Abstract
Many effective drugs for metastatic and/or advanced-stage cancers have been developed over the past decade, although the evolution of resistance remains the major barrier to disease control or cure. In large, diverse populations such as the cells that compose metastatic cancers, the emergence of cells that are resistant or that can quickly develop resistance is virtually inevitable and most likely cannot be prevented. However, clinically significant resistance occurs only when the pre-existing resistant phenotypes are able to proliferate extensively, a process governed by eco-evolutionary dynamics. Attempts to disrupt the molecular mechanisms of resistance have generally been unsuccessful in clinical practice. In this Review, we focus on the Darwinian processes driving the eco-evolutionary dynamics of treatment-resistant cancer populations. We describe a variety of evolutionarily informed strategies designed to increase the probability of disease control or cure by anticipating and steering the evolutionary dynamics of acquired resistance.
Key points
-
Despite the increasing numbers of effective agents for treating metastatic cancers, evolution of resistance remains the fundamental barrier to cure and long-term disease control.
-
Efforts to overcome resistance by blocking the molecular machinery of resistance have had little clinical success, probably because many alternative evolutionary resistance pathways are accessible to cancer cells.
-
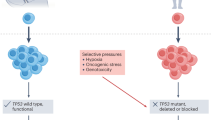
Resistant cells are almost inevitably present in the large heterogeneous populations of metastatic cancers, and the subsequent fates of those cells (proliferation, quiescence or death) are governed by evolutionary forces.
-
Adaptive therapy cycles the application of treatment to synchronize with patient-specific intratumoural evolutionary dynamics to suppress proliferation of resistant cells and prolong response to treatment.
-
Extinction therapy involves the aggressive application of new perturbations (second strikes) to the resistant cells following the initial (first strike) therapy to exploit the vulnerabilities of small populations with the explicit goal of cure.
-
Clinical trial designs based on evolutionary mathematical models enable the detailed evaluation of outcomes in individual patients, thus promoting an understanding of why the trial failed or succeeded and providing guidance for new strategies designed to optimize patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Strebhardt, K. & Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 8, 473–480 (2008).
Simon, R. & Norton, L. The Norton-Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat. Clin. Pract. Oncol. 3, 406–407 (2006).
Bayat Mokhtari, R. et al. Combination therapy in combating cancer. Oncotarget 8, 38022–38043 (2017).
DeVita, V. T. Jr. & Chu, E. A history of cancer chemotherapy. Cancer Res. 68, 8643–8653 (2008).
Lippman, M. E. High-dose chemotherapy plus autologous bone marrow transplantation for metastatic breast cancer. N. Engl. J. Med. 342, 1119–1120 (2000).
Al Hamed, R., Bazarbachi, A. H., Malard, F., Harousseau, J. L. & Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 9, 44 (2019).
Fernandez, H. F. et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 361, 1249–1259 (2009).
Bregni, M., Badoglio, M., Pedrazzoli, P. & Lanza, F. Is allogeneic transplant for solid tumors still alive? Bone Marrow Transpl. 51, 751–752 (2016).
Vasan, N., Baselga, J. & Hyman, D. M. A view on drug resistance in cancer. Nature 575, 299–309 (2019).
Diaz, L. A. Jr. et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012).
Mostaghel, E. A. et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 17, 5913–5925 (2011).
Kmetova Sivonova, M. et al. The role of CYP17A1 in prostate cancer development: structure, function, mechanism of action, genetic variations and its inhibition. Gen. Physiol. Biophys. 36, 487–499 (2017).
Brown, J. S., Cunningham, J. J. & Gatenby, R. A. Aggregation effects and population-based dynamics as a source of therapy resistance in cancer. IEEE Trans. Biomed. Eng. 64, 512–518 (2017).
Labadie, B. W., Bao, R., Luke, J. J. & Reimagining, I. D. O. Pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon axis. Clin. Cancer Res. 25, 1462–1471 (2019).
Chen, J. et al. HIF-1alpha inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS ONE 9, e98882 (2014).
Coley, H. M. Overcoming multidrug resistance in cancer: clinical studies of P-glycoprotein inhibitors. Methods Mol. Biol. 596, 341–358 (2010).
Baer, M. R. et al. A phase 3 study of the multidruig resistance modulator PSC-833 in Previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B study 9720. Blood 100, 1224–1232 (2002).
Merlo, L. M., Pepper, J. W., Reid, B. J. & Maley, C. C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935 (2006).
Aktipis, C. A., Kwan, V. S., Johnson, K. A., Neuberg, S. L. & Maley, C. C. Overlooking evolution: a systematic analysis of cancer relapse and therapeutic resistance research. PLoS ONE 6, e26100 (2011).
Heikamp, E. B. & Pui, C. H. Next-Generation evaluation and treatment of pediatric acute lymphoblastic leukemia. J. Pediatr. 203, 14–24 e12 (2018).
Pui, C. H. et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J. Clin. Oncol. 33, 2938–2948 (2015).
Terwilliger, T. & Abdul-Hay, M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 7, e577 (2017).
Cairns, J. Mutation selection and the natural history of cancer. Nature 255, 197–200 (1975).
Gatenby, R. A., Gillies, R. J. & Brown, J. S. Of cancer and cave fish. Nat. Rev. Cancer 11, 237–238 (2011).
Ma, Y. et al. The relationship between early embryo development and tumourigenesis. J. Cell Mol. Med. 14, 2697–2701 (2010).
Dashwood, R. H. Xenobiotic metabolism relevance to cancer. J. Nutr. 136, 2681S–2682S (2006).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 3, 177–182 (1997).
Mullon, C. D., Wakano, J. Y. & Ohtsuki, H. Evolutionary feedbacks between genes and their extended effects under limited gene flow and biased ecological inheritance. Preprint at bioRxiv https://doi.org/10.1101/2020.03.10.985671 (2020).
Archetti, M. & Pienta, K. J. Cooperation among cancer cells: applying game theory to cancer. Nat. Rev. Cancer 19, 110–117 (2019).
Stankova, K., Brown, J. S., Dalton, W. S. & Gatenby, R. A. Optimizing cancer treatment using game theory: a review. JAMA Oncol. 5, 96–103 (2019).
Stackelberg, H. V., Bazin, D., Urch, L. & Hill, R. Market Structure and Equilibrium. (Springer, 2011).
Luh, P. B., C., S.-C. & Chang, T.-S. Solutions and properties of multi-stage Stackelberg games. Automatica 20, 251–256 (1984).
Shen, F. et al. Quantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cells. J. Pharmacol. Exp. Ther. 324, 95–102 (2008).
Gatenby, R. A., Cunningham, J. J. & Brown, J. S. Evolutionary triage governs fitness in driver and passenger mutations and suggests targeting never mutations. Nat. Commun. 5, 5499 (2014).
Chmielecki, J. et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci. Transl Med. 3, 90ra59 (2011).
Cunningham, J. J. A call for integrated metastatic management. Nat. Ecol. Evol. 3, 996–998 (2019).
Hussain, M. et al. Intermittent versus continuous androgen deprivation in prostate cancer. N. Engl. J. Med. 368, 1314–1325 (2013).
Crook, J. M. et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N. Engl. J. Med. 367, 895–903 (2012).
Gatenby, R. A. A change of strategy in the war on cancer. Nature 459, 508–509 (2009).
Gatenby, R. Perspective: Finding cancer’s first principles. Nature 491, S55 (2012).
Cunningham, J. J., Gatenby, R. A. & Brown, J. S. Evolutionary dynamics in cancer therapy. Mol. Pharm. 8, 2094–2100 (2011).
Gatenby, R. A., Silva, A. S., Gillies, R. J. & Frieden, B. R. Adaptive therapy. Cancer Res. 69, 4894–4903 (2009).
Enriquez-Navas, P. M. et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci. Transl Med. 8, 327ra324 (2016).
Gatenby, R. & Brown, J. The evolution and ecology of resistance in cancer therapy. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a033415 (2018).
Zhang, J., Cunningham, J. J., Brown, J. S. & Gatenby, R. A. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat. Commun. 8, 1816 (2017).
Cunningham, J. J., Brown, J. S., Gatenby, R. A. & Stankova, K. Optimal control to develop therapeutic strategies for metastatic castrate resistant prostate cancer. J. Theor. Biol. 459, 67–78 (2018).
Dong, Z. et al. Organ-specific modulation of steady-state mdr gene expression and drug resistance in murine colon cancer cells. J. Natl Cancer Inst. 86, 913–920 (1994).
Abolhoda, A. et al. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin. Cancer Res. 5, 3352–3356 (1999).
Marusyk, A., Janiszewska, M. & Polyak, K. Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell 37, 471–484 (2020).
Maley, C. C., Reid, B. J. & Forrest, S. Cancer prevention strategies that address the evolutionary dynamics of neoplastic cells: simulating benign cell boosters and selection for chemosensitivity. Cancer Epidemiol. Biomarkers Prev. 13, 1375–1384 (2004).
Basanta, D. & Anderson, A. R. A. Exploiting ecological principles to better understand cancer progression and treatment. Interface Focus https://doi.org/10.1098/rsfs.2013.0020 (2013).
Schweizer, M. T. et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Sci. Transl Med. 7, 269ra262 (2015).
Basanta, D., Gatenby, R. A. & Anderson, A. R. Exploiting evolution to treat drug resistance: combination therapy and the double bind. Mol. Pharm. 9, 914–921 (2012).
Gatenby, R. A., Brown, J. & Vincent, T. Lessons from applied ecology: cancer control using an evolutionary double bind. Cancer Res. 69, 7499–7502 (2009).
Acar, A. et al. Exploiting evolutionary herding to control drug resistance in cancer. Preprint at bioRxiv https://doi.org/10.1101/566950 (2019).
Antonia, S. J. et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin. Cancer Res. 12, 878–88 (2006).
Hardin, G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science 162, 1243–1248 (1968).
Archetti, M. Evolutionary game theory of growth factor production: implications for tumour heterogeneity and resistance to therapies. Br. J. Cancer 109, 1056–1062 (2013).
Archetti, M. Evolutionarily stable anti-cancer therapies by autologous cell defection. Evol. Med. Public Health 2013, 161–172 (2013).
Archetti, M., Ferraro, D. A. & Christofori, G. Heterogeneity for IGF-II production maintained by public goods dynamics in neuroendocrine pancreatic cancer. Proc. Natl Acad. Sci. USA 112, 1833–1838 (2015).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
You, L. et al. Spatial vs. non-spatial eco-evolutionary dynamics in a tumor growth model. J. Theor. Biol. 435, 78–97 (2017).
Gallaher, J. A., Enriquez-Navas, P. M., Luddy, K. A., Gatenby, R. A. & Anderson, A. R. A. Spatial heterogeneity and evolutionary dynamics modulate time to recurrence in continuous and adaptive cancer therapies. Cancer Res. 78, 2127–2139 (2018).
Walther, V. et al. Can oncology recapitulate paleontology? Lessons from species extinctions. Nat. Rev. Clin. Oncol. 12, 273–285 (2015).
Korolev, K. S., Xavier, J. B. & Gore, J. Turning ecology and evolution against cancer. Nat. Rev. Cancer 14, 371–380 (2014).
Hull, P. Life in the Aftermath of Mass Extinctions. Curr. Biol. 25, R941–R952 (2015).
Otto, S. P. Adaptation, speciation and extinction in the Anthropocene. Proc. Biol. Sci. https://doi.org/10.1098/rspb.2018.2047 (2018).
Raup, D. M. Extinction: Bad Genes or Bad Luck? (W.W. Norton, 1991).
Armas, O. A. et al. Clinical and pathobiological effects of neoadjuvant total androgen ablation therapy on clinically localized prostatic adenocarcinoma. Am. J. Surg. Pathol. 18, 979–991 (1994).
Fagan, W. F. & Holmes, E. E. Quantifying the extinction vortex. Ecol. Lett. 9, 51–60 (2006).
Lynch, M., Conery, J. & Burger, R. Mutational meltdowns in sexual populations. Evolution 49, 1067–1080 (1995).
Normark, B. B. & Moran, N. A. Testing for the accumulation of deleterious mutations in asexual eukaryote genomes using molecular sequences. J. Nat. History 34, 1719–1729 (2000).
Neiman, M., Hehman, G., Miller, J. T., Logsdon, J. M. Jr. & Taylor, D. R. Accelerated mutation accumulation in asexual lineages of a freshwater snail. Mol. Biol. Evol. 27, 954–963 (2010).
Ha, K. et al. Histone deacetylase inhibitor treatment induces ‘BRCAness’ and synergistic lethality with PARP inhibitor and cisplatin against human triple negative breast cancer cells. Oncotarget 5, 5637–5650 (2014).
Eigen, M. & Schuster, P. The hypercycle. a principle of natural self-organization. part a: emergence of the hypercycle. Naturwissenschaften 64, 541–565 (1977).
Johnson, J. A. & Dunn, P. O. Low genetic variation in the heath hen prior to extinction and implications for the conservation of prairie-chicken populations. Conserv. Genet. 7, 37–48 (2006).
Murray, G. G. R. et al. Natural selection shaped the rise and fall of passenger pigeon genomic diversity. Science 358, 951–954 (2017).
Allee, W. C. Animal Aggregations, a Study in General Sociology (The University of Chicago Press, 1931).
Johnson, K. E. et al. Cancer cell population growth kinetics at low densities deviate from the exponential growth model and suggest an Allee effect. PLoS Biol. 17, e3000399 (2019).
Bottger, K. et al. An emerging allee effect is critical for tumor initiation and persistence. PLoS Comput. Biol. 11, e1004366 (2015).
Boukal, D. S. & Berec, L. Single-species models of the Allee effect: extinction boundaries, sex ratios and mate encounters. J. Theor. Biol. 218, 375–394 (2002).
Neufeld, Z. et al. The role of Allee effect in modelling post resection recurrence of glioblastoma. PLoS Comput. Biol. 13, e1005818 (2017).
Konstorum, A., Hillen, T. & Lowengrub, J. Feedback regulation in a cancer stem cell model can cause an allee effect. Bull. Math. Biol. 78, 754–785 (2016).
Gillies, R. J., Brown, J. S., Anderson, A. R. A. & Gatenby, R. A. Eco-evolutionary causes and consequences of temporal changes in intratumoural blood flow. Nat. Rev. Cancer 18, 576–585 (2018).
Alllee, W. C. B. & Bowen, E. S. Studies in animal aggregations: mass protection against colloidal silver among goldfishes. J. Exp. Zool. 61, 185–207 (1932).
Parrish, J. K. & E.-K., L. Complexity, pattern and evolutionary trade-offs in animal aggregation. Science 284, 99–100 (1999).
Perkins, S. M., Shinohara, E. T., DeWees, T. & Frangoul, H. Outcome for children with metastatic solid tumors over the last four decades. PLoS ONE 9, e100396 (2014).
Fujii, T. et al. Effectiveness of an adjuvant chemotherapy regimen for early-stage breast cancer: a systematic review and network meta-analysis. JAMA Oncol. 1, 1311–1318 (2015).
Carrato, A. Adjuvant treatment of colorectal cancer. Gastrointest. Cancer Res. 2, S42–S46 (2008).
Park, C. K., Jung, W. H. & Koo, J. S. Pathologic evaluation of breast cancer after neoadjuvant therapy. J. Pathol. Transl Med. 50, 173–180 (2016).
Gatenby, R. A., Artzy-Randrup, Y., Epstein, T., Reed, D. R. & Brown, J. S. Eradicating metastatic cancer and the eco-evolutionary dynamics of Anthropocene extinctions. Cancer Res. 80, 613–623 (2020).
Gatenby, R. A., Zhang, J. & Brown, J. S. First strike-second strike strategies in metastatic cancer: lessons from the evolutionary dynamics of extinction. Cancer Res. 79, 3174–3177 (2019).
Koinis, F., Kotsakis, A. & Georgoulias, V. Small cell lung cancer (SCLC): no treatment advances in recent years. Trans. Lung Cancer Res. 5, 39–20 (2016).
Sweeney, C. J. et al. Chemohormonal therapy for metastatic hormone sensitive prostate cancer. N. Engl. J. Med. 373, 737–746 (2015).
Rudin S., Marable M., Huang R. The promise of pharmacogenomics in reducing toxicity during acute lymphoblastic leukemia maintenance treatment. Genomics Proteomics Bioinformatics https://doi.org/10.1016/j.gpb.2016.11.003 (2017).
Bhojwani, D., Yang, J. J. & Pui, C. H. Biology of childhood acute lymphoblastic leukemia. Pediatr. Clin. North. Am. 62, 47–60 (2015).
Campana, D. & Pui, C. H. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood 129, 1913–1918 (2017).
Ehler, L. E. Integrated pest management (IPM): definition, historical development and implementation, and the other IPM. Pest. Manag. Sci. 62, 787–789 (2006).
Jansen, G., Gatenby, R. & Aktipis, C. A. Opinion: control vs. eradication: applying infectious disease treatment strategies to cancer. Proc. Natl Acad. Sci. USA 112, 937–938 (2015).
Pannell, J. H. The heath hen. Science 98, 174 (1943).
Headstrom, B. R. Preservation of the heath hen. Science https://doi.org/10.1126/science.68.1749.15-a (1928).
Greenberg, J. A Feathered River Across The Sky: The Passenger Pigeon’s Flight to Extinction (Bloomsbury, 2014).
Acknowledgements
The authors thank D. Lemanne, Oregon Integrative Oncology, for providing a steady stream of good ideas for framing and applying extinction therapy. This work was supported by the European Commission Horizon 2020 research and innovation programme (grant agreement no. 690817), a James S. McDonnell Foundation grant (“Cancer Therapy: Perturbing a Complex Adaptive System”), a V Foundation grant, NIH/National Cancer Institute (NCI) grants R01CA170595 (“Application of Evolutionary Principles to Maintain Cancer Control (PQ21)”), U54CA143970-05 (Physical Science Oncology Network; “Cancer as a Complex Adaptive System”) and R01CA187532-01 (“Imaging Habitats in Sarcoma”), the National Paediatric Cancer Foundation and the Jacobson Foundation (“Integrating Evolution into Cancer Therapy”).
Author information
Authors and Affiliations
Contributions
Both authors made a substantial contribution to all aspects of the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gatenby, R.A., Brown, J.S. Integrating evolutionary dynamics into cancer therapy. Nat Rev Clin Oncol 17, 675–686 (2020). https://doi.org/10.1038/s41571-020-0411-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-0411-1
This article is cited by
-
Characterization of dabrafenib-induced drug insensitivity via cellular barcoding and collateral sensitivity to second-line therapeutics
Scientific Reports (2024)
-
Tumor microenvironment-responsive DNA-based nanomedicine triggers innate sensing for enhanced immunotherapy
Journal of Nanobiotechnology (2023)
-
The regulation loop of MARVELD1 interacting with PARP1 in DNA damage response maintains genome stability and promotes therapy resistance of cancer cells
Cell Death & Differentiation (2023)
-
Modeling cancer’s ecological and evolutionary dynamics
Medical Oncology (2023)
-
Potential of green-synthesized ZnO NPs against human ovarian teratocarcinoma: an in vitro study
Molecular Biology Reports (2023)