Abstract
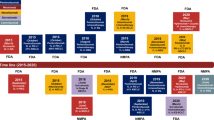
Immune-checkpoint inhibitors (ICIs) are approved in the first-line and third-line settings for patients with extensive-stage or relapsed small-cell lung cancer (SCLC), respectively. In the first-line setting, the addition of the anti-programmed cell death 1 ligand 1 (PD-L1) antibody atezolizumab to chemotherapy improves overall survival (OS). In patients with relapsed disease, data from nonrandomized trials have revealed promising responses, although a significant improvement in OS over that obtained with conventional chemotherapy was not achieved in a randomized trial in this setting. Substantial research interest exists in identifying predictive biomarkers that could guide the use of ICIs in patients with SCLC. PD-L1 expression is typically low or absent in SCLC, which has precluded its use as a predictive biomarker. Tumour mutational burden might have some predictive value, although blood-based measures of tumour mutational burden did not have predictive value in patients receiving atezolizumab plus chemotherapy in the first-line setting. After three decades, ICIs have finally enabled an improvement in OS for patients with SCLC; however, a substantial amount of research remains to be done, including identifying the optimal therapeutic strategy and predictive biomarkers. In this Review, we describe the available data on clinical efficacy, the emerging evidence regarding biomarkers and ongoing clinical trials using ICIs and other immunotherapies in patients with SCLC.
Key points
-
Immune-checkpoint inhibitors (ICIs) are approved as first-line and third-line therapies for patients with advanced-stage small-cell lung cancer (SCLC).
-
In the first-line setting, the anti-programmed cell death 1 ligand 1 ICI atezolizumab plus chemotherapy has been shown to improve overall survival, relative to chemotherapy alone.
-
In the relapsed setting, nonrandomized data reveal promising responses to several ICIs that have not been corroborated in randomized trials.
-
No broadly accepted biomarkers that predict benefit from ICI have been identified to date.
-
Many ongoing trials are evaluating the performance of immune-based treatment strategies in patients with SCLC; these will hopefully enable the optimization of immune-based treatment strategies in this patient population.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bernhardt, E. B. & Jalal, S. I. Small cell lung cancer. Cancer Treat. Res. 170, 301–322 (2016).
Gazdar, A. F. & Minna, J. D. Developing new, rational therapies for recalcitrant small cell lung cancer. J. Natl Cancer Inst. 108, djw119 (2016).
Horn, L. et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 379, 2220–2229 (2018).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Antonia, S. J. et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 17, 883–895 (2016).
Schultheis, A. M. et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur. J. Cancer 51, 421–426 (2015).
Hellmann, M. D. et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 33, 853–861.e4 (2018).
Boumber, Y. Tumor mutational burden (TMB) as a biomarker of response to immunotherapy in small cell lung cancer. J. Thorac. Dis. China 10, 4689–4693 (2018).
Gadgeel, S. M. et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J. Thorac. Oncol. 13, 1393–1399 (2018).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377, 2500–2501 (2017).
Ott, P. A. et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J. Clin. Oncol. 35, 3823–3829 (2017).
Carter, L. et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 23, 114–119 (2016).
Rizvi, H. et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J. Clin. Oncol. 36, 633–641 (2018).
Nowak, A. K. et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol. 170, 4905–4913 (2003).
Nowak, A. K., Robinson, B. W. & Lake, R. A. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 62, 2353–2358 (2002).
van der Most, R. G. et al. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol. Immunother. 58, 1219–1228 (2009).
Bonanno, L. et al. The role of immune microenvironment in small-cell lung cancer: distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes. Eur. J. Cancer 101, 191–200 (2018).
Wang, H. et al. Prognostic significance of PD-L1 expression and CD8+ T cell infiltration in pulmonary neuroendocrine tumors. Diagn. Pathol. 13, 30 (2018).
Berghoff, A. S. et al. Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J. Neurooncol. 130, 19–29 (2016).
Iams, W. T. et al. Improved prognosis and increased tumor-infiltrating lymphocytes in patients who have SCLC with neurologic paraneoplastic syndromes. J. Thorac. Oncol. 14, 1970–1981 (2019).
Ishii, H. et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J. Thorac. Oncol. 10, 426–430 (2015).
George, J. et al. Comprehensive genomic profiles of small cell lung cancer. Nature 524, 47–53 (2015).
Poirier, J. T. et al. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene 34, 5869–5878 (2015).
Huang, Y. H. et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 32, 915–928 (2018).
Carney, D. N. et al. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 45, 2913–2923 (1985).
Wooten, D. J. et al. Systems-level network modeling of small cell lung cancer subtypes identifies master regulators and destabilizers. Preprint at bioRxiv https://doi.org/10.1101/506402 (2018).
McColl, K. et al. Reciprocal expression of INSM1 and YAP1 defines subgroups in small cell lung cancer. Oncotarget 8, 73745–73756 (2017).
Poirier, J. T. et al. Selective tropism of Seneca Valley virus for variant subtype small cell lung cancer. J. Natl Cancer Inst. 105, 1059–1065 (2013).
Borromeo, M. D. et al. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep. 16, 1259–1272 (2016).
Mollaoglu, G. et al. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell 31, 270–285 (2017).
Rudin, C. M. et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat. Rev. Cancer 19, 289–297 (2019).
Wargo, J. A., Reuben, A., Cooper, Z. A., Oh, K. S. & Sullivan, R. J. Immune effects of chemotherapy, radiation, and targeted therapy and opportunities for combination with immunotherapy. Semin. Oncol. 42, 601–616 (2015).
Reck, M. et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol. 34, 3740–3748 (2016).
Reck, M. L. S. et al. IMPower133: updated overall survival (OS) analysis of first-line (1L) atezolizumab (atezo) + carboplatin + etoposide in extensive-stage SCLC (ES-SCLC). Ann. Oncol. 30, mdz264 (2019).
Paz-Ares, L. et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394, 1929–1939 (2019).
Paz-Ares, L. et al. Overall survival with durvalumab plus etoposide-platinum in first-line extensive-stage SCLC: results from the CASPIAN study. J. Thorac. Oncol. 14, S7–S8 (2019).
Rossi, A. et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J.Clin. Oncol. 30, 1692–1698 (2012).
Darnell, R. B. & Posner, J. B. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 349, 1543–1554 (2003).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Paz-Ares, L. et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379, 2040–2051 (2018).
Owonikoko, T. K. et al. Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): results from the double-blind, randomized phase III CheckMate 451 study (LBA1_PR). Ann. Oncol. 30, mdz094 (2019).
Ready, N. et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J. Thorac. Oncol. 14, 237–244 (2019).
Reck, M. V. D. et al. Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy in recurrent small cell lung cancer (SCLC): results from CheckMate 331. Ann. Oncol. 29 (suppl. 10), x39–x43 (2018).
Chung et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J. Clin. Oncol. 36, 8506 (2018).
Pujol, J. L. et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J. Thorac. Oncol. 14, 903–913 (2019).
Ready, N. E. et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J. Thorac. Oncol. https://doi.org/10.1016/j.jtho.2019.10.004 (2019).
Tsao, M. S. et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J. Thorac. Oncol. 13, 1302–1311 (2018).
Ott, P. A. et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 37, 318–327 (2019).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Danaher, P. et al. Pan-cancer adaptive immune resistance as defined by the tumor inflammation signature (TIS): results from The Cancer Genome Atlas (TCGA). J. Immunother. Cancer 6, 63 (2018).
Frampton, G. M. et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 31, 1023–1031 (2013).
Gandara, D. R. et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 24, 1441–1448 (2018).
Chalmers, Z. R. et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9, 34 (2017).
Garassino, M. et al. KEYNOTE 189: tumor mutation burden not significantly associated with efficacy of pembrolizumab. Presented at IASLC World Conference on Lung Cancer 2019.
Langer, C. et al. KEYNOTE 021: tumor mutation burden not significantly associated with efficacy of pembrolizumab. Presented at IASLC World Conference on Lung Cancer 2019.
Sadelain, M., Brentjens, R. & Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 3, 388–398 (2013).
Springuel, L. et al. Chimeric antigen receptor-T cells for targeting solid tumors: current challenges and existing strategies. BioDrugs 33, 515–537 (2019).
Slaney, C. Y., Wang, P., Darcy, P. K. & Kershaw, M. H. CARs versus BiTEs: a comparison between T cell-redirection strategies for cancer treatment. Cancer Discov. 8, 924–934 (2018).
NCCN Guidelines: Small Cell Lung Cancer Version 1 (National Comprehensive Cancer Network, 2019).
Acknowledgements
W.T.I. acknowledges support from the National Institutes of Health and National Cancer Institute Vanderbilt Clinical Oncology Research Career Development Award (VCORCDP) 2K12CA090625-17 and an American Society of Clinical Oncology/Conquer Cancer Foundation Young Investigator Award.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
W.T.I. has acted as a consultant for Defined Health, Genentech and Outcomes Insights. L.H. reports clinical trial funding from BMS, Boehringer Ingelheim and Xcovery; and has acted as a consultant for AbbVie, AstraZeneca, EMD Serono, Incyte, Merck, Pfizer, Roche-Genentech, Tesaro and Xcovery. J.P. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Iams, W.T., Porter, J. & Horn, L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol 17, 300–312 (2020). https://doi.org/10.1038/s41571-019-0316-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-019-0316-z
This article is cited by
-
A telomere-targeting drug depletes cancer initiating cells and promotes anti-tumor immunity in small cell lung cancer
Nature Communications (2024)
-
FAK-LINC01089 negative regulatory loop controls chemoresistance and progression of small cell lung cancer
Oncogene (2024)
-
Inhibition of proteinase-activated receptor 2 (PAR2) decreased the malignant progression of lung cancer cells and increased the sensitivity to chemotherapy
Cancer Chemotherapy and Pharmacology (2024)
-
Circulating Osteopontin Predicts Clinical and Radiological Response in First-Line Treatment of Advanced Non-Small Cell Lung Cancer
Lung (2024)
-
Effect of immune checkpoint inhibitors at different treatment time periods on prognosis of patients with extensive-stage small-cell lung cancer
Clinical and Translational Oncology (2024)