. M., Yuzugullu, H. & Zhao, J. J. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 15, 7–24 (2015).
Vasan, N. et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kα inhibitors. Science 366, 714–723 (2019).
Ruiz-Saenz, A. et al. HER2 amplification in tumors activates PI3K/Akt signaling independent of HER3. Cancer Res. 78, 3645–3658 (2018).
Tsay, J. J. et al. Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am. J. Respir. Crit. Care Med. 198, 1188–1198 (2018).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018).
Hopkins, B. D., Goncalves, M. D. & Cantley, L. C. Insulin-PI3K signalling: an evolutionarily insulated metabolic driver of cancer. Nat. Rev. Endocrinol. 16, 276–283 (2020).
André, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Lockney, N. A. et al. PIK3CA mutation is associated with increased local failure in lung stereotactic body radiation therapy (SBRT). Clin. Transl. Radiat. Oncol. 7, 91–93 (2017).
Lockney, N. A. et al. Phosphatidylinositol-3-kinase mutations are associated with increased local failure in brain metastases treated with radiation. Int. J. Radiat. Oncol. Biol. Phys. 101, 833–844 (2018).
Zafarana, G. et al. Copy number alterations of c-MYC and PTEN are prognostic factors for relapse after prostate cancer radiotherapy. Cancer 118, 4053–4062 (2012).
Ang, K. K. et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 62, 7350–7356 (2002).
Chua, D. T., Nicholls, J. M., Sham, J. S. & Au, G. K. Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 59, 11–20 (2004).
Brollo, J. et al. Locoregional recurrence in patients with HER2 positive breast cancer. Breast 22, 856–862 (2013).
Green, M. M. et al. Expression of vascular endothelial growth factor (VEGF) in locally invasive prostate cancer is prognostic for radiotherapy outcome. Int. J. Radiat. Oncol. Biol. Phys. 67, 84–90 (2007).
Yoshimoto, Y. et al. Mutation profiling of uterine cervical cancer patients treated with definitive radiotherapy. Gynecol. Oncol. 159, 546–553 (2020).
Darwis, N. D. M. et al. FGFR signaling as a candidate therapeutic target for cancers resistant to carbon ion radiotherapy. Int. J. Mol. Sci. 20, 4563 (2019).
Chen, D. J. & Nirodi, C. S. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin. Cancer Res. 13, 6555–6560 (2007).
Li, H. F., Kim, J. S. & Waldman, T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat. Oncol. 4, 43 (2009).
Chinnaiyan, P. et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 65, 3328–3335 (2005).
Park, C. M. et al. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res. 66, 8511–8519 (2006).
Cao, N. et al. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat. Res. 171, 9–21 (2009).
Rose, Li,Y. et al. Mutational signatures in tumours induced by high and low energy radiation in Trp53 deficient mice. Nat. Commun. 11, 394 (2020).
De Bacco, F. et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J. Natl Cancer Inst. 103, 645–661 (2011).
Luttich, L. et al. Tyrosine kinase c-MET as therapeutic target for radiosensitization of head and neck squamous cell carcinomas. Cancers 13, 1865 (2021).
Nisa, L. et al. Targeting the MET receptor tyrosine kinase as a strategy for radiosensitization in locoregionally advanced head and neck squamous cell carcinoma. Mol. Cancer Ther. 19, 614–626 (2020).
Sofia Vala, I. et al. Low doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesis. PLoS ONE 5, e11222 (2010).
Gorski, D. H. et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 59, 3374–3378 (1999).
Knizetova, P. et al. Autocrine regulation of glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle 7, 2553–2561 (2008).
Gomez-Roman, N. et al. Radiation responses of 2D and 3D glioblastoma cells: a novel, 3D-specific radioprotective role of VEGF/Akt signaling through functional activation of NHEJ. Mol. Cancer Ther. 19, 575–589 (2020).
Ma, J. et al. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell 35, 504–518.e7 (2019).
Lammering, G., Valerie, K., Lin, P. S., Hewit, T. H. & Schmidt-Ullrich, R. K. Radiation-induced activation of a common variant of EGFR confers enhanced radioresistance. Radiother. Oncol. 72, 267–273 (2004).
Lammering, G. et al. EGFRvIII-mediated radioresistance through a strong cytoprotective response. Oncogene 22, 5545–5553 (2003).
Chang, L. et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 4, e875 (2013).
Ni, J. et al. Epithelial cell adhesion molecule (EpCAM) is associated with prostate cancer metastasis and chemo/radioresistance via the PI3K/Akt/mTOR signaling pathway. Int. J. Biochem. Cell Biol. 45, 2736–2748 (2013).
Fruman, D. A. & Rommel, C. PI3K and cancer: lessons, challenges and opportunities. Nat. Rev. Drug Discov. 13, 140–156 (2014).
Janku, F., Yap, T. A. & Meric-Bernstam, F. Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol. 15, 273–291 (2018).
Kim, I. A. et al. Selective inhibition of Ras, phosphoinositide 3 kinase, and Akt isoforms increases the radiosensitivity of human carcinoma cell lines. Cancer Res. 65, 7902–7910 (2005).
Brognard, J., Clark, A. S., Ni, Y. & Dennis, P. A. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 61, 3986–3997 (2001).
Hasslacher, S. et al. Inhibition of PI3K signalling increases the efficiency of radiotherapy in glioblastoma cells. Int. J. Oncol. 53, 1881–1896 (2018).
Shi, F. et al. The PI3K inhibitor GDC-0941 enhances radiosensitization and reduces chemoresistance to temozolomide in GBM cell lines. Neuroscience 346, 298–308 (2017).
Park, J. H. et al. Radiosensitization of the PI3K inhibitor HS-173 through reduction of DNA damage repair in pancreatic cancer. Oncotarget 8, 112893–112906 (2017).
Zumsteg, Z. S. et al. Taselisib (GDC-0032), a potent β-sparing small molecule inhibitor of PI3K, radiosensitizes head and neck squamous carcinomas containing activating PIK3CA alterations. Clin. Cancer Res. 22, 2009–2019 (2016).
Glorieux, M., Dok, R. & Nuyts, S. The influence of PI3K inhibition on the radiotherapy response of head and neck cancer cells. Sci. Rep. 10, 16208 (2020).
Djuzenova, C. S. et al. Differential effects of the Akt inhibitor MK-2206 on migration and radiation sensitivity of glioblastoma cells. BMC Cancer 19, 299 (2019).
Miyasaka, A. et al. PI3K/mTOR pathway inhibition overcomes radioresistance via suppression of the HIF1-α/VEGF pathway in endometrial cancer. Gynecol. Oncol. 138, 174–180 (2015).
Yu, C. C. et al. Targeting the PI3K/AKT/mTOR signaling pathway as an effectively radiosensitizing strategy for treating human oral squamous cell carcinoma in vitro and in vivo. Oncotarget 8, 68641–68653 (2017).
Eke, I. et al. Exploiting radiation-induced signaling to increase the susceptibility of resistant cancer cells to targeted drugs: AKT and mTOR inhibitors as an example. Mol. Cancer Ther. 17, 355–367 (2018).
Chuang, F. C. et al. PI3k inhibitors (BKM120 and BYL719) as radiosensitizers for head and neck squamous cell carcinoma during radiotherapy. PLoS ONE 16, e0245715 (2021).
Juvekar, A. et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2, 1048–1063 (2012).
Gonzalez-Billalabeitia, E. et al. Vulnerabilities of PTEN-TP53-deficient prostate cancers to compound PARP-PI3K inhibition. Cancer Discov. 4, 896–904 (2014).
Bian, X. et al. PTEN deficiency sensitizes endometrioid endometrial cancer to compound PARP-PI3K inhibition but not PARP inhibition as monotherapy. Oncogene 37, 341–351 (2018).
Philip, C. A. et al. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer 17, 638 (2017).
Lammering, G. et al. Inhibition of the type III epidermal growth factor receptor variant mutant receptor by dominant-negative EGFR-CD533 enhances malignant glioma cell radiosensitivity. Clin. Cancer Res. 10, 6732–6743 (2004).
Akashi, Y. et al. Enhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor status. Br. J. Cancer 98, 749–755 (2008).
Raben, D. et al. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin. Cancer Res. 11, 795–805 (2005).
Yu, T. et al. Radiosensitizing effect of lapatinib in human epidermal growth factor receptor 2-positive breast cancer cells. Oncotarget 7, 79089–79100 (2016).
Huang, T. et al. Pyrotinib enhances the radiosensitivity of HER2‑overexpressing gastric and breast cancer cells. Oncol. Rep. 44, 2634–2644 (2020).
Wu, S. et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat. Cell Biol. 21, 1027–1040 (2019).
Candas-Green, D. et al. Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells. Nat. Commun. 11, 4591 (2020).
Cao, C. et al. Vascular endothelial growth factor tyrosine kinase inhibitor AZD2171 and fractionated radiotherapy in mouse models of lung cancer. Cancer Res. 66, 11409–11415 (2006).
Melsens, E. et al. The VEGFR inhibitor cediranib improves the efficacy of fractionated radiotherapy in a colorectal cancer xenograft model. Eur. Surg. Res. 58, 95–108 (2017).
Liao, J. et al. Apatinib potentiates irradiation effect via suppressing PI3K/AKT signaling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 38, 454 (2019).
Chen, L. et al. VEGF knockdown enhances radiosensitivity of nasopharyngeal carcinoma by inhibiting autophagy through the activation of mTOR pathway. Sci. Rep. 10, 16328 (2020).
SenthilKumar, G. et al. FGFR inhibition enhances sensitivity to radiation in non-small cell lung cancer. Mol. Cancer Ther. 19, 1255–1265 (2020).
De Bacco, F. et al. MET inhibition overcomes radiation resistance of glioblastoma stem-like cells. EMBO Mol. Med. 8, 550–568 (2016).
Truman, J. P. et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS ONE 5, e12310 (2010).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Bonner, J. A. et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 11, 21–28 (2010).
Vermorken, J. B. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 359, 1116–1127 (2008).
Mesia, R. et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 16, 208–220 (2015).
Giralt, J. et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 16, 221–232 (2015).
Bonomo, P. et al. Incidence of skin toxicity in squamous cell carcinoma of the head and neck treated with radiotherapy and cetuximab: a systematic review. Crit. Rev. Oncol. Hematol. 120, 98–110 (2017).
Tougeron, D. et al. Skin inflammatory response and efficacy of anti-epidermal growth factor receptor therapy in metastatic colorectal cancer (CUTACETUX). Oncoimmunology 9, 1848058 (2020).
Zheng, M. H. et al. Combining whole-brain radiotherapy with gefitinib/erlotinib for brain metastases from non-small-cell lung cancer: a meta-analysis. Biomed. Res. Int. 2016, 5807346 (2016).
Kulinich, D. P. et al. Radiotherapy versus combination radiotherapy-bevacizumab for the treatment of recurrent high-grade glioma: a systematic review. Acta Neurochir. 163, 1921–1934 (2021).
Andronesi, O. C. et al. Early changes in glioblastoma metabolism measured by MR spectroscopic imaging during combination of anti-angiogenic cediranib and chemoradiation therapy are associated with survival. NPJ Precis. Oncol. 1, 20 (2017).
Batchelor, T. T. et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl Acad. Sci. USA 110, 19059–19064 (2013).
Zhao, F. et al. Apatinib alone or combined with radiotherapy in metastatic prostate cancer: results from a pilot, multicenter study. Oncotarget 8, 110774–110784 (2017).
Yamamoto, N. et al. Phase 2 study of nimotuzumab in combination with concurrent chemoradiotherapy in patients with locally advanced non-small-cell lung cancer. Clin. Lung Cancer 22, 134–141 (2021).
Du, X. J. et al. Efficacy and safety of nimotuzumab in addition to radiotherapy and temozolomide for cerebral glioblastoma: a phase II multicenter clinical trial. J. Cancer 10, 3214–3223 (2019).
Fleischhack, G. et al. Nimotuzumab and radiotherapy for treatment of newly diagnosed diffuse intrinsic pontine glioma (DIPG): a phase III clinical study. J. Neurooncol 143, 107–113 (2019).
Dunn, L. A. et al. A phase 1b study of cetuximab and BYL719 (Alpelisib) concurrent with intensity modulated radiation therapy in stage III-IVB head and neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 106, 564–570 (2020).
Day, D. et al. Phase I trial of alpelisib in combination with concurrent cisplatin-based chemoradiotherapy in patients with locoregionally advanced squamous cell carcinoma of the head and neck. Oral. Oncol. 108, 104753 (2020).
McGowan, D. R. et al. Buparlisib with thoracic radiotherapy and its effect on tumour hypoxia: a phase I study in patients with advanced non-small cell lung carcinoma. Eur. J. Cancer 113, 87–95 (2019).
Wen, P. Y. et al. Phase I, open-label, multicentre study of buparlisib in combination with temozolomide or with concomitant radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. ESMO Open 5, e000673 (2020).
Vanacker, H., Cassier, P. A. & Bachelot, T. The complex balance of PI3K inhibition. Ann. Oncol. 32, 127–128 (2021).
Wise-Draper, T. M. et al. A phase Ib study of the dual PI3K/mTOR inhibitor dactolisib (BEZ235) combined with everolimus in patients with advanced solid malignancies. Target. Oncol. 12, 323–332 (2017).
Rodon, J. et al. Phase 1/1b dose escalation and expansion study of BEZ235, a dual PI3K/mTOR inhibitor, in patients with advanced solid tumors including patients with advanced breast cancer. Cancer Chemother. Pharmacol. 82, 285–298 (2018).
Salazar, R. et al. Phase II study of BEZ235 versus everolimus in patients with mammalian target of rapamycin inhibitor-naive advanced pancreatic neuroendocrine tumors. Oncologist 23, 766–e90 (2018).
Carlo, M. I. et al. A phase Ib study of BEZ235, a dual inhibitor of phosphatidylinositol 3-Kinase (PI3K) and mammalian target of rapamycin (mTOR), in patients with advanced renal cell carcinoma. Oncologist 21, 787–788 (2016).
Narayan, V. et al. Phase 1 trial of everolimus and radiation therapy for salvage treatment of biochemical recurrence in prostate cancer patients following prostatectomy. Int. J. Radiat. Oncol. Biol. Phys. 97, 355–361 (2017).
Gelsomino, F. et al. A dose-finding and biomarker evaluation phase Ib study of everolimus in association with 5-fluorouracil and pelvic radiotherapy as neoadjuvant treatment of locally advanced rectal cancer (E-LARC study). Clin. Colorectal Cancer 16, 410–415.e1 (2017).
Chinnaiyan, P. et al. A randomized phase II study of everolimus in combination with chemoradiation in newly diagnosed glioblastoma: results of NRG Oncology RTOG 0913. Neuro Oncol. 20, 666–673 (2018).
Cao, L. et al. Trastuzumab improves locoregional control in HER2-positive breast cancer patients following adjuvant radiotherapy. Medicine 95, e4230 (2016).
Jeon, S. H. et al. Effects of trastuzumab on locoregional recurrence in human epidermal growth factor receptor 2-overexpressing breast cancer patients treated with chemotherapy and radiotherapy. Breast Cancer Res. Treat. 172, 619–626 (2018).
Sun, G. Y. et al. Trastuzumab provides a comparable prognosis in patients with HER2-positive breast cancer to those with HER2-negative breast cancer: post hoc analyses of a randomized controlled trial of post-mastectomy hypofractionated radiotherapy. Front. Oncol. 10, 605750 (2020).
Abi Jaoude, J. et al. De-intensifying radiation therapy in HER-2 positive breast cancer: to boost or not to boost? Int. J. Radiat. Oncol. Biol. Phys. 108, 1040–1046 (2020).
Chumsri, S. et al. Incidence of late relapses in patients with HER2-positive breast cancer receiving adjuvant trastuzumab: combined analysis of NCCTG N9831 (Alliance) and NRG Oncology/NSABP B-31. J. Clin. Oncol. 37, 3425–3435 (2019).
Bonzano, E., Guenzi, M. & Corvò, R. Cardiotoxicity assessment after different adjuvant hypofractionated radiotherapy concurrently associated with trastuzumab in early breast cancer. In Vivo 32, 879–882 (2018).
Sayan, M. et al. Acute cardiotoxicity with concurrent trastuzumab and hypofractionated radiation therapy in breast cancer patients. Front. Oncol. 9, 970 (2019).
Khan, M., Zhao, Z., Arooj, S., Zheng, T. & Liao, G. Lapatinib plus local radiation therapy for brain metastases from HER-2 positive breast cancer patients and role of trastuzumab: a systematic review and meta-analysis. Front. Oncol. 10, 576926 (2020).
Harrington, K. et al. Randomised phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. Eur. J. Cancer 49, 1609–1618 (2013).
Harrington, K. et al. Postoperative adjuvant lapatinib and concurrent chemoradiotherapy followed by maintenance lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: a phase III, randomized, double-blind, placebo-controlled study. J. Clin. Oncol. 33, 4202–4209 (2015).
Lolkema, M. P. et al. The c-Met tyrosine kinase inhibitor JNJ-38877605 causes renal toxicity through species-specific insoluble metabolite formation. Clin. Cancer Res. 21, 2297–2304 (2015).
Derynck, R., Turley, S. J. & Akhurst, R. J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 18, 9–34 (2021).
Kirshner, J. et al. Inhibition of transforming growth factor-β1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res. 66, 10861–10869 (2006).
Liu, Q. et al. Subjugation of TGFβ signaling by human papilloma virus in head and neck squamous cell carcinoma shifts DNA repair from homologous recombination to alternative end joining. Clin. Cancer Res. 24, 6001–6014 (2018).
Bouquet, F. et al. TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin. Cancer Res. 17, 6754–6765 (2011).
Hardee, M. E. et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 72, 4119–4129 (2012).
Zhang, M. et al. Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 71, 7155–7167 (2011).
Gonzalez-Junca, A. et al. Positron emission tomography imaging of functional transforming growth factor β (TGFβ) activity and benefit of TGFβ inhibition in irradiated intracranial tumors. Int. J. Radiat. Oncol. Biol. Phys. 109, 527–539 (2021).
Du, S. et al. Attenuation of the DNA damage response by transforming growth factor-beta inhibitors enhances radiation sensitivity of non-small-cell lung cancer cells in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 91, 91–99 (2015).
Bellomo, C., Caja, L. & Moustakas, A. Transforming growth factor β as regulator of cancer stemness and metastasis. Br. J. Cancer 115, 761–769 (2016).
Biswas, S. et al. Inhibition of TGF-β with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J. Clin. Invest. 117, 1305–1313 (2007).
Vanpouille-Box, C. et al. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 75, 2232–2242 (2015).
Rodríguez-Ruiz, M. E. et al. TGFβ blockade enhances radiotherapy abscopal efficacy effects in combination with anti-PD1 and anti-CD137 immunostimulatory monoclonal antibodies. Mol. Cancer Ther. 18, 621–631 (2019).
Rodriguez-Ruiz, M. E. et al. Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. Oncoimmunology 8, e1655964 (2019).
Lind, H. et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J. Immunother. Cancer 8, e000433 (2020).
Lan, Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 10, eaan5488 (2018).
Wang, S. et al. Plasma Levels of IL-8 and TGF-β1 predict radiation-induced lung toxicity in non-small cell lung cancer: a validation study. Int. J. Radiat. Oncol. Biol. Phys. 98, 615–621 (2017).
Kim, H. et al. LXA(4)-FPR2 signaling regulates radiation-induced pulmonary fibrosis via crosstalk with TGF-β/Smad signaling. Cell Death Dis. 11, 653 (2020).
Han, G. et al. Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis. Nat. Med. 19, 421–428 (2013).
Lee, J. W. et al. Inhibition of Smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch. Otolaryngol. Head. Neck Surg. 136, 714–719 (2010).
Boerma, M., Wang, J., Sridharan, V., Herbert, J. M. & Hauer-Jensen, M. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS ONE 8, e70479 (2013).
Flechsig, P. et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-β and BMP-associated proinflammatory and proangiogenic signals. Clin. Cancer Res. 18, 3616–3627 (2012).
Luo, J. et al. Smad7 promotes healing of radiotherapy-induced oral mucositis without compromising oral cancer therapy in a xenograft mouse model. Clin. Cancer Res. 25, 808–818 (2019).
Ciardiello, D., Elez, E., Tabernero, J. & Seoane, J. Clinical development of therapies targeting TGFβ: current knowledge and future perspectives. Ann. Oncol. 31, 1336–1349 (2020).
Formenti, S. C. et al. Focal irradiation and systemic TGFβ blockade in metastatic breast cancer. Clin. Cancer Res. 24, 2493–2504 (2018).
Formenti, S. C. et al. Baseline T cell dysfunction by single cell network profiling in metastatic breast cancer patients. J. Immunother. Cancer 7, 177 (2019).
De Martino, M. et al. Activin a promotes regulatory T-cell-mediated immunosuppression in irradiated breast cancer. Cancer Immunol. Res. 9, 89–102 (2021).
Wick, A. et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-beta receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Invest. New Drugs 38, 1570–1579 (2020).
Vanpouille-Box, C. & Formenti, S. C. Dual transforming growth factor-β and programmed death-1 blockade: a strategy for immune-excluded tumors? Trends Immunol. 39, 435–437 (2018).
Levy, J. M. M., Towers, C. G. & Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017).
Galluzzi, L., Bravo-San, J. M. P., Levine, B., Green, D. R. & Kroemer, G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 16, 487–511 (2017).
Jin, X. et al. Role of autophagy in high linear energy transfer radiation-induced cytotoxicity to tumor cells. Cancer Sci. 105, 770–778 (2014).
Galati, S., Boni, C., Gerra, M. C., Lazzaretti, M. & Buschini, A. Autophagy: a player in response to oxidative stress and DNA damage. Oxid. Med. Cell Longev. 2019, 5692958 (2019).
Wang, W. J. et al. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro. Acta Pharmacol. Sin. 34, 681–690 (2013).
Cao, C. et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 66, 10040–10047 (2006).
Albert, J. M., Kim, K. W., Cao, C. & Lu, B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol. Cancer Ther. 5, 1183–1189 (2006).
Woo, Y. et al. Rapamycin promotes ROS-mediated cell death via functional inhibition of xCT expression in melanoma under γ-irradiation. Front. Oncol. 11, 665420 (2021).
Zhuang, W. et al. Induction of autophagy promotes differentiation of glioma-initiating cells and their radiosensitivity. Int. J. Cancer 129, 2720–2731 (2011).
Saleh, A. D. et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle 12, 1955–1963 (2013).
Simone, B. A. et al. Caloric restriction coupled with radiation decreases metastatic burden in triple negative breast cancer. Cell Cycle 15, 2265–2274 (2016).
Classen, F. et al. Autophagy induced by ionizing radiation promotes cell death over survival in human colorectal cancer cells. Exp. Cell Res. 374, 29–37 (2019).
Galluzzi, L. & Green, D. R. Autophagy-independent functions of the autophagy machinery. Cell 177, 1682–1699 (2019).
Kimmelman, A. C. & White, E. Autophagy and tumor metabolism. Cell Metab. 25, 1037–1043 (2017).
Liu, E. Y. et al. Loss of autophagy causes a synthetic lethal deficiency in DNA repair. Proc. Natl Acad. Sci. USA 112, 773–778 (2015).
Hewitt, G. & Korolchuk, V. I. Repair, reuse, recycle: the expanding role of autophagy in genome maintenance. Trends Cell Biol. 27, 340–351 (2017).
Wang, Y. et al. Autophagy regulates chromatin ubiquitination in DNA damage response through elimination of SQSTM1/p62. Mol. Cell 63, 34–48 (2016).
Park, J. M., Tougeron, D., Huang, S., Okamoto, K. & Sinicrope, F. A. Beclin 1 and UVRAG confer protection from radiation-induced DNA damage and maintain centrosome stability in colorectal cancer cells. PLoS One 9, e100819 (2014).
Chen, X. et al. Autophagy enhanced the radioresistance of non-small cell lung cancer by regulating ROS level under hypoxia condition. Int. J. Radiat. Biol. 93, 764–770 (2017).
Chaachouay, H. et al. AMPK-independent autophagy promotes radioresistance of human tumor cells under clinical relevant hypoxia in vitro. Radiother. Oncol. 116, 409–416 (2015).
Jing, Q. et al. Wnt3a promotes radioresistance via autophagy in squamous cell carcinoma of the head and neck. J. Cell Mol. Med. 23, 4711–4722 (2019).
Hu, J. L. et al. Inhibition of ATG12-mediated autophagy by miR-214 enhances radiosensitivity in colorectal cancer. Oncogenesis 7, 16 (2018).
Zheng, X. et al. Inhibiting autophagy with chloroquine enhances the anti-tumor effect of high-LET carbon ions via ER stress-related apoptosis. Med. Oncol. 34, 25 (2017).
Tseng, H. C. et al. Sensitizing effect of 3-methyladenine on radiation-induced cytotoxicity in radio-resistant HepG2 cells in vitro and in tumor xenografts. Chem. Biol. Interact. 192, 201–208 (2011).
Chen, Y. et al. Combining radiation with autophagy inhibition enhances suppression of tumor growth and angiogenesis in esophageal cancer. Mol. Med. Rep. 12, 1645–1652 (2015).
Ko, A. et al. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 21, 92–99 (2014).
Lin, W. et al. Autophagy confers DNA damage repair pathways to protect the hematopoietic system from nuclear radiation injury. Sci. Rep. 5, 12362 (2015).
Xu, F. et al. Autophagy promotes the repair of radiation-induced DNA damage in bone marrow hematopoietic cells via enhanced STAT3 signaling. Radiat. Res. 187, 382–396 (2017).
Xu, F. et al. Nuclear localization of Beclin 1 promotes radiation-induced DNA damage repair independent of autophagy. Sci. Rep. 7, 45385 (2017).
Janji, B., Hasmim, M., Parpal, S., Berchem, G. & Noman, M. Z. Firing up the cold tumors by targeting Vps34. Oncoimmunology 9, 1809936 (2020).
Arensman, M. D. et al. Anti-tumor immunity influences cancer cell reliance upon ATG7. Oncoimmunology 9, 1800162 (2020).
Pietrocola, F., Bravo-San Pedro, J. M., Galluzzi, L. & Kroemer, G. Autophagy in natural and therapy-driven anticancer immunosurveillance. Autophagy 13, 2163–2170 (2017).
Manic, G., Obrist, F., Kroemer, G., Vitale, I. & Galluzzi, L. Chloroquine and hydroxychloroquine for cancer therapy. Mol. Cell Oncol. 1, e29911 (2014).
Muller, R. Systemic toxicity of chloroquine and hydroxychloroquine: prevalence, mechanisms, risk factors, prognostic and screening possibilities. Rheumatol. Int. 41, 1189–1202 (2021).
Rosenfeld, M. R. et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy 10, 1359–1368 (2014).
Compter, I. et al. Chloroquine combined with concurrent radiotherapy and temozolomide for newly diagnosed glioblastoma: a phase IB trial. Autophagy 17, 2604–2612 (2020).
Rojas-Puentes, L. L. et al. Phase II randomized, double-blind, placebo-controlled study of whole-brain irradiation with concomitant chloroquine for brain metastases. Radiat. Oncol. 8, 209 (2013).
Eldredge, H. B. et al. Concurrent whole brain radiotherapy and short-course chloroquine in patients with brain metastases: a pilot trial. J. Radiat. Oncol. 2, 315–321 (2013).
Clarke, A. J. & Simon, A. K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 19, 170–183 (2019).
Vanpouille-Box, C. et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618 (2017).
Roach, M. C., Bradley, J. D. & Robinson, C. G. Optimizing radiation dose and fractionation for the definitive treatment of locally advanced non-small cell lung cancer. J. Thorac. Dis. 10, S2465–S2473 (2018).
Stollings, L. M. et al. Immune modulation by volatile anesthetics. Anesthesiology 125, 399–411 (2016).
Byrne, A. T. et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 17, 254–268 (2017).
Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Demaria, S. et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 58, 862–870 (2004).
Lhuillier, C., Vanpouille-Box, C., Galluzzi, L., Formenti, S. C. & Demaria, S. Emerging biomarkers for the combination of radiotherapy and immune checkpoint blockers. Semin. Cancer Biol. 52, 125–134 (2018).
Landman, Y. et al. Durvalumab after concurrent chemotherapy and high-dose radiotherapy for locally advanced non-small cell lung cancer. Oncoimmunology 10, 1959979 (2021).
Antonia, S. J. et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 377, 1919–1929 (2017).
Nishino, M., Ramaiya, N. H., Hatabu, H. & Hodi, F. S. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 14, 655–668 (2017).
Paz-Ares, L. et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. 31, 798–806 (2020).
Theelen, W. et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 5, 1276–1282 (2019).
Sun, X. S. et al. Debio 1143 and high-dose cisplatin chemoradiotherapy in high-risk locoregionally advanced squamous cell carcinoma of the head and neck: a double-blind, multicentre, randomised, phase 2 study. Lancet Oncol. 21, 1173–1187 (2020).
Le Tourneau, C. et al. Phase I trial of debio 1143, an antagonist of inhibitor of apoptosis proteins, combined with cisplatin chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck. Clin. Cancer Res. 26, 6429–6436 (2020).
Galluzzi, L., Yamazaki, T. & Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 19, 731–745 (2018).
Vitale, I., Galluzzi, L., Castedo, M. & Kroemer, G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 12, 385–392 (2011).
Lei, G. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 30, 146–162 (2020).
Ye, L. F. et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem. Biol. 15, 469–484 (2020).
Lhuillier, C. et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Invest. 131, e138740 (2021).
Wennerberg, E. et al. Immune recognition of irradiated cancer cells. Immunol. Rev. 280, 220–230 (2017).
Yamazaki, T. & Galluzzi, L. Mitochondrial control of innate immune signaling by irradiated cancer cells. Oncoimmunology 9, 1797292 (2020).
Golden, E. B. et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 3, e28518 (2014).
Turchan, W. T., Pitroda, S. P. & Weichselbaum, R. R. Radiotherapy and immunotherapy combinations in the treatment of patients with metastatic disease: current status and future focus. Clin. Cancer Res. 27, 5188 (2021).
Batlle, E. & Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 (2019).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Chakravarthy, A., Khan, L., Bensler, N. P., Bose, P. & De Carvalho, D. D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9, 4692 (2018).
Galluzzi, L., Chan, T. A., Kroemer, G., Wolchok, J. D. & Lopez-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 10, eaat7807 (2018).
Jobling, M. F. et al. Isoform-specific activation of latent transforming growth factor β (LTGF-β) by reactive oxygen species. Radiat. Res. 166, 839–848 (2006).
Barcellos-Hoff, M. H. & Cucinotta, F. A. New tricks for an old fox: impact of TGFβ on the DNA damage response and genomic stability. Sci. Signal. 7, re5 (2014).
Wiegman, E. M., Blaese, M. A., Loeffler, H., Coppes, R. P. & Rodemann, H. P. TGFβ-1 dependent fast stimulation of ATM and p53 phosphorylation following exposure to ionizing radiation does not involve TGFβ-receptor I signalling. Radiother. Oncol. 83, 289–295 (2007).
Galluzzi, L. et al. Molecular definitions of autophagy and related processes. EMBO J. 36, 1811–1836 (2017).
Galluzzi, L. et al. Autophagy in malignant transformation and cancer progression. EMBO J. 34, 856–880 (2015).
White, E., Lattime, E. C. & Guo, J. Y. Autophagy regulates stress responses, metabolism, and anticancer immunity. Trends Cancer 7, 778–789 (2021).
Acknowledgements
The work of L.C.C. is supported by US National Institutes of Health (NIH) P01 (#P01CA120964) and R35 (#R35CA197588) grants. The work of S.C.F. is supported by a Breakthrough Level 2 grant from the US Department of Defense (DoD), Breast Cancer Research Program (BRCP) (#BC180476) and by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177) from Stand Up to Cancer (SU2C). The work of L.G. is supported by a Breakthrough Level 2 grant from the US DoD BRCP (#BC180476P1), by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177, PI: Formenti) from SU2C, by a Mantle Cell Lymphoma Research Initiative (MCL-RI, PI: Chen-Kiang) grant from the Leukaemia and Lymphoma Society (LLS), by a startup grant from the Department of Radiation Oncology at Weill Cornell Medicine (New York, USA), by a Rapid Response Grant from the Functional Genomics Initiative (New York, USA), by industrial collaborations with Lytix (Oslo, Norway) and Phosplatin (New York, USA), and by donations from Phosplatin (New York, USA), the Luke Heller TECPR2 Foundation (Boston, USA), Onxeo (Paris, France), Ricerchiamo (Brescia, Italy) and Sotio a.s. (Prague, Czech Republic).
Author information
Authors and Affiliations
Contributions
L.G. and S.C.F. conceived the article. G.P. and L.G. wrote the first version of the manuscript with critical input from L.C.C., L.S. and S.C.F. G.P. prepared display items under supervision from L.G. All authors approve the final version of the article.
Corresponding author
Ethics declarations
Competing interests
L.S. has received research funding from Puretech. L.C.C. has acted as a consultant and/or advisor to Agios Pharmaceuticals, Faeth Therapeutics, Larkspur Therapeutics and Volastra Therapeutics, has received research funding from Petra Pharmaceuticals and is a co-founder of, and holds equity in, Agios Pharmaceuticals, Faeth Therapeutics, Larkspur Therapeutics and Volastra Therapeutics. S.C.F. has acted as a consultant and/or advisor to AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Elekta, EMD Serono, GlaxoSmithKline, Janssen, MedImmune, Merck US, Regeneron, Varian and ViewRay, and has received research funding from Bristol Myers Squibb, Eli-Lilly, Merck, Regeneron and Varian; and other support from Pfizer. L.G. has acted as a consultant and/or advisor to AstraZeneca, Boehringer Ingelheim, Inzen, The Longevity Labs, the Luke Heller TECPR2 Foundation, OmniSEQ and Onxeo, and has received research funding from Lytix, and Phosplatin. G.P. declares no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks E. Dikomey, Y. Li, Y. Tao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Petroni, G., Cantley, L.C., Santambrogio, L. et al. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nat Rev Clin Oncol 19, 114–131 (2022). https://doi.org/10.1038/s41571-021-00579-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00579-w
This article is cited by
-
CYLD induces high oxidative stress and DNA damage through class I HDACs to promote radiosensitivity in nasopharyngeal carcinoma
Cell Death & Disease (2024)
-
Oncolytic mineralized bacteria as potent locally administered immunotherapeutics
Nature Biomedical Engineering (2024)
-
Cellular senescence in the response of HR+ breast cancer to radiotherapy and CDK4/6 inhibitors
Journal of Translational Medicine (2023)
-
Myoglobin-loaded gadolinium nanotexaphyrins for oxygen synergy and imaging-guided radiosensitization therapy
Nature Communications (2023)
-
Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications
Signal Transduction and Targeted Therapy (2023)