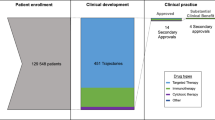
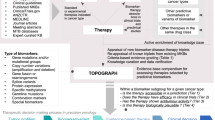
Abstract
The traditional regulatory drug approval paradigm comprising discrete phases of clinical testing that culminate in a large randomized superiority trial has historically been predominant in oncology. However, this approach has evolved in the current era of drug development, with multiple other development pathways now being utilized. Indeed, treatment approaches designed on the basis of an improved understanding of cancer biology have led to unprecedented responses in early phase trials, sometimes resulting in drug approvals in the absence of large-scale trials. At the same time, improved molecular diagnostic technologies have led to the identification of ever-smaller patient subgroups for molecularly targeted therapy. Moreover, new FDA regulatory paradigms have enabled the rapid review and accelerated approval of certain drugs in the absence of survival data. Regulatory approvals based on large-cohort trials with surrogate or intermediate clinical end points or on non-inferiority trials, as well as new tumour-agnostic indications, also set important precedents in the field. In this Viewpoint, we asked two leading oncologists involved in clinical drug development, an expert in regulatory science and prescription drug policy and a prominent patient advocate, to provide their opinions on the implications of these changes in regulatory practices for patient care.
The contributors
R.K. is the Director of the Center for Personalized Cancer Therapy and founder/director of the Rare Tumor Clinic (as well as Associate Director of the Clinical Science division and Leader of the Experimental Therapeutics Program) at the University of California San Diego Moores Cancer Center. Previously, at MD Anderson Cancer Center, she founded, built up and chaired one of the world’s largest clinical trials departments. Her expertise and interests are in patient-focused precision medicine.
H.M.K. is the Chair of the Leukaemia Department at MD Anderson Cancer Center. He joined the institution in 1981 as a fellow and over the past 38 years has continued his clinical-translational research in leukaemia. He is also a Special Fellow on Healthcare Policies at the Rice Baker Institute and advocates for health-care issues pertinent to patients with cancer and leukaemia.
A.S.K. is a Professor of Medicine at Harvard Medical School and Director of the Program On Regulation, Therapeutics, And Law (PORTAL) in the Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital. PORTAL is an interdisciplinary research centre focusing on intersections among prescription drugs and medical devices, patient health outcomes, and regulatory practices and the law.
E.V.S. is the Chairperson and Founder of Friends of Cancer Research, and in addition is the Chair of the Board of Directors at the Reagan–Udall Foundation for the FDA, board member of the Foundation for the National Institute of Health, where she chairs the Portfolio Oversight Committee, and is on the Board of Governors of the Patient-Centered Outcomes Research Institute (PCORI). She also holds leadership positions with a broad range of cancer advocacy, public policy organizations and academic health centres including: MD Anderson Cancer Center External Advisory Board, the Duke University Cancer Center Board of Overseers, and The Sidney Kimmel Comprehensive Cancer Center Advisory Council.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Stadtmauer, E. A. et al. Conventional-dose chemotherapy compared with high dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. Philadelphia Bone Marrow Transplant Group. N. Engl. J. Med. 342, 1069–1076 (2000).
Kurzrock, R., Kantarjian, H. & Stewart, D. J. A cancer trial scandal and its regulatory backlash. Nat. Biotechnol. 32, 27–31 (2014).
Stewart, D. J., Whitney, S. N. & Kurzrock, R. Equipoise lost: ethics, costs, and the regulation of cancer clinical research. J. Clin. Oncol. 28, 2925–2935 (2010).
Tsimberidou, A. M., Braiteh, F., Stewart, D. J. & Kurzrock, R. Ultimate fate of oncology drugs approved by the US Food and Drug Administration without a randomized trial. J. Clin. Oncol. 27, 6243–6250 (2009).
FDA. CFR - Code of Federal Regulations Title 21 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=312&showFR=1&subpartNode=21:5.0.1.1.3.5 (2019).
Ravandi, F. & Kantarjian, H. Gemtuzumab ozogamicin in acute myeloid leukaemia. Nat. Rev. Clin. Oncol. 9, 310–311 (2012).
Ravandi, F. et al. Gemtuzumab ozogamicin: time to resurrect? J. Clin. Oncol. 30, 3921–3923 (2012).
Kantarjian, H. et al. Decitabine in older adults with acute myeloid leukemia: why was the dream broken? J. Clin. Oncol. 31, 1795–1796 (2013).
Kantarjian, H. & Estey, E. Drug approvals in acute myeloid leukemia: can we do better? The ASCO Post https://www.ascopost.com/issues/may-15-2013/drug-approvals-in-acute-myeloid-leukemia-can-we-do-better/ (2013).
Kantarjian, H. M. et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 30, 2670–2677 (2012).
Naqvi, K. et al. Early results of lower dose dasatinib (50 mg daily) as frontline therapy for newly diagnosed chronic-phase chronic myeloid leukemia. Cancer 124, 2740–2747 (2018).
Kantarjian, H. M. et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N. Engl. J. Med. 375, 740–753 (2016).
Kantarjian, H. et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 19, 240–248 (2018).
Jabbour, E. et al. Salvage chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD for patients with relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: a phase 2 clinical trial. JAMA Oncol. 4, 230–234 (2018).
Jabbour, E. et al. Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer 124, 4044–4055 (2018).
Kantarjian, H. & O'Brien, S. Poor penetration of existing effective chemoimmunotherapy for the treatment of older patients with newly diagnosed acute lymphocytic leukemia (ALL) or refractory/relapsed ALL. J. Oncol. Pract. 15, 77–79 (2019).
Stein, E. et al. Ivosidenib or enasidenib combined with induction and consolidation chemotherapy in patients with newly diagnosed AML with an IDH1 or IDH2 mutation is safe, effective, and leads to MRD-negative complete remissions. Blood 132, 560 (2018).
DiNardo, C. D. et al. Mutant IDH (MIDH) inhibitors, ivosidenib or enasidenib, with azacitidine (AZA) in patients with acute myeloid leukemia (AML). J. Clin. Oncol. 36 (suppl.), 7042–7042 (2018).
Hwang, T. J. et al. Efficacy, safety, and regulatory approval of Food and Drug Administration-designated breakthrough and nonbreakthrough cancer medicines. J. Clin. Oncol. 36, 1805–1812 (2018).
Olson, M. K. The risk we bear: the effects of review speed and industry user fees on new drug safety. J. Health Econ. 27, 175–200 (2008).
Grabowski, H. & Wang, Y. R. Do faster Food and Drug Administration drug reviews adversely affect patient safety? An analysis of the 1992 Prescription Drug User Fee Act. J. Law Econ. 51, 377–406 (2008).
Mostaghim, S., Gagne, J. J. & Kesselheim, A. S. Safety-related label changes for new drugs after approval in the US through expedited regulatory pathways: retrospective cohort study. BMJ 358, j3837 (2017).
Kesselheim, A. S., Wang, B., Franklin, J. M. & Darrow, J. J. Trends in utilization of FDA expedited drug development and approval programs, 1987–2014: cohort study. BMJ 351, h4633 (2015).
FDA. 2016 novel drugs summary https://www.fda.gov/media/102618/download (2017).
FDA. Advancing health through innovation: 2017 new drug therapy approvals https://www.fda.gov/media/110526/download (2018).
FDA. Advancing health through innovation: 2018 new drug therapy approvals https://www.fda.gov/media/120357/download (2019).
Chen, E. Y., Joshi, S. K., Tran, A. & Prasad, V. Estimation of study time reduction using surrogate end points rather than overall survival in oncology clinical trials. JAMA Intern. Med. 179, 642–647 (2019).
Johnson, K. R. et al. Response rate or time to progression as predictors of survival in trials of metastatic colorectal cancer or non-small-cell lung cancer: a meta-analysis. Lancet Oncol. 7, 741–746 (2006).
Kurzrock, R. & Stewart, D. J. Equipoise abandoned? Randomization and clinical trials. Ann. Oncol. 24, 2471–2474 (2013).
Kim, C. & Prasad, V. Strength of validation for surrogate end points used in the US Food and Drug Administration’s approval of oncology drugs. Mayo Clin. Proc. 91, 713–725 (2016).
Kesselheim, A. S., Myers, J. A. & Avorn, J. Characteristics of clinical trials to support approval of orphan vs nonorphan drugs for cancer. JAMA 305, 2320–2326 (2011).
[No authors listed]. New drug, antibiotic, and biological drug product regulations; accelerated approval--FDA. Fed. Reg. 57, 13234–13242 (1992).
Micheel, C. M. & Ball, J. R. (eds) Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease (National Academies Press, 2010).
Vokinger, K. N. & Kesselheim, A. S. Characteristics of trials and regulatory pathways leading to US approval of innovative vs. non-innovative oncology drugs. Health Policy 123, 721–727 (2019).
Powers, J. H., Evans, S. R. & Kesselheim, A. S. Studying new antibiotics for multidrug resistant infections: are today’s patients paying for unproved future benefits? BMJ 360, k587 (2018).
Gyawali, B. & Kesselheim, A. S. FDA approval of new drugs based on non-inferiority trials in oncology: a dangerous precedent? JAMA Oncol. 5, 607–608 (2019).
Naci, H., Smalley, K. R. & Kesselheim, A. S. Characteristics of preapproval and postapproval studies for drugs granted accelerated approval by the US Food and Drug Administration. JAMA 318, 626–636 (2017).
Gyawali, B., Hey, S. P. & Kesselheim, A. S. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern. Med. 179, 906–913 (2019).
Gellad, W. F. & Kesselheim, A. S. Accelerated approval and expensive drugs — a challenging combination. N. Engl. J. Med. 376, 2001–2004 (2017).
FDA. CFR - Code of Federal Regulations Title 21 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=601.41 (2019).
Stewart, D. J. & Kurzrock, R. Cancer: the road to Amiens. J. Clin. Oncol. 27, 328–333 (2009).
Subbiah, V. & Kurzrock, R. The marriage between genomics and immunotherapy: mismatch meets its match. Oncologist 24, 1–3 (2019).
Shah, P. et al. Artificial intelligence and machine learning in clinical development: a translational perspective. NPJ Digit. Med. 2, 69 (2019).
European Medicines Agency. ICH E6 (R2) Good clinical practice https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice (2016).
FDA. CFR - Code of Federal Regulations Title 21 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm (2019).
FDA. CFR - Code of Federal Regulations Title 21 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.64 (2019).
Good clinical practice network. 4. Investigator https://ichgcp.net/4-investigator/ (2016).
Kantarjian, H. M. et al. Cancer research in the United States: a critical review of current status and proposal for alternative models. Cancer 124, 2881–2889 (2018).
Kim, E. S. et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J. Clin. Oncol. 35, 3737–3744 (2017).
Sherman, R. E. et al. Real-world evidence — what is it and what can it tell us? N. Engl. J. Med. 375, 2293–2297 (2016).
Acknowledgements
The work of R.K. is funded in part by the Joan and Irwin Jacobs Fund and by National Cancer Institute grant P30 CA023100. The work of A.S.K. is funded by Arnold Ventures, the Harvard-MIT Center for Regulatory Science, and the Engelberg Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R.K. receives research funding from Foundation Medicine, Genentech, Grifols, Guardant Health, Incyte, Konica Minolta, Merck Serono, Pfizer and Sequenom, and consultant fees from Actuate Therapeutics, Genentech, LOXO, NeoMed and X-Biotech. She receives speaker fees from Roche and has an equity interest in Curematch and IDbyDNA. H.M.K. has received research funding from Abbvie, Agios, Amgen, Ariad, Astex, BMS, Cyclacel, Daiichi–Sankyo, Immunogen, Jazz pharma, Novartis and Pfizer, and declares honoraria from Abbvie, Actinium, Agios, Amgen, Pfizer and Takeda. The other contributors declare no competing interests.
Additional information
Disclaimer
All comments by Hagop Kantarjian are his personal opinions and do not reflect the opinions of his institutional affiliation.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
FDA Adverse Event Reporting System: https://open.fda.gov/data/faers/
Rights and permissions
About this article
Cite this article
Kurzrock, R., Kantarjian, H.M., Kesselheim, A.S. et al. New drug approvals in oncology. Nat Rev Clin Oncol 17, 140–146 (2020). https://doi.org/10.1038/s41571-019-0313-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-019-0313-2
This article is cited by
-
Selection of clinically relevant drug concentrations for in vitro studies of candidates drugs for cancer repurposing: a proposal
Clinical and Translational Oncology (2023)
-
The evidence for repurposing anti-epileptic drugs to target cancer
Molecular Biology Reports (2023)
-
Mitochondrial adaptation in cancer drug resistance: prevalence, mechanisms, and management
Journal of Hematology & Oncology (2022)
-
Harnessing the predictive power of preclinical models for oncology drug development
Nature Reviews Drug Discovery (2022)
-
The paediatric cancer clinical research landscape in Spain: a 13-year multicentre experience of the new agents group of the Spanish Society of Paediatric Haematology and Oncology (SEHOP)
Clinical and Translational Oncology (2021)