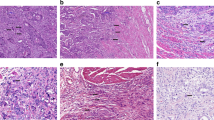
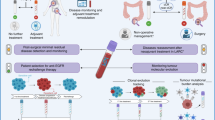
Abstract
Tumour budding is an emerging prognostic biomarker in colorectal cancer (CRC) and other solid cancers. Tumour buds are usually defined as isolated single cancer cells or clusters of up to four cancer cells located at the invasive tumour front. The prognostic value of tumour budding is now supported by a large body of evidence, whereas the utility of this phenotype as a predictive biomarker remains under investigation. The application of tumour budding indices in clinical practice requires a standardized scoring system that can be tailored to specific tumour types and clinical scenarios. In the context of CRC, tumour budding can be assessed according to the method agreed at the International Tumour Budding Consensus Conference (ITBCC) in 2016. Using the ITBCC scoring system, tumour budding is an independent predictor of lymph node metastasis in patients with pT1 CRC and of unfavourable survival in patients with stage II colon cancer. Regardless of the clinical scenario or tumour type, the assertion that ‘the more tumour buds, the worse the clinical outcome’ applies. In this Review, we provide an overview of tumour budding in solid cancers, highlighting the molecular and biological aspects of this phenomenon, including its associations with epithelial–mesenchymal transition and features of the tumour microenvironment. We also describe the available evidence demonstrating the value of tumour budding as a biomarker across various solid cancers.
Key points
-
Tumour budding is an independent prognostic factor across a variety of solid cancers.
-
In general, the higher the tumour bud count, the worse the clinical outcome.
-
Tumour budding is included as a prognostic factor in published cancer classification guidelines of the Union for International Cancer Control (UICC), the American Joint Committee on Cancer (AJCC) and the World Health Organization (WHO).
-
Grading systems for tumour budding vary between different types of solid cancers.
-
Tumour budding is strongly associated with epithelial–mesenchymal transition and various factors in the tumour microenvironment, where individual tumour buds interact with diverse components of the tumour stroma and immune system.
-
The development of international, evidence-based, standardized scoring systems for tumour budding is essential for future multicentre retrospective clinical studies and prospective randomized clinical trials in order to better define the different prognostic groups.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brierley, J. D., Gospodarowicz, M. K. & Wittekind, C. TNM Classification of Malignant Tumours. 8th edn (Wiley Blackwell, 2017).
Amin, M. B. et al. (eds). AJCC Cancer Staging Manual. 8th edn (Springer, 2017).
WHO Classification of Tumours Editorial Board. in Digestive System Tumours. 5th edn (IARC, 2019).
Koelzer, V. H., Langer, R., Zlobec, I. & Lugli, A. Tumor budding in upper gastrointestinal carcinomas. Front. Oncol. 4, 216 (2014).
Kadota, K. et al. Tumor budding correlates with the protumor immune microenvironment and is an independent prognostic factor for recurrence of stage I lung adenocarcinoma. Chest 148, 711–721 (2015).
Mitrovic, B., Schaeffer, D. F., Riddell, R. H. & Kirsch, R. Tumor budding in colorectal carcinoma: time to take notice. Mod. Pathol. 25, 1315–1325 (2012).
Imai, T. Growth patterns in human carcinoma. Their classification and relation to prognosis. Obstet. Gynecol. 16, 296–308 (1960).
Berg, K. B. & Schaeffer, D. F. Tumor budding as a standardized parameter in gastrointestinal carcinomas: more than just the colon. Mod. Pathol. 31, 862–872 (2018).
Almangush, A., Salo, T., Hagstrom, J. & Leivo, I. Tumour budding in head and neck squamous cell carcinoma - a systematic review. Histopathology 65, 587–594 (2014).
Zlobec, I. & Lugli, A. Tumour budding in colorectal cancer: molecular rationale for clinical translation. Nat. Rev. Cancer 18, 203–204 (2018).
Lugli, A. et al. Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum. Pathol. 42, 1833–1840 (2011).
Lugli, A. et al. Recommendations for reporting tumor budding in colorectal cancer based on the international tumor budding consensus conference (ITBCC) 2016. Mod. Pathol. 30, 1299–1311 (2017).
Loughrey, M. et al. Colorectal Cancer Histopathology Reporting Guide. (International Collaboration on Cancer Reporting, 2020).
Burgart, L. et al. Protocol for the examination of excisional biopsy specimens from patients with primary carcinoma of the colon and rectum. Version 4.1.0.0. College of American Pathologists https://documents.cap.org/protocols/cp-gilower-colonrectum-resection-20-4100.pdf (2020).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Klymkowsky, M. W. & Savagner, P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am. J. Pathol. 174, 1588–1593 (2009).
Polyak, K. & Weinberg, R. A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265–273 (2009).
Yilmaz, M. & Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 28, 15–33 (2009).
Attramadal, C. G. et al. Tumor budding, EMT and cancer stem cells in T1-2/N0 oral squamous cell carcinomas. Anticancer Res. 35, 6111–6120 (2015).
Kohler, I. et al. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol. 30, 78–84 (2015).
Nakagawa, Y. et al. Tumor budding and E-cadherin expression are useful predictors of nodal involvement in T1 esophageal squamous cell carcinoma. Anticancer Res. 33, 5023–5029 (2013).
Lee, S. J. et al. Combined aberrant expression of E-cadherin and S100A4, but not beta-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn. Pathol. 8, 99 (2013).
Zlobec, I. et al. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J. Pathol. 212, 260–268 (2007).
Koyuncuoglu, M. et al. Tumor budding and E-Cadherin expression in endometrial carcinoma: are they prognostic factors in endometrial cancer? Gynecol. Oncol. 125, 208–213 (2012).
Jensen, D. H. et al. Molecular profiling of tumour budding implicates TGFβ-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J. Pathol. 236, 505–516 (2015).
Galvan, J. A. et al. Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br. J. Cancer 112, 1944–1950 (2015).
Hong, K. O. et al. Tumor budding is associated with poor prognosis of oral squamous cell carcinoma and histologically represents an epithelial-mesenchymal transition process. Hum. Pathol. 80, 123–129 (2018).
Dardare, J., Witz, A., Merlin, J. L., Gilson, P. & Harle, A. SMAD4 and the TGFβ pathway in patients with pancreatic ductal adenocarcinoma. Int. J. Mol. Sci. 21, 3534 (2020).
Oyanagi, H. et al. SMAD4 alteration associates with invasive-front pathological markers and poor prognosis in colorectal cancer. Histopathology 74, 873–882 (2019).
Gosens, M. J., van Kempen, L. C., van de Velde, C. J., van Krieken, J. H. & Nagtegaal, I. D. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod. Pathol. 20, 221–232 (2007).
Lugli, A. et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br. J. Cancer 103, 382–390 (2010).
Lawlor, R. T. et al. Prognostic role of high-grade tumor budding in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis with a focus on epithelial to mesenchymal transition. Cancers 11, 113 (2019).
Maffeis, V. et al. Tumor budding is an adverse prognostic marker in intestinal-type sinonasal adenocarcinoma and seems to be unrelated to epithelial-mesenchymal transition. Virchows Arch. 477, 241–248 (2020).
Galvan, J. A. et al. TWIST1 and TWIST2 promoter methylation and protein expression in tumor stroma influence the epithelial-mesenchymal transition-like tumor budding phenotype in colorectal cancer. Oncotarget 6, 874–885 (2015).
Yamada, N. et al. Tumor budding at the invasive front of colorectal cancer may not be associated with the epithelial-mesenchymal transition. Hum. Pathol. 60, 151–159 (2017).
Derynck, R. & Weinberg, R. A. EMT and cancer: more than meets the eye. Dev. Cell 49, 313–316 (2019).
Zhou, B. et al. Interaction between laminin-5gamma2 and integrin beta1 promotes the tumor budding of colorectal cancer via the activation of Yes-associated proteins. Oncogene 39, 1527–1542 (2020).
Katayama, M. & Sekiguchi, K. Laminin-5 in epithelial tumour invasion. J. Mol. Histol. 35, 277–286 (2004).
Shinto, E., Mochizuki, H., Ueno, H., Matsubara, O. & Jass, J. R. A novel classification of tumour budding in colorectal cancer based on the presence of cytoplasmic pseudo-fragments around budding foci. Histopathology 47, 25–31 (2005).
Shinto, E. et al. Tumor buds show reduced expression of laminin-5 gamma 2 chain in DNA mismatch repair deficient colorectal cancer. Dis. Colon. Rectum 49, 1193–1202 (2006).
Okado, Y. et al. Tumor budding and laminin5-gamma2 in squamous cell carcinoma of the external auditory canal are associated with shorter survival. Springerplus 4, 814 (2015).
Marangon Junior, H. et al. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J. Oral Pathol. Med. 43, 199–204 (2014).
Masuda, R. et al. Laminin-5gamma2 chain expression is associated with tumor cell invasiveness and prognosis of lung squamous cell carcinoma. Biomed. Res. 33, 309–317 (2012).
Taira, T. et al. Characterization of the immunophenotype of the tumor budding and its prognostic implications in squamous cell carcinoma of the lung. Lung Cancer 76, 423–430 (2012).
Kevans, D. et al. Epithelial-mesenchymal transition (EMT) protein expression in a cohort of stage II colorectal cancer patients with characterized tumor budding and mismatch repair protein status. Int. J. Surg. Pathol. 19, 751–760 (2011).
Masaki, T. et al. Laminin-5 gamma 2 chain and matrix metalloproteinase-2 may trigger colorectal carcinoma invasiveness through formation of budding tumor cells. Anticancer Res. 23, 4113–4119 (2003).
Sordat, I. et al. Tumor cell budding and laminin-5 expression in colorectal carcinoma can be modulated by the tissue micro-environment. Int. J. Cancer 88, 708–717 (2000).
Hlubek, F., Spaderna, S., Jung, A., Kirchner, T. & Brabletz, T. -catenin activates a coordinated expression of the proinvasive factors laminin-5 γ2 chain and MT1-MMP in colorectal carcinomas. Int. J. Cancer 108, 321–326 (2004).
Prall, F. & Ostwald, C. High-degree tumor budding and podia-formation in sporadic colorectal carcinomas with K-ras gene mutations. Hum. Pathol. 38, 1696–1702 (2007).
Shinto, E. et al. Differential prognostic significance of morphologic invasive markers in colorectal cancer: tumor budding and cytoplasmic podia. Dis. Colon. Rectum 49, 1422–1430 (2006).
Rizzi, C. et al. The expression of the high-mobility group A2 protein in colorectal cancer and surrounding fibroblasts is linked to tumor invasiveness. Hum. Pathol. 44, 122–132 (2013).
Sugai, T. et al. Vascular invasion and stromal S100A4 expression at the invasive front of colorectal cancer are novel determinants and tumor prognostic markers. J. Cancer 8, 1552–1561 (2017).
Markl, B. et al. Tumour budding, uPA and PAI-1 are associated with aggressive behaviour in colon cancer. J. Surg. Oncol. 102, 235–241 (2010).
Guzinska-Ustymowicz, K. MMP-9 and cathepsin B expression in tumor budding as an indicator of a more aggressive phenotype of colorectal cancer (CRC). Anticancer Res. 26, 1589–1594 (2006).
Karamitopoulou, E. et al. Loss of Raf-1 kinase inhibitor protein (RKIP) is strongly associated with high-grade tumor budding and correlates with an aggressive phenotype in pancreatic ductal adenocarcinoma (PDAC). J. Transl Med. 11, 311 (2013).
Banias, L. et al. Nuclear maspin expression: A biomarker for budding assessment in colorectal cancer specimens. Pathol. Res. Pract. 213, 1227–1230 (2017).
Dawson, H. et al. Tyrosine kinase receptor B (TrkB) expression in colorectal cancers highlights anoikis resistance as a survival mechanism of tumour budding cells. Histopathology 66, 715–725 (2015).
Tanaka, K. et al. Tropomyosin-related receptor kinase B at the invasive front and tumour cell dedifferentiation in gastric cancer. Br. J. Cancer 110, 2923–2934 (2014).
Righi, A. et al. Tumour budding is associated with hypoxia at the advancing front of colorectal cancer. Histopathology 66, 982–990 (2015).
Hatzikirou, H., Basanta, D., Simon, M., Schaller, K. & Deutsch, A. ‘Go or grow’: the key to the emergence of invasion in tumour progression? Math. Med. Biol. 29, 49–65 (2012).
Dawson, H. et al. The apoptotic and proliferation rate of tumour budding cells in colorectal cancer outlines a heterogeneous population of cells with various impacts on clinical outcome. Histopathology 64, 577–584 (2014).
Rubio, C. A. Arrest of cell proliferation in budding tumor cells ahead of the invading edge of colonic carcinomas. A preliminary report. Anticancer Res. 28, 2417–2420 (2008).
Marangon Junior, H. et al. Cell proliferation is associated with intensity of tumor budding in oral squamous cell carcinoma. J. Oral Pathol. Med. 47, 128–135 (2018).
Hacking, S. et al. Tumor budding in colorectal carcinoma showing a paradoxical mitotic index (Via PHH3) with possible association to the tumor stromal microenvironment. Appl. Immunohistochem. Mol. Morphol. https://doi.org/10.1097/PAI.0000000000000805 (2019).
Boxberg, M. et al. Immunohistochemical expression of CD44 in oral squamous cell carcinoma in relation to histomorphological parameters and clinicopathological factors. Histopathology 73, 559–572 (2018).
Zheng, Z. et al. Heterogeneous expression of Lgr5 as a risk factor for focal invasion and distant metastasis of colorectal carcinoma. Oncotarget 9, 30025–30033 (2018).
Zhou, Y. et al. Cancer stem cells in progression of colorectal cancer. Oncotarget 9, 33403–33415 (2018).
Hostettler, L., Zlobec, I., Terracciano, L. & Lugli, A. ABCG5-positivity in tumor buds is an indicator of poor prognosis in node-negative colorectal cancer patients. World J. Gastroenterol. 16, 732–739 (2010).
Meyer, S. N. et al. Co-expression of cytokeratin and vimentin in colorectal cancer highlights a subset of tumor buds and an atypical cancer-associated stroma. Hum. Pathol. 87, 18–27 (2019).
Chouat, E. et al. Tumor budding is a prognostic factor linked to epithelial mesenchymal transition in pancreatic ductal adenocarcinoma. Study report and literature review. Pancreatology 18, 79–84 (2018).
Wang, C. et al. Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma. J. Oral Pathol. Med. 40, 545–551 (2011).
Carr, I., Levy, M. & Watson, P. The invasive edge: invasion in colorectal cancer. Clin. Exp. Metastasis 4, 129–139 (1986).
Jass, J. R., Love, S. B. & Northover, J. M. A new prognostic classification of rectal cancer. Lancet 1, 1303–1306 (1987).
Bronsert, P. et al. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J. Pathol. 234, 410–422 (2014).
Prall, F., Ostwald, C. & Linnebacher, M. Tubular invasion and the morphogenesis of tumor budding in colorectal carcinoma. Hum. Pathol. 40, 1510–1512 (2009).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Trinh, A. et al. Tumour budding is associated with the mesenchymal colon cancer subtype and RAS/RAF mutations: a study of 1320 colorectal cancers with Consensus Molecular Subgroup (CMS) data. Br. J. Cancer 119, 1244–1251 (2018).
De Smedt, L. et al. Expression profiling of budding cells in colorectal cancer reveals an EMT-like phenotype and molecular subtype switching. Br. J. Cancer 116, 58–65 (2017).
Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018).
Arner, E. N., Du, W. & Brekken, R. A. Behind the wheel of epithelial plasticity in KRAS-Driven Cancers. Front. Oncol. 9, 1049 (2019).
Steinestel, K., Lennerz, J. K., Eder, S., Kraft, K. & Arndt, A. Invasion pattern and histologic features of tumor aggressiveness correlate with MMR protein expression, but are independent of activating KRAS and BRAF mutations in CRC. Virchows Arch. 465, 155–163 (2014).
Barresi, V., Bonetti, L. R. & Bettelli, S. KRAS, NRAS, BRAF mutations and high counts of poorly differentiated clusters of neoplastic cells in colorectal cancer: observational analysis of 175 cases. Pathology 47, 551–556 (2015).
Jang, S. et al. KRAS and PIK3CA mutations in colorectal adenocarcinomas correlate with aggressive histological features and behavior. Hum. Pathol. 65, 21–30 (2017).
Jass, J. R. et al. APC mutation and tumour budding in colorectal cancer. J. Clin. Pathol. 56, 69–73 (2003).
Xie, N. et al. Decreased miR-320a promotes invasion and metastasis of tumor budding cells in tongue squamous cell carcinoma. Oncotarget 7, 65744–65757 (2016).
Karamitopoulou, E. et al. MicroRNA dysregulation in the tumor microenvironment influences the phenotype of pancreatic cancer. Mod. Pathol. 30, 1116–1125 (2017).
Moller, T. et al. Co-detection of miR-21 and TNF-α mRNA in budding cancer cells in colorectal cancer. Int. J. Mol. Sci. 20, 1907 (2019).
Knudsen, K. N. et al. miR-21 expression analysis in budding colon cancer cells by confocal slide scanning microscopy. Clin. Exp. Metastasis 35, 819–830 (2018).
Mongroo, P. S. & Rustgi, A. K. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol. Ther. 10, 219–222 (2010).
Martinez-Ciarpaglini, C. et al. Low miR200c expression in tumor budding of invasive front predicts worse survival in patients with localized colon cancer and is related to PD-L1 overexpression. Mod. Pathol. 32, 306–313 (2019).
Boland, C. R. & Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 138, 2073–2087.e3 (2010).
Koelzer, V. H. et al. Active immunosurveillance in the tumor microenvironment of colorectal cancer is associated with low frequency tumor budding and improved outcome. Transl Res. 166, 207–217 (2015).
Koelzer, V. H. et al. Phenotyping of tumor-associated macrophages in colorectal cancer: impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology 5, e1106677 (2016).
Zlobec, I., Minoo, P., Terracciano, L., Baker, K. & Lugli, A. Characterization of the immunological microenvironment of tumour buds and its impact on prognosis in mismatch repair-proficient and -deficient colorectal cancers. Histopathology 59, 482–495 (2011).
Orhan, A. et al. The prognostic value of tumour-infiltrating lymphocytes in pancreatic cancer: a systematic review and meta-analysis. Eur. J. Cancer 132, 71–84 (2020).
Zhao, Y. et al. Prognostic value and clinicopathological roles of phenotypes of tumour-associated macrophages in colorectal cancer. J. Cancer Res. Clin. Oncol. 145, 3005–3019 (2019).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Idos, G. E. et al. The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: a systematic review and meta-analysis. Sci. Rep. 10, 3360 (2020).
Kuwahara, T. et al. Intratumoural-infiltrating CD4+ and FOXP3+ T cells as strong positive predictive markers for the prognosis of resectable colorectal cancer. Br. J. Cancer 121, 659–665 (2019).
Pages, F. et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139 (2018).
Lugli, A. et al. CD8+lymphocytes/ tumour-budding index: an independent prognostic factor representing a ‘pro-/anti-tumour’ approach to tumour host interaction in colorectal cancer. Br. J. Cancer 101, 1382–1392 (2009).
Karamitopoulou, E. Tumour microenvironment of pancreatic cancer: immune landscape is dictated by molecular and histopathological features. Br. J. Cancer 121, 5–14 (2019).
Dawson, H. et al. Tumour budding/T cell infiltrates in colorectal cancer: proposal of a novel combined score. Histopathology 76, 572–580 (2019).
Nearchou, I. P. et al. Automated analysis of lymphocytic infiltration, tumor budding, and their spatial relationship improves prognostic accuracy in colorectal cancer. Cancer Immunol. Res. 7, 609–620 (2019).
Wartenberg, M. et al. Integrated genomic and immunophenotypic classification of pancreatic cancer reveals three distinct subtypes with prognostic/predictive significance. Clin. Cancer Res. 24, 4444–4454 (2018).
Ueno, H. et al. Histologic categorization of desmoplastic reaction: its relevance to the colorectal cancer microenvironment and prognosis. Ann. Surg. Oncol. 22, 1504–1512 (2015).
Rogers, A. C. et al. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br. J. Cancer 115, 831–840 (2016).
Bosch, S. L., Teerenstra, S., de Wilt, J. H., Cunningham, C. & Nagtegaal, I. D. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy 45, 827–834 (2013).
Ueno, H. et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 127, 385–394 (2004).
Cappellesso, R. et al. Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: a meta-analysis. Hum. Pathol. 65, 62–70 (2017).
Betge, J. et al. Tumor budding is an independent predictor of outcome in AJCC/UICC stage II colorectal cancer. Ann. Surg. Oncol. 19, 3706–3712 (2012).
Zlobec, I. et al. Intratumoural budding (ITB) in preoperative biopsies predicts the presence of lymph node and distant metastases in colon and rectal cancer patients. Br. J. Cancer 110, 1008–1013 (2014).
Giger, O. T., Comtesse, S. C., Lugli, A., Zlobec, I. & Kurrer, M. O. Intra-tumoral budding in preoperative biopsy specimens predicts lymph node and distant metastasis in patients with colorectal cancer. Mod. Pathol. 25, 1048–1053 (2012).
Rogers, A. C. et al. Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod. Pathol. 27, 156–162 (2014).
Almangush, A. et al. Evaluation of the budding and depth of invasion (BD) model in oral tongue cancer biopsies. Virchows Arch. 472, 231–236 (2018).
Almangush, A. et al. Does evaluation of tumour budding in diagnostic biopsies have a clinical relevance? A systematic review. Histopathology 74, 536–544 (2019).
Jesinghaus, M. et al. Pre-operative cellular dissociation grading in biopsies is highly predictive of post-operative tumour stage and patient outcome in head and neck squamous cell carcinoma. Br. J. Cancer 122, 835–846 (2020).
Lino-Silva, L. S. et al. Mismatch repair protein expression and intratumoral budding in rectal cancer are associated with an increased pathological complete response to preoperative chemoradiotherapy: a case-control study. World J. Clin. Oncol. 9, 133–139 (2018).
Seki, M., Sano, T., Yokoo, S. & Oyama, T. Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck 38, E1582–E1590 (2016).
Backes, Y. et al. Histologic factors associated with need for surgery in patients with pedunculated T1 colorectal carcinomas. Gastroenterology 154, 1647–1659 (2018).
Barel, F. et al. Histopathological factors help to predict lymph node metastases more efficiently than extra-nodal recurrences in submucosa invading pT1 colorectal cancer. Sci. Rep. 9, 8342 (2019).
Slik, K. et al. Combined epithelial marker analysis of tumour budding in stage II colorectal cancer. J. Pathol. Clin. Res. 5, 63–78 (2019).
Nearchou, I. P. et al. Novel internationally verified method reports desmoplastic reaction as the most significant prognostic feature for disease-specific survival in stage II colorectal cancer. Am. J. Surg. Pathol. 43, 1239–1248 (2019).
Romiti, A. et al. Study of histopathologic parameters to define the prognosis of stage II colon cancer. Int. J. Colorectal Dis. 34, 905–913 (2019).
Lee, V. W. K. & Chan, K. F. Tumor budding and poorly-differentiated cluster in prognostication in Stage II colon cancer. Pathol. Res. Pract. 214, 402–407 (2018).
Ueno, H. et al. Prospective multicenter study on the prognostic and predictive impact of tumor budding in stage II colon cancer: results from the SACURA trial. J. Clin. Oncol. 37, 1886–1894 (2019).
van Wyk, H. C. et al. The relationship between tumor budding, tumor microenvironment, and survival in patients with primary operable colorectal cancer. Ann. Surg. Oncol. 26, 4397–4404 (2019).
Almangush, A., Karhunen, M., Hautaniemi, S., Salo, T. & Leivo, I. Prognostic value of tumour budding in oesophageal cancer: a meta-analysis. Histopathology 68, 173–182 (2016).
Roh, M. S., Lee, J. I. & Choi, P. J. Tumor budding as a useful prognostic marker in esophageal squamous cell carcinoma. Dis. Esophagus 17, 333–337 (2004).
Koike, M. et al. Multivariate analysis of the pathologic features of esophageal squamous cell cancer: tumor budding is a significant independent prognostic factor. Ann. Surg. Oncol. 15, 1977–1982 (2008).
Miyata, H. et al. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer 115, 3324–3334 (2009).
Brown, M. et al. Tumour budding and a low host inflammatory response are associated with a poor prognosis in oesophageal and gastro-oesophageal junction cancers. Histopathology 56, 893–899 (2010).
Nakanishi, Y. et al. Correlation between tumor budding and post-resection prognosis in patients with invasive squamous cell carcinoma of the thoracic esophagus. World J. Surg. 35, 349–356 (2011).
Ito, E. et al. New invasive patterns as a prognostic factor for superficial esophageal cancer. J. Gastroenterol. 47, 1279–1289 (2012).
Teramoto, H. et al. Tumor budding as a useful prognostic marker in T1-stage squamous cell carcinoma of the esophagus. J. Surg. Oncol. 108, 42–46 (2013).
Niwa, Y. et al. Epithelial to mesenchymal transition correlates with tumor budding and predicts prognosis in esophageal squamous cell carcinoma. J. Surg. Oncol. 110, 764–769 (2014).
Jesinghaus, M. et al. A novel grading system based on tumor budding and cell nest size is a strong predictor of patient outcome in esophageal squamous cell carcinoma. Am. J. Surg. Pathol. 41, 1112–1120 (2017).
Jesinghaus, M. et al. Cellular dissociation grading based on the parameters tumor budding and cell nest size in pretherapeutic biopsy specimens allows for prognostic patient stratification in esophageal squamous cell carcinoma independent from clinical staging. Am. J. Surg. Pathol. 43, 618–627 (2019).
Min, B. H. et al. Nomogram for prediction of lymph node metastasis in patients with superficial esophageal squamous cell carcinoma. J. Gastroenterol. Hepatol. 3, 1009–1015 (2020).
Mitobe, J. et al. Clinicopathological investigation of lymph node metastasis predictors in superficial esophageal squamous cell carcinoma with a focus on evaluation of lympho-vascular invasion. Scand. J. Gastroenterol. 48, 1173–1182 (2013).
Fuchinoue, K. et al. Immunohistochemical analysis of tumor budding as predictor of lymph node metastasis from superficial esophageal squamous cell carcinoma. Esophagus 17, 168–174 (2020).
Landau, M. S. et al. Tumor budding is associated with an increased risk of lymph node metastasis and poor prognosis in superficial esophageal adenocarcinoma. Mod. Pathol. 27, 1578–1589 (2014).
Thies, S. et al. Impact of peritumoral and intratumoral budding in esophageal adenocarcinomas. Hum. Pathol. 52, 1–8 (2016).
Gabbert, H. E., Meier, S., Gerharz, C. D. & Hommel, G. Tumor-cell dissociation at the invasion front: a new prognostic parameter in gastric cancer patients. Int. J. Cancer 50, 202–207 (1992).
Kemi, N., Eskuri, M., Ikalainen, J., Karttunen, T. J. & Kauppila, J. H. Tumor budding and prognosis in gastric adenocarcinoma. Am. J. Surg. Pathol. 43, 229–234 (2019).
Olsen, S., Jin, L., Fields, R. C., Yan, Y. & Nalbantoglu, I. Tumor budding in intestinal-type gastric adenocarcinoma is associated with nodal metastasis and recurrence. Hum. Pathol. 68, 26–33 (2017).
Ulase, D., Heckl, S., Behrens, H. M., Kruger, S. & Rocken, C. Prognostic significance of tumour budding assessed in gastric carcinoma according to the criteria of the International Tumour Budding Consensus Conference. Histopathology 76, 433–446 (2020).
Guo, Y. X., Zhang, Z. Z., Zhao, G. & Zhao, E. H. Prognostic and pathological impact of tumor budding in gastric cancer: A systematic review and meta-analysis. World J. Gastrointest. Oncol. 11, 898–908 (2019).
Che, K. et al. Prognostic significance of tumor budding and single cell invasion in gastric adenocarcinoma. Onco. Targets Ther. 10, 1039–1047 (2017).
Du, M. et al. Tumor budding and other risk factors of lymph node metastasis in submucosal early gastric carcinoma: a multicenter clinicopathologic study in 621 radical gastrectomies of chinese patients. Am. J. Surg. Pathol. 43, 1074–1082 (2019).
Gulluoglu, M. et al. Tumor budding is independently predictive for lymph node involvement in early gastric cancer. Int. J. Surg. Pathol. 23, 349–358 (2015).
Bailey, P. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016).
Puleo, F. et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology 155, 1999–2013.e3 (2018).
Lang-Schwarz, C. et al. Budding and tumor-infiltrating lymphocytes - combination of both parameters predicts survival in colorectal cancer and leads to new prognostic subgroups. Hum. Pathol. 79, 160–167 (2018).
Ogino, M. et al. Impact of tumour budding grade in 310 patients who underwent surgical resection for extrahepatic cholangiocarcinoma. Histopathology 74, 861–872 (2019).
Ohike, N. et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am. J. Surg. Pathol. 34, 1417–1424 (2010).
Okubo, S. et al. The prognostic impact of differentiation at the invasive front of biliary tract cancer. J. Surg. Oncol. 117, 1278–1287 (2018).
Tanaka, M. et al. Tumor budding in intrahepatic cholangiocarcinoma: a predictor of postsurgery outcomes. Am. J. Surg. Pathol. 43, 1180–1190 (2019).
Ueno, H., Murphy, J., Jass, J. R., Mochizuki, H. & Talbot, I. C. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40, 127–132 (2002).
Kai, K. et al. Tumor budding and dedifferentiation in gallbladder carcinoma: potential for the prognostic factors in T2 lesions. Virchows Arch. 459, 449–456 (2011).
Almangush, A. et al. Tumour budding in oral squamous cell carcinoma: a meta-analysis. Br. J. Cancer 118, 577–586 (2018).
Yu, P. et al. A novel prognostic model for tongue squamous cell carcinoma based on the characteristics of tumour and its microenvironment: iBD score. Histopathology 74, 766–779 (2019).
Ho, Y. Y., Wu, T. Y., Cheng, H. C., Yang, C. C. & Wu, C. H. The significance of tumor budding in oral cancer survival and its relevance to the eighth edition of the American Joint committee on cancer staging system. Head Neck 41, 2991–3001 (2019).
Xie, N. et al. Validation of the International Tumor Budding Consensus Conference (2016) recommendations in oral tongue squamous cell carcinoma. J. Oral Pathol. Med. 48, 451–458 (2019).
Elseragy, A. et al. A proposal to revise the histopathologic grading system of early oral tongue cancer incorporating tumor budding. Am. J. Surg. Pathol. 43, 703–709 (2019).
Luo, W. R., Gao, F., Li, S. Y. & Yao, K. T. Tumour budding and the expression of cancer stem cell marker aldehyde dehydrogenase 1 in nasopharyngeal carcinoma. Histopathology 61, 1072–1081 (2012).
Marangon Junior, H. et al. Immunolocalization of cancer stem cells marker ALDH1 and its association with tumor budding in oral squamous cell carcinoma. Head. Neck Pathol. 13, 535–542 (2019).
Makitie, A. A., Almangush, A., Rodrigo, J. P., Ferlito, A. & Leivo, I. Hallmarks of cancer: tumor budding as a sign of invasion and metastasis in head and neck cancer. Head Neck 41, 3712–3718 (2019).
Neppl, C., Zlobec, I., Schmid, R. A. & Berezowska, S. Validation of the International Tumor Budding Consensus Conference (ITBCC) 2016 recommendation in squamous cell carcinoma of the lung-a single-center analysis of 354 cases. Mod. Pathol. 33, 802–811 (2020).
Yamaguchi, Y. et al. Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J. Thorac. Oncol. 5, 1361–1368 (2010).
Masuda, R. et al. Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. Mol. Med. Rep. 6, 937–943 (2012).
Kadota, K. et al. Comprehensive pathological analyses in lung squamous cell carcinoma: single cell invasion, nuclear diameter, and tumor budding are independent prognostic factors for worse outcomes. J. Thorac. Oncol. 9, 1126–1139 (2014).
Gujam, F. J., McMillan, D. C., Mohammed, Z. M., Edwards, J. & Going, J. J. The relationship between tumour budding, the tumour microenvironment and survival in patients with invasive ductal breast cancer. Br. J. Cancer 113, 1066–1074 (2015).
Sun, Y. et al. Prognostic value of poorly differentiated clusters in invasive breast cancer. World J. Surg. Oncol. 12, 310 (2014).
Liang, F. et al. The prognostic value of tumor budding in invasive breast cancer. Pathol. Res. Pract. 209, 269–275 (2013).
Salhia, B. et al. High tumor budding stratifies breast cancer with metastatic properties. Breast Cancer Res. Treat. 150, 363–371 (2015).
Laedrach, C., Salhia, B., Cihoric, N., Zlobec, I. & Tapia, C. Immunophenotypic profile of tumor buds in breast cancer. Pathol. Res. Pract. 214, 25–29 (2018).
Park, J. Y., Hong, D. G., Chong, G. O. & Park, J. Y. Tumor budding is a valuable diagnostic parameter in prediction of disease progression of endometrial endometrioid carcinoma. Pathol. Oncol. Res. 25, 723–730 (2019).
Huang, B., Cai, J., Xu, X., Guo, S. & Wang, Z. High-grade tumor budding stratifies early-stage cervical cancer with recurrence risk. PLoS ONE 11, e0166311 (2016).
Satabongkoch, N. et al. Prognostic value of tumor budding in early-stage cervical adenocarcinomas. Asian Pac. J. Cancer Prev. 18, 1717–1722 (2017).
Jesinghaus, M. et al. Introducing a novel highly prognostic grading scheme based on tumour budding and cell nest size for squamous cell carcinoma of the uterine cervix. J. Pathol. Clin. Res. 4, 93–102 (2018).
Kucuk, U. et al. Prognostic significance of tumor budding in muscle invasive urothelial carcinomas of the bladder. Turk. J. Urol. 45, 273–278 (2018).
Koelzer, V. H. et al. Tumor budding in colorectal cancer revisited: results of a multicenter interobserver study. Virchows Arch. 466, 485–493 (2015).
Hase, K., Shatney, C., Johnson, D., Trollope, M. & Vierra, M. Prognostic value of tumor “budding” in patients with colorectal cancer. Dis. Colon Rectum 36, 627–635 (1993).
Ueno, H. et al. A new prognostic staging system for rectal cancer. Ann. Surg. 240, 832–839 (2004).
Nakamura, T., Mitomi, H., Kikuchi, S., Ohtani, Y. & Sato, K. Evaluation of the usefulness of tumor budding on the prediction of metastasis to the lung and liver after curative excision of colorectal cancer. Hepatogastroenterology 52, 1432–1435 (2005).
Lugli, A., Karamitopoulou, E. & Zlobec, I. Tumour budding: a promising parameter in colorectal cancer. Br. J. Cancer 106, 1713–1717 (2012).
Rieger, G. et al. Comprehensive assessment of tumour budding by cytokeratin staining in colorectal cancer. Histopathology 70, 1044–1051 (2017).
Bokhorst, J. M. et al. Assessment of individual tumor buds using keratin immunohistochemistry: moderate interobserver agreement suggests a role for machine learning. Mod. Pathol. 33, 825–833 (2020).
Fauzi, M. F. A. et al. Tumor budding detection system in whole slide pathology images. J. Med. Syst. 44, 38 (2019).
Takamatsu, M. et al. Immunohistochemical evaluation of tumor budding for stratifying T1 colorectal cancer: optimal cut-off value and a novel computer-assisted semiautomatic method. Mod. Pathol. 32, 675–683 (2019).
Weis, C. A. et al. Automatic evaluation of tumor budding in immunohistochemically stained colorectal carcinomas and correlation to clinical outcome. Diagn. Pathol. 13, 64 (2018).
Jepsen, R. K. et al. Digital image analysis of pan-cytokeratin stained tumor slides for evaluation of tumor budding in pT1/pT2 colorectal cancer: results of a feasibility study. Pathol. Res. Pract. 214, 1273–1281 (2018).
Brieu, N. et al. Automated tumour budding quantification by machine learning augments TNM staging in muscle-invasive bladder cancer prognosis. Sci. Rep. 9, 5174 (2019).
Pedersen, N. J. et al. Construction of a pathological risk model of occult lymph node metastases for prognostication by semi-automated image analysis of tumor budding in early-stage oral squamous cell carcinoma. Oncotarget 8, 18227–18237 (2017).
Karamitopoulou, E. et al. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur. J. Cancer 49, 1032–1039 (2013).
Liu, D. N. et al. Superior mesenteric artery margin in pancreaticoduodenectomy for pancreatic adenocarcinoma. Oncotarget 8, 7766–7776 (2017).
Lohneis, P. et al. Tumour buds determine prognosis in resected pancreatic ductal adenocarcinoma. Br. J. Cancer 118, 1485–1491 (2018).
O’Connor, K. et al. Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma. Am. J. Surg. Pathol. 39, 472–478 (2015).
Zhang, L., Guo, L., Tao, M., Fu, W. & Xiu, D. Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma. Chin. J. Cancer Res. 28, 180–186 (2016).
Zhao, Y. et al. Invasion types are associated with poor prognosis in lung squamous carcinoma patients. Medicine 94, e1634 (2015).
Weichert, W. et al. Proposal of a prognostically relevant grading scheme for pulmonary squamous cell carcinoma. Eur. Respir. J. 47, 938–946 (2016).
Kadota, K. et al. Tumor spread through air spaces is an independent predictor of recurrence-free survival in patients with resected lung squamous cell carcinoma. Am. J. Surg. Pathol. 41, 1077–1086 (2017).
Kadota, K. et al. A grading system combining tumor budding and nuclear diameter predicts prognosis in resected lung squamous cell carcinoma. Am. J. Surg. Pathol. 41, 750–760 (2017).
Acknowledgements
The authors acknowledge research funding from the KWF Kankerbestrijding (Dutch Cancer Society; grant 10602 to A.L., I.Z., R.K. and I.D.N.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks Alhadi Almangush, Moritz Jesinghaus and Dongya Jia for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lugli, A., Zlobec, I., Berger, M.D. et al. Tumour budding in solid cancers. Nat Rev Clin Oncol 18, 101–115 (2021). https://doi.org/10.1038/s41571-020-0422-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-0422-y
This article is cited by
-
Assessing the applicability and interobserver variability of tumor budding and poorly differentiated clusters in colorectal cancer
Surgical and Experimental Pathology (2024)
-
Tumour budding in head and neck cancer: what have we learnt and the next steps towards clinical implementation
British Journal of Cancer (2024)
-
Predicting 5-year recurrence risk in colorectal cancer: development and validation of a histology-based deep learning approach
British Journal of Cancer (2024)
-
Establish a novel tumor budding-related signature to predict prognosis and guide clinical therapy in colorectal cancer
Scientific Reports (2024)
-
Personalizing adjuvant therapy for patients with colorectal cancer
Nature Reviews Clinical Oncology (2024)