Abstract
Cancer of unknown primary (CUP) is an enigmatic disease entity encompassing heterogeneous malignancies without a detectable primary tumour, despite a thorough diagnostic workup. A minority of patients with CUP (15–20%) can be assigned a putative primary tissue of origin according to clinical and histopathological findings and typically have a more favourable prognosis with the use of corresponding tumour type-specific therapies. Thus, the majority of patients with CUP have disease that cannot be assigned to a culprit primary tumour, are treated with empirical chemotherapy and have a poor prognosis. In the molecular era, the use of (epi)genomic or transcriptomic CUP classifiers and DNA or RNA sequencing offers two, sometimes overlapping, therapeutic strategies: tumour type-specific therapy and biomarker-guided therapy. Published data reveal that the accuracy of site-of-origin predictions made using CUP classifiers ranges between 54% and 98% when compared with the assignment made according to the recommended clinicopathological criteria. These advances have led to promising results in non-randomized prospective studies evaluating the efficacy of tumour type-specific therapy; however, the favourable outcomes were not confirmed in randomized controlled studies comparing this approach with standard empirical chemotherapy. Currently, the evidence supporting the use of biomarker-guided therapies is limited to case reports and small case series. In this Review, we discuss the clinical management of CUP in the era of precision medicine. We focus on the advances in understanding the biology of CUP, the implications for the diagnosis and classification of CUP according to the tissue of origin and the shift away from empirical therapy towards tailored therapy.
Key points
Cancer of unknown primary (CUP) is a clinically well-recognized, but biologically enigmatic, disease entity that encompasses a heterogeneous group of metastatic cancers without an identifiable primary tumour, despite extensive investigations.
The current era is witnessing a decrease in the proportion of patients with cancer who are diagnosed with CUP to 1–2%, in comparison to 3–5% in the early 1990s.
The recommended diagnostic workup includes a thorough physical examination, basic blood analyses, evaluation of tumour biomarkers (guided by the clinical scenario) and CT scans of the thorax, abdomen and pelvis.
Multiple assays have been developed to predict the putative tissue of origin by alignment with prominent molecular profiles established for cancers with a known primary.
Together with the overall clinical picture and results of pathology investigations, molecular profiling of CUP can have therapeutic implications by guiding treatment decisions according to the putative primary tumour type and the detection of potential driver mutations.
The design of future prospective randomized trials should address the minority of patients that present with targetable mutations, especially driver mutations, based on the findings of molecular analyses and the predicted primary tumour type.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fizazi, K. et al. Cancers of unknown primary site: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26 (Suppl. 5), v133–v138 (2015).
Shu, X., Sundquist, K., Sundquist, J. & Hemminki, K. Time trends in incidence, causes of death, and survival of cancer of unknown primary in Sweden. Eur. J. Cancer Prev. 21, 281–288 (2012).
Urban, D., Rao, A., Bressel, M., Lawrence, Y. R. & Mileshkin, L. Cancer of unknown primary: a population-based analysis of temporal change and socioeconomic disparities. Br. J. Cancer 109, 1318–1324 (2013).
Brewster, D. H., Lang, J., Bhatti, L. A., Thomson, C. S. & Oien, K. A. Descriptive epidemiology of cancer of unknown primary site in Scotland, 1961–2010. Cancer Epidemiol. 38, 227–234 (2014).
Randén, M., Rutqvist, L.-E. & Johansson, H. Cancer patients without a known primary: incidence and survival trends in Sweden 1960–2007. Acta Oncol. 48, 915–920 (2009).
Levi, F., Te, V. C., Erler, G., Randimbison, L. & La Vecchia, C. Epidemiology of unknown primary tumours. Eur. J. Cancer 38, 1810–1812 (2002).
Rassy, E. & Pavlidis, N. The currently declining incidence of cancer of unknown primary. Cancer Epidemiol. 61, 139–141 (2019).
Bochtler, T. & Krämer, A. Does cancer of unknown primary (CUP) truly exist as a distinct cancer entity? Front Oncol. 9, 402 (2019).
Bochtler, T. et al. Comparative genetic profiling aids diagnosis and clinical decision making in challenging cases of CUP syndrome. Int. J. Cancer 145, 2963–2973 (2019).
Rassy, E., Kattan, J. & Pavlidis, N. Familial cancer of unknown primary. Int. J. Clin. Oncol. 24, 1328–1331 (2019).
Conway, A.-M. et al. Molecular characterisation and liquid biomarkers in carcinoma of unknown primary (CUP): taking the ‘U’ out of ‘CUP’. Br. J. Cancer 120, 141–153 (2019).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Pavlidis, N. & Pentheroudakis, G. Cancer of unknown primary site. Lancet 379, 1428–1435 (2012).
Rassy, E., Assi, T., Kattan, J. & Pavlidis, N. Paraneoplastic syndromes in cancers of unknown primary: an unknown field for oncologists. Bull Cancer 106, 590–603 (2019).
Vikeså, J. et al. Cancers of unknown primary origin (CUP) are characterized by chromosomal instability (CIN) compared to metastasis of known origin. BMC Cancer 15, 151 (2015).
Pavlidis, N., Briasoulis, E., Hainsworth, J. & Greco, F. A. Diagnostic and therapeutic management of cancer of an unknown primary. Eur. J. Cancer 39, 1990–2005 (2003).
Pavlidis, N. & Fizazi, K. Carcinoma of unknown primary (CUP). Crit. Rev. Oncol. Hematol. 69, 271–278 (2009).
Pentheroudakis, G., Briasoulis, E. & Pavlidis, N. Cancer of unknown primary site: missing primary or missing biology? Oncologist 12, 418–425 (2007).
Hemminki, K., Bevier, M., Sundquist, J. & Hemminki, A. Cancer of unknown primary (CUP): does cause of death and family history implicate hidden phenotypically changed primaries? Ann. Oncol. 23, 2720–2724 (2012).
Hemminki, K., Sundquist, K., Sundquist, J., Hemminki, A. & Ji, J. Location of metastases in cancer of unknown primary are not random and signal familial clustering. Sci. Rep. 6, 22891 (2016).
Rassy, E. et al. The role of site-specific therapy for cancers of unknown of primary: a meta-analysis. Eur. J. Cancer 127, 118–122 (2020).
Hainsworth, J. D. et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J. Clin. Oncol. 31, 217–223 (2013).
Moran, S. et al. Epigenetic profiling to classify cancer of unknown primary: a multicentre, retrospective analysis. Lancet Oncol. 17, 1386–1395 (2016).
Gross-Goupil, M. et al. Identifying the primary site using gene expression profiling in patients with carcinoma of an unknown primary (CUP): a feasibility study from the GEFCAPI. Onkologie 35, 54–55 (2012).
Rassy, E. & Pavlidis, N. The current evidence for a biomarker-based approach in cancer of unknown primary. Cancer Treat. Rev. 67, 21–28 (2018).
Hayashi, H. et al. Randomized phase II trial comparing site-specific treatment based on gene expression profiling with carboplatin and paclitaxel for patients with cancer of unknown primary site. J. Clin. Oncol. 37, 570–579 (2019).
Fizazi, K. et al. A phase III trial of empiric chemotherapy with cisplatin and gemcitabine or systemic treatment tailored by molecular gene expression analysis in patients with carcinomas of an unknown primary (CUP) site (GEFCAPI 04) [abstract LBA15_PR]. Ann. Oncol. 30 (Suppl. 5), v851–v934 (2019).
Losa, F. et al. SEOM clinical guideline on unknown primary cancer (2017). Clin. Transl. Oncol. 20, 89–96 (2018).
NICE. Metastatic malignant disease of unknown primary origin in adults: diagnosis and management. Clinical guideline. https://www.nice.org.uk/guidance/cg104/resources/metastatic-malignant-disease-of-unknown-primary-origin-in-adults-diagnosis-and-management-pdf-35109328970437 (2010).
NCCN. Occult primary (cancer of unknown primary (CUP)) version 2.2020 https://www.nccn.org/professionals/physician_gls/pdf/occult.pdf. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) (2020).
Pentheroudakis, G. & Pavlidis, N. in Wick metastatic carcinomas of unknown origin (ed. Mark, R) 165–175 (Demos Medical Publishing, 2008).
Hainsworth, J. D. & Greco, F. A. Gene expression profiling in patients with carcinoma of unknown primary site: from translational research to standard of care. Virchows Arch. 464, 393–402 (2014).
Kwee, T. C. & Kwee, R. M. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur. Radiol. 19, 731–744 (2009).
Rassy, E., Kattan, J. & Pavlidis, N. A new entity of abdominal squamous cell carcinoma of unknown primary. Eur. J. Clin. Invest. 49, e13111 (2019).
Rassy, E., Nicolai, P. & Pavlidis, N. Comprehensive management of HPV-related squamous cell carcinoma of the head and neck of unknown primary. Head Neck 41, 3700–3711 (2019).
Rassy, E. et al. New rising entities in cancer of unknown primary: is there a real therapeutic benefit? Crit. Rev. Oncol. Hematol. 147, 102882 (2020).
Varadhachary, G. R. et al. Carcinoma of unknown primary with gastrointestinal profile: immunohistochemistry and survival data for this favorable subset. Int. J. Clin. Oncol. 19, 479–484 (2014).
Lazaridis, G., Pentheroudakis, G., Fountzilas, G. & Pavlidis, N. Liver metastases from cancer of unknown primary (CUPL): a retrospective analysis of presentation, management and prognosis in 49 patients and systematic review of the literature. Cancer Treat. Rev. 34, 693–700 (2008).
Golfinopoulos, V. et al. Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: multiple-treatments meta-analysis. Cancer Treat. Rev. 35, 570–573 (2009).
Hess, K. R., Abbruzzese, M. C., Lenzi, R., Raber, M. N. & Abbruzzese, J. L. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin. Cancer Res. 5, 3403–3410 (1999).
Greco, F. A. et al. Gemcitabine, carboplatin, and paclitaxel for patients with carcinoma of unknown primary site: a Minnie Pearl Cancer Research Network study. J. Clin. Oncol. 20, 1651–1656 (2002).
Huebner, G. et al. Paclitaxel and carboplatin vs gemcitabine and vinorelbine in patients with adeno- or undifferentiated carcinoma of unknown primary: a randomised prospective phase II trial. Br. J. Cancer 100, 44–49 (2009).
Hemminki, K., Bevier, M., Hemminki, A. & Sundquist, J. Survival in cancer of unknown primary site: population-based analysis by site and histology. Ann. Oncol. 23, 1854–1863 (2012).
Jones, W. et al. Cancers of unknown primary diagnosed during hospitalization: a population-based study. BMC Cancer 17, 85 (2017).
Hemminki, K., Pavlidis, N., Tsilidis, K. K., Sundquist, K. & Ji, J. Age-dependent metastatic spread and survival: cancer of unknown primary as a model. Sci. Rep. 6, 23725 (2016).
Pavlidis, N., Rassy, E. & Smith-Gagen, J. Cancer of unknown primary: incidence rates, risk factors and survival among adolescents and young adults. Int. J. Cancer 146, 1490–1498 (2019).
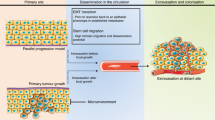
Rassy, E., Assi, T. & Pavlidis, N. Exploring the biological hallmarks of cancer of unknown primary: where do we stand today? Br. J. Cancer 122, 1124–1132 (2020).
Frost, P. Unknown primary tumors: an example of accelerated (type 2) tumor progression. Basic. Life Sci. 57, 233–237 (1991).
Brabletz, T., Jung, A., Spaderna, S., Hlubek, F. & Kirchner, T. Opinion: migrating cancer stem cells — an integrated concept of malignant tumour progression. Nat. Rev. Cancer 5, 744–749 (2005).
Scadden, D. T. The stem-cell niche as an entity of action. Nature 441, 1075–1079 (2006).
López-Lázaro, M. The migration ability of stem cells can explain the existence of cancer of unknown primary site. Rethinking metastasis. Oncoscience 2, 467–475 (2015).
Califano, J. et al. Unknown primary head and neck squamous cell carcinoma: molecular identification of the site of origin. J. Natl Cancer Inst. 91, 599–604 (1999).
Suzuki, M., Mose, E. S., Montel, V. & Tarin, D. Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. Am. J. Pathol. 169, 673–681 (2006).
Tarin, D. Clinical and biological implications of the tumor microenvironment. Cancer Microenviron. 5, 95–112 (2012).
Dasgupta, A., Lim, A. R. & Ghajar, C. M. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol. Oncol. 11, 40–61 (2017).
Stoyianni, A. et al. Immunohistochemical study of the epithelial–mesenchymal transition phenotype in cancer of unknown primary: incidence, correlations and prognostic utility. Anticancer Res. 32, 1273–1281 (2012).
Stoyianni, A. et al. Insights into the epithelial mesenchymal transition phenotype in cancer of unknown primary from a global microRNA profiling study. Clin. Transl. Oncol. 16, 725–731 (2014).
Kamposioras, K., Pentheroudakis, G. & Pavlidis, N. Exploring the biology of cancer of unknown primary: breakthroughs and drawbacks. Eur. J. Clin. Invest. 43, 491–500 (2013).
Vanharanta, S. & Massagué, J. Origins of metastatic traits. Cancer Cell 24, 410–421 (2013).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Albertini, R. J., Nicklas, J. A., O’Neill, J. P. & Robison, S. H. In vivo somatic mutations in humans: measurement and analysis. Annu. Rev. Genet. 24, 305–326 (1990).
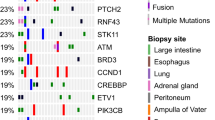
Clynick, B. et al. Genetic characterisation of molecular targets in carcinoma of unknown primary. J. Transl. Med. 16, 185 (2018).
Tothill, R. W. et al. Massively-parallel sequencing assists the diagnosis and guided treatment of cancers of unknown primary. J. Pathol. 231, 413–423 (2013).
Gatalica, Z. et al. Comprehensive tumor profiling identifies numerous biomarkers of drug response in cancers of unknown primary site: analysis of 1806 cases. Oncotarget 5, 12440–12447 (2014).
Penson, A. et al. Development of genome-derived tumor type prediction to inform clinical cancer care. JAMA Oncol. 6, 84–91 (2020).
Centeno, B. A. et al. Hybrid model integrating immunohistochemistry and expression profiling for the classification of carcinomas of unknown primary site. J. Mol. Diagn. 12, 476–486 (2010).
Pavlidis, N. Forty years experience of treating cancer of unknown primary. Acta Oncol. 46, 592–601 (2007).
Kim, C. S. et al. Survival outcome differences based on treatments used and knowledge of the primary tumour site for patients with cancer of unknown and known primary in Ontario. Curr. Oncol. 25, 307–316 (2018).
Daud, A. I. Removing the unknown from the carcinoma of unknown primary. J. Clin. Oncol. 31, 174–175 (2012).
Greco, F. A. & Hainsworth, J. D. Renal cell carcinoma presenting as carcinoma of unknown primary site: recognition of a treatable patient subset. Clin. Genitourin. Cancer 16, e893–e898 (2018).
Overby, A., Duval, L., Ladekarl, M., Laursen, B. E. & Donskov, F. Carcinoma of unknown primary site (CUP) with metastatic renal-cell carcinoma (mRCC) histologic and immunohistochemical characteristics (CUP-mRCC): results from consecutive patients treated with targeted therapy and review of literature. Clin. Genitourin. Cancer 17, e32–e37 (2019).
Thamcharoen, N. & Chaiwiriyawong, W. Papillary renal cell carcinoma presented with supraclavicular lymph node metastasis without renal primary lesion. World J. Oncol. 4, 50–53 (2013).
Honda, A. et al. Successful control of carcinoma of unknown primary with axitinib, a novel molecular-targeted agent: a case report. Chemotherapy 60, 342–345 (2014).
Escudier, B. Combination therapy as first-line treatment in metastatic renal-cell carcinoma. N. Engl. J. Med. 380, 1176–1178 (2019).
Michielin, O., van Akkooi, A., Ascierto, P., Dummer, R. & Keilholz, U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30, 1884–1901 (2019).
Simile, M. M. et al. Targeted therapies in cholangiocarcinoma: emerging evidence from clinical trials. Medicina 55, 42 (2019).
Jardim, D. L. et al. Impact of a biomarker-based strategy on oncology drug development: a meta-analysis of clinical trials leading to FDA approval. J. Natl Cancer Inst. 107, djv253 (2015).
Schwaederle, M. et al. Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J. Clin. Oncol. 33, 3817–3825 (2015).
Hoadley, K. A. et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 158, 929–944 (2014).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Bogenberger, J. M., DeLeon, T. T., Arora, M., Ahn, D. H. & Borad, M. J. Emerging role of precision medicine in biliary tract cancers. NPJ Precis. Onc. 2, 21 (2018).
Brown, N. A., Aisner, D. L. & Oxnard, G. R. Precision medicine in non-small cell lung cancer: current standards in pathology and biomarker interpretation. Am. Soc. Clin. Oncol. Educ. Book 38, 708–715 (2018).
Guler, I., Askan, G., Klostergaard, J. & Sahin, I. H. Precision medicine for metastatic colorectal cancer: an evolving era. Expert Rev. Gastroenterol. Hepatol. 13, 919–931 (2019).
Binder, C., Matthes, K. L., Korol, D., Rohrmann, S. & Moch, H. Cancer of unknown primary — epidemiological trends and relevance of comprehensive genomic profiling. Cancer Med. 7, 4814–4824 (2018).
Chakravarty, D. et al. OncoKB: a precision oncology knowledge base. JCO Precis. Oncol. 1, 1–16 (2017).
Mateo, J. et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 29, 1895–1902 (2018).
Ross, J. S. et al. Comprehensive genomic profiling of carcinoma of unknown primary site: new routes to targeted therapies. JAMA Oncol. 1, 40–49 (2015).
Löffler, H. et al. Molecular driver alterations and their clinical relevance in cancer of unknown primary site. Oncotarget 7, 44322–44329 (2016).
Kato, S. et al. Utility of genomic analysis in circulating tumor DNA from patients with carcinoma of unknown primary. Cancer Res. 77, 4238–4246 (2017).
Varghese, A. M. et al. Clinical and molecular characterization of patients with cancer of unknown primary in the modern era. Ann. Oncol. 28, 3015–3021 (2017).
Gatalica, Z., Xiu, J., Swensen, J. & Vranic, S. Comprehensive analysis of cancers of unknown primary for the biomarkers of response to immune checkpoint blockade therapy. Eur. J. Cancer 94, 179–186 (2018).
Hainsworth, J. D. et al. Phase II trial of bevacizumab and erlotinib in carcinomas of unknown primary site: the Minnie Pearl Cancer Research Network. J. Clin. Oncol. 25, 1747–1752 (2007).
Hainsworth, J. D. et al. Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist 14, 1189–1197 (2009).
Dietlein, F. et al. Identification of cancer driver genes based on nucleotide context. Nat. Genet. 52, 208–218 (2020).
Hainsworth, J. D., Lennington, W. J. & Greco, F. A. Overexpression of Her-2 in patients with poorly differentiated carcinoma or poorly differentiated adenocarcinoma of unknown primary site. J. Clin. Oncol. 18, 632–635 (2000).
Massard, C. et al. Carcinoma of an unknown primary: are EGF receptor, Her-2/neu, and c-Kit tyrosine kinases potential targets for therapy? Br. J. Cancer 97, 857–861 (2007).
Dova, L. et al. Targeting c-KIT, PDGFR in cancer of unknown primary: a screening study for molecular markers of benefit. J. Cancer Res. Clin. Oncol. 134, 697 (2008).
Dova, L. et al. Global profiling of EGFR gene mutation, amplification, regulation and tissue protein expression in unknown primary carcinomas: to target or not to target? Clin. Exp. Metastasis 24, 79–86 (2007).
Koo, J. S. & Kim, H. Hypoxia-related protein expression and its clinicopathologic implication in carcinoma of unknown primary. Tumour Biol. 32, 893–904 (2011).
Pavlidis, N., Briassoulis, E., Bai, M., Fountzilas, G. & Agnantis, N. Overexpression of C-myc, Ras and C-erbB-2 oncoproteins in carcinoma of unknown primary origin. Anticancer Res. 15, 2563–2567 (1995).
Mauri, G. et al. TRKA expression and NTRK1 gene copy number across solid tumours. J. Clin. Pathol. 71, 926–931 (2018).
Krikelis, D. et al. Profiling immunohistochemical expression of NOTCH1-3, JAGGED1, cMET, and phospho-MAPK in 100 carcinomas of unknown primary. Clin. Exp. Metastasis 29, 603–614 (2012).
Gatalica, Z. et al. Molecular profiling of cancers of unknown primary site (CUP): paradigm shift in management of CUP [abstract LBA39]. Eur. J. Cancer 49, S17 (2013).
Pentheroudakis, G. et al. Mutational profiling of the RAS, PI3K, MET and b-catenin pathways in cancer of unknown primary: a retrospective study of the Hellenic Cooperative Oncology Group. Clin. Exp. Metastasis 31, 761–769 (2014).
Stella, G. M. et al. MET mutations in cancers of unknown primary origin (CUPs). Hum. Mutat. 32, 44–50 (2011).
Golfinopoulos, V. et al. Intracellular signalling via the AKT axis and downstream effectors is active and prognostically significant in cancer of unknown primary (CUP): a study of 100 CUP cases. Ann. Oncol. 23, 2725–2730 (2012).
Pentheroudakis, G. et al. Immunohistochemical profiling of signalling pathways in cancer of unknown primary (CUP). Eur. J. Cancer 47, S184 (2011).
Briasoulis, E. et al. Bcl2 and p53 protein expression in metastatic carcinoma of unknown primary origin: biological and clinical implications. A Hellenic Co-operative Oncology Group study. Anticancer Res. 18, 1907–1914 (1998).
van de Wouw, A. J., Jansen, R. L. H., Griffioen, A. W. & Hillen, H. F. P. Clinical and immunohistochemical analysis of patients with unknown primary tumour. A search for prognostic factors in UPT. Anticancer Res. 24, 297–301 (2004).
Rashid, A. et al. Overexpression and prevalence of molecular markers in patients with cancer of unknown primary (CUP). J. Clin. Oncol. 24, 9683 (2005).
Karavasilis, V. et al. Angiogenesis in cancer of unknown primary: clinicopathological study of CD34, VEGF and TSP-1. BMC Cancer 5, 25 (2005).
Karavasilis, V. et al. Matrix metalloproteinases in carcinoma of unknown primary. Cancer 104, 2282–2287 (2005).
Hemminki, K. et al. Germline genetics of cancer of unknown primary (CUP) and its specific subtypes. Oncotarget 7, 22140–22149 (2016).
Hedley, D. W., Leary, J. A. & Kirsten, F. Metastatic adenocarcinoma of unknown primary site: abnormalities of cellular DNA content and survival. Eur. J. Cancer Clin. Oncol. 21, 185–189 (1985).
Tothill, R. W. et al. An expression-based site of origin diagnostic method designed for clinical application to cancer of unknown origin. Cancer Res. 65, 4031–4040 (2005).
Horlings, H. M. et al. Gene expression profiling to identify the histogenetic origin of metastatic adenocarcinomas of unknown primary. J. Clin. Oncol. 26, 4435–4441 (2008).
Bridgewater, J., van Laar, R., Floore, A. & Van’T Veer, L. Gene expression profiling may improve diagnosis in patients with carcinoma of unknown primary. Br. J. Cancer 98, 1425–1430 (2008).
van Laar, R. K. et al. Implementation of a novel microarray-based diagnostic test for cancer of unknown primary. Int. J. Cancer 125, 1390–1397 (2009).
Monzon, F. A., Medeiros, F., Lyons-Weiler, M. & Henner, W. D. Identification of tissue of origin in carcinoma of unknown primary with a microarray-based gene expression test. Diagn. Pathol. 5, 3 (2010).
Ades, F. et al. Comparison of a gene expression profiling strategy to standard clinical work-up for determination of tumour origin in cancer of unknown primary (CUP). J. Chemother 25, 239–246 (2013).
Tothill, R. W. et al. Development and validation of a gene expression tumour classifier for cancer of unknown primary. Pathology 47, 7–12 (2015).
Mileshkin, L. R. et al. Development of a histology-guided gene expression tumor classifier for cancer of unknown primary (CUP). J. Clin. Oncol. 32, 11108–11108 (2014).
Varadhachary, G. R. et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J. Clin. Oncol. 26, 4442–4448 (2008).
Greco, F. A., Lennington, W. J., Spigel, D. R. & Hainsworth, J. D. Molecular profiling diagnosis in unknown primary cancer: accuracy and ability to complement standard pathology. J. Natl Cancer Inst. 105, 782–790 (2013).
Raghav, K. P. S., Poage, G. M., Schnabel, C. A. & Varadhachary, G. R. Resolving diagnostic uncertainty in bone-predominant metastases in cancer of unknown primary (CUP) using the 92-gene assay. J. Clin. Oncol. 36, 12064–12064 (2018).
Thompson, D. S. et al. Molecular tumor profiling (MTP) in cancer of unknown primary site (CUP): a complement to standard pathologic diagnosis. J. Clin. Oncol. 29, 10560–10560 (2011).
Ferracin, M. et al. MicroRNA profiling for the identification of cancers with unknown primary tissue-of-origin. J. Pathol. 225, 43–53 (2011).
Varadhachary, G. R. et al. Prospective gene signature study using microRNA to identify the tissue of origin in patients with carcinoma of unknown primary. Clin. Cancer Res. 17, 4063–4070 (2011).
Sanden, M. O. et al. Observational study of real world clinical performance of microRNA molecular profiling for cancer of unknown primary (CUP). J. Clin. Oncol. 31, e22173–e22173 (2013).
Fernandez, A. F. et al. A DNA methylation fingerprint of 1628 human samples. Genome Res. 22, 407–419 (2012).
Mileshkin, L. R. et al. Clinical impact of tissue of origin testing and mutation profiling in the solving unknown primary cancer (SUPER) national prospective study: experience of the first two years. J. Clin. Oncol. 37, 3072–3072 (2019).
Culine, S. et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study-trial for the French Study Group on carcinomas of unknown primary (GEFCAPI 01). J. Clin. Oncol. 21, 3479–3482 (2003).
Greco, F. A. et al. Carcinoma of unknown primary site: phase II trials with docetaxel plus cisplatin or carboplatin. Ann. Oncol. 11, 211–215 (2000).
Dowell, J. E., Garrett, A. M., Shyr, Y., Johnson, D. H. & Hande, K. R. A randomized phase II trial in patients with carcinoma of an unknown primary site. Cancer 91, 592–597 (2001).
Briasoulis, E. et al. Multicenter phase-II trial of irinotecan plus oxaliplatin (IROX regimen) in patients with poor-prognosis cancer of unknown primary: a Hellenic Cooperative Oncology Group study. Cancer Chemother. Pharmacol. 62, 277–284 (2008).
Schuette, K. et al. Phase II trial of capecitabine and oxaliplatin in patients with adeno- and undifferentiated carcinoma of unknown primary. Onkologie 32, 162–166 (2009).
Møller, A. K. H., Pedersen, K. D., Abildgaard, J., Petersen, B. L. & Daugaard, G. Capecitabine and oxaliplatin as second-line treatment in patients with carcinoma of unknown primary site. Acta Oncol. 49, 431–435 (2010).
Hainsworth, J. D. et al. Oxaliplatin and capecitabine in the treatment of patients with recurrent or refractory carcinoma of unknown primary site. Cancer 116, 2448–2454 (2010).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Clinical Oncology thanks Kari Hemminki, Kanwal Raghav and the other, anonymous, reviewer for their contribution to the peer review of this work.
Related links
OncoKB: https://www.oncokb.org/
Rights and permissions
About this article
Cite this article
Rassy, E., Pavlidis, N. Progress in refining the clinical management of cancer of unknown primary in the molecular era. Nat Rev Clin Oncol 17, 541–554 (2020). https://doi.org/10.1038/s41571-020-0359-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-0359-1
This article is cited by
-
Long-read transcriptome landscapes of primary and metastatic liver cancers at transcript resolution
Biomarker Research (2024)
-
Tissue of origin prediction for cancer of unknown primary using a targeted methylation sequencing panel
Clinical Epigenetics (2024)
-
Prediction of tumor origin in cancers of unknown primary origin with cytology-based deep learning
Nature Medicine (2024)
-
„Cancer of unknown primary“ – Stellenwert der Chirurgie
Die Onkologie (2024)
-
Cancer of unknown primary—state of the art
memo - Magazine of European Medical Oncology (2024)