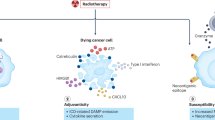
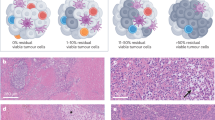
Abstract
Considerable interest is being directed toward combining immune-checkpoint inhibition (ICI) with radiotherapy to improve response rates to ICI, which have been disappointingly low at around 15–30% among patients with advanced-stage cancers other than melanoma. Since a case report published in 2012, in which authors described the resolution of metastatic disease after irradiation of a single lesion in a patient who had been receiving ICI, hundreds of clinical trials have been launched with the aim of testing the safety and/or efficacy of radiotherapy in combination with immunotherapy, nearly all of which use this single-site irradiation, or ‘abscopal’, approach. However, emerging preclinical and clinical evidence suggests that this approach likely produces suboptimal results. In this Perspective, we describe this evidence and provide a biological rationale supporting the abandonment of the single-site abscopal approach. We instead advocate exploring comprehensive irradiation of multiple/all lesions in order to enhance the likelihood of obtaining meaningful clinical outcomes — if such a clinical synergy between radiation and ICI does exist — before the failure of the current, single-site approach leads to the potential premature and inappropriate abandonment of radiotherapy in combination with ICI altogether.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Seiwert, T. Y. et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17, 956–965 (2016).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Postow, M. A. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Hellmann, M. D. et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 18, 31–41 (2017).
Demaria, S., Golden, E. B. & Formenti, S. C. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 1, 1325–1332 (2015).
Demaria, S. et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 58, 862–870 (2004). This reference (and reference 17, from the same group) helped to provide the basis for describing the biological mechanism by which radiation potentiates and enhances an immunological antitumour response.
Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006).
Kalbasi, A., June, C. H., Haas, N. & Vapiwala, N. Radiation and immunotherapy: a synergistic combination. J. Clin. Invest. 123, 2756–2763 (2013).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activates non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015). This report helped to further establish the biological mechanisms of synergy between radiation and ICI, and how synergy between them can differ depending on the biological mechanisms of the agents used. This report drew attention to the importance of the timing and/or sequencing of selected combinations.
Pardoll, D. & Allison, J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat. Med. 10, 887–892 (2004).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Andrews, M. C. & Wargo, J. A. Immunotherapy resistance: the answers lie ahead — not in front — of us. J. Immunother. Cancer. 5, 10 (2017).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti–CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Demaria, S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Verbrugge, I. et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 72, 3163–3174 (2012).
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Golden, E. B. et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 3, e28518 (2014).
Gameiro, S. R. et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T cell killing. Oncotarget 5, 403 (2014).
Golden, E. B., Pellicciotta, I., Demaria, S., Barcellos-Hoff, M. H. & Formenti, S. C. The convergence of radiation and immunogenic cell death signaling pathways. Front. Oncol. 2, 88 (2012).
Akashi, M., Hachiya, M., Koeffler, H. P. & Suzuki, G. Irradiation increases levels of GM-CSF through RNA stabilization which requires an AU-rich region in cancer cells. Biochem. Biophys. Res. Comm. 189, 986–993 (1992).
Kim, J. Y. et al. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp. Mol. Med. 38, 474–484 (2006).
Torihata, H. et al. Irradiation up-regulates CD80 expression through two different mechanisms in spleen B cells, B lymphoma cells, and dendritic cells. Immunology 112, 219–227 (2004).
Tsai, M. H. et al. Gene expression profiling of breast, prostate, and glioma cells following single versus fractionated doses of radiation. Cancer Res. 67, 3845–3852 (2007).
Filatenkov, A. et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 21, 3727–3739 (2015).
Stamell, E. F., Wolchok, J. D., Gnjatic, S., Lee, N. Y. & Brownell, I. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 85, 293–295 (2013).
Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 925–931 (2012). The abscopal effect was first described by R. H. Mole in 1953, although this seminal case report from Memorial Sloan Kettering Cancer Center reignited attention on the potential clinical scalability of immune-checkpoint inhibitors to induce abscopal effects.
Hiniker, S. M., Chen, D. S. & Knox, S. J. Abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 2035–2036 (2012).
Golden, E. B., Demaria, S., Schiff, P. B., Chachoua, A. & Formenti, S. C. An abscopal response to radiation and ipilimumab in a patient with metastatic non–small cell lung cancer. Cancer Immunol. Res. 1, 365–372 (2013).
Crittenden, M. et al. Current clinical trials testing combinations of immunotherapy and radiation. Semin. Radiat. Oncol. 25, 54–64 (2015).
Kwon, E. D. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 700–712 (2014). This first phase III trial testing the efficacy of radiotherapy in combination with anti-CTLA-4 immunotherapy showed negative results when only a single bony metastasis was targeted with radiotherapy. These findings suggest either that single-site irradiation or anti-CTLA4 antibodies are not an optimal approach for patients with prostate cancer. However, subgroup analyses showed that patients who did benefit had favourable prognostic factors and a lower disease burden (oligometastatic disease), suggesting that radiotherapy might be optimal when used in patients with smaller disease burdens.
Luke, J. J. et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J. Clin. Oncol. 36, 1611–1618 (2018). Data from this study demonstrated that irradiation of one or a few lesions within a larger disease burden leads to response rates similar to those of historical results obtained with immunotherapy alone. These data suggest that, rather than using subtotal irradiation (irradiation of only one or a few sites), a new strategy should be adopted. In our opinion, that strategy should be comprehensive, multisite radiotherapy with immunotherapy.
Gomez, D. R. et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 17, 1672–1682 (2016). This crucial report from a phase II randomized trial showed that consolidative radiotherapy alone offers independent benefits (in terms of prolonged progression-free survival and time to new metastases) for patients with oligometastatic disease. These findings confirm the safety of a consolidative approach and provide a clinical rationale for such an approach in appropriately selected patients.
Huang, A. C. et al. T cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017). Using an IFNγ-related gene assay, these investigators showed that invigoration and continuation of an immune response prompted by immune-checkpoint inhibitors is related to disease burden. Specifically, the lower the burden, the greater the response to anti-PD-1 antibodies in patients with melanoma. This finding provides further support for the suggestion that reducing disease burden to the greatest possible extent could help to optimize responses to ICI, a strategy that could be achieved using radiation of all sites, but not with single-site abscopal approaches.
Mole, R. H. Whole body irradiation — radiobiology or medicine? Br. J. Radiol. 26, 234–241 (1953).
Mikuriya, S. et al. Study of abscopal effect and cellular infiltration of tumor nests using less-fractionated, large-dose radiation [Japanese]. Gan No Rinsho 33, 1239–1252 (1987).
Finkelstein, S. E. et al. Serial assessment of lymphocytes and apoptosis in the prostate during coordinated intraprostatic dendritic cell injection and radiotherapy. Immunotherapy 4, 373–382 (2012).
Antoniades, J., Brady, L. W. & Lightfoot, D. A. Lymphangiographic demonstration of the abscopal effect in patients with malignant lymphomas. Int. J. Radiat. Oncol. Biol. Phys. 2, 141–147 (1977).
Rees, G. J. Abscopal regression in lymphoma: a mechanism in common with total body irradiation? Clin. Radiol. 32, 475–480 (1981).
Nobler, M. P. The abscopal effect in malignant lymphoma and its relationship to lymphocyte circulation. Radiology 93, 410–412 (1969).
Sham, R. L. The abscopal effect and chronic lymphocytic leukemia. Am. J. Med. 98, 307–308 (1995).
Mikuriya, S. et al. Pathologic and immunologic analysis for a case with carcinoma of aberrant breast of the axilla showed “abscopal effect” after the radiotherapy (author’s translation) [Japanese]. Nihon Gan Chiryo Gakkai Shi 13, 406–413 (1978).
Rees, G. J. & Ross, C. M. Abscopal regression following radiotherapy for adenocarcinoma. Br. J. Radiol. 56, 63–66 (1983).
Nakanishi, M. et al. Abscopal effect on hepatocellular carcinoma. Am. J. Gastroenterol. 103, 1320–1321 (2008).
Cotter, S. E. et al. Abscopal effect in a patient with metastatic Merkel cell carcinoma following radiation therapy: potential role of induced antitumor immunity. Arch. Dermatol. 147, 870–872 (2011).
Ishiyama, H. et al. Spontaneous regression of thoracic metastases while progression of brain metastases after stereotactic radiosurgery and stereotactic body radiotherapy for metastatic renal cell carcinoma: abscopal effect prevented by the blood-brain barrier? Clin. Genitourin. Cancer. 10, 196–198 (2012).
Takaya, M. et al. Abscopal effect of radiation on toruliform para-aortic lymph node metastases of advanced uterine cervical carcinoma — a case report. Anticancer Res. 27, 499–503 (2007).
Okuma, K. et al. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J. Med. Case Rep. 5, 111 (2011).
Okwan-Duodu, D. et al. Role of radiation therapy as immune activator in the era of modern immunotherapy for metastatic malignant melanoma. Am. J. Clin. Oncol. 38, 119–125 (2015).
Tsui, J. M., Mihalcioiu, C. & Cury, F. L. Abscopal effect in a stage IV melanoma patient who progressed on pembrolizumab. Cureus 10, e2238 (2018).
Chicas-Sett, R., Morales-Orue, I., Rodriguez-Abreu, D. & Lara-Jimenez, P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: a systematic review. Clin. Transl Radiat. Oncol. 9, 5–11 (2017).
Deipolyi, A. R. et al. Abscopal effect after radioembolization for metastatic breast cancer in the setting of immunotherapy. J. Vasc. Interv. Radiol. 29, 432–433 (2018).
Ngwa, W. et al. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer. 18, 313–322 (2018).
Joe, M. B. et al. Radiation generates an abscopal response and complete resolution of metastatic squamous cell carcinoma of the anal canal: a case report. J. Gastrointest. Oncol. 8, E84 (2017).
Leung, H. W., Wang, S. Y., Jin-Jhih, H. & Chan, A. L. Abscopal effect of radiation on bone metastases of breast cancer: a case report. Cancer Biol. Ther. 19, 20–24 (2018).
Ebner, D. K., Kamada, T. & Yamada, S. Abscopal effect in recurrent colorectal cancer treated with carbon-ion radiation therapy: 2 case reports. Adv. Radiat. Oncol. 2, 333 (2017).
Xie, G., Gu, D., Zhang, L., Chen, S. & Wu, D. A rapid and systemic complete response to stereotactic body radiation therapy and pembrolizumab in a patient with metastatic renal cell carcinoma. Cancer Biol. Ther. 18, 547–551 (2017).
Sharabi, A. et al. Exceptional response to nivolumab and stereotactic body radiation therapy (SBRT) in neuroendocrine cervical carcinoma with high tumor mutational burden: management considerations from the center for personalized cancer therapy at UC San Diego Moores Cancer Center. Oncologist 22, 631–637 (2017).
Sato, H. et al. An abscopal effect in a case of concomitant treatment of locally and peritoneally recurrent gastric cancer using adoptive T-cell immunotherapy and radiotherapy. Clin. Case Rep. 5, 380–384 (2017).
Shi, F., Wang, X., Teng, F., Kong, L. & Yu, J. Abscopal effect of metastatic pancreatic cancer after local radiotherapy and granulocyte-macrophage colony-stimulating factor therapy. Cancer Biol. Ther. 18, 137–141 (2017).
Cong, Y., Shen, G., Wu, S. & Hao, R. Abscopal regression following SABR for non-small-cell-lung cancer: a case report. Cancer Biol. Ther. 18, 1–3 (2017).
Orton, A., Wright, J., Buchmann, L., Randall, L. & Hitchcock, Y. J. A case of complete abscopal response in high-grade pleiomorphic sarcoma treated with radiotherapy alone. Cureus 8, e821 (2016).
Gomes, J. R. et al. Analysis of the abscopal effect with anti-PD1 therapy in patients with metastatic solid tumors. J. Immunother. 39, 367–372 (2016).
Barton, S. M. et al. Abscopal effect in congenital fibrosarcoma with novel EML4-NTRK3 fusion. Int. J. Radiat. Oncol. Biol. Phys. 96, S87 (2016).
Carvalho, R. F. et al. Abscopal effect in patients with metastatic melanoma treated with checkpoint inhibitors (anti-CTLA-4 and anti-PD1): a retrospective analysis of a single institution. Int. J. Radiat. Oncol. Biol. Phys. 96, S159 (2016).
Nakajima, K. et al. The abscopal effect in patients with multiple metastases treated with combination of dendritic cell-based immunotherapy and focal radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 96, E570–E571 (2016).
Michot, J. M. et al. Abscopal effect in a Hodgkin lymphoma patient treated by an anti-programmed death 1 antibody. Eur. J. Cancer. 66, 91–94 (2016).
Van de Walle, M., Demol, J., Staelens, L. & Rottey, S. Abscopal effect in metastatic renal cell carcinoma. Acta Clin. Belg. 72, 245–249 (2017).
Saba, R., Saleem, N. & Peace, D. Long-term survival consequent on the abscopal effect in a patient with multiple myeloma. BMJ Case Rep. 2016, bcr2016215237 (2016).
Lock, M. et al. Abscopal effects: case report and emerging opportunities. Cureus 7, e344 (2015).
Panje, C. & Guckenberger, M. Abscopal responses of local radiotherapy combined with systemic immunotherapy in patients with metastatic solid tumors [German]. Strahlenther. Onkol. 192, 72–74 (2016).
Chandra, R. A. et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 4, e1046028 (2015).
Golden, E. B. et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 16, 795–803 (2015).
Thallinger, C. et al. Abscopal effect in the treatment of malignant melanoma [German]. Hautarzt 66, 545–548 (2015).
Brix, N., Tiefenthaller, A., Anders, H., Belka, C. & Lauber, K. Abscopal, immunological effects of radiotherapy: narrowing the gap between clinical and preclinical experiences. Immunol. Rev. 280, 249–279 (2017).
Abuodeh, Y., Venkat, P. & Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 40, 25–37 (2016).
Nikitina, E. Y. & Gabrilovich, D. I. Combination of γ-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int. J. Cancer. 94, 825–833 (2001).
Dewan, M. Z. et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin. Cancer Res. 18, 6668–6678 (2012).
Vatner, R. E., Cooper, B. T., Vanpouille-Box, C., Demaria, S. & Formenti, S. C. Combinations of immunotherapy and radiation in cancer therapy. Front. Oncol. 4, 325 (2014).
Demaria, S., Vanpouille-Box, C., Formenti, S. C. & Adams, S. The TLR7 agonist imiquimod as an adjuvant for radiotherapy-elicited in situ vaccination against breast cancer. Oncoimmunology 2, e25997 (2013).
Demaria, S., Pilones, K. A., Formenti, S. C. & Dustin, M. L. Exploiting the stress response to radiation to sensitize poorly immunogenic tumors to anti-CTLA-4 treatment. Oncoimmunology 2, e23127 (2013).
Yasuda, K., Nirei, T., Tsuno, N. H., Nagawa, H. & Kitayama, J. Intratumoral injection of interleukin-2 augments the local and abscopal effects of radiotherapy in murine rectal cancer. Cancer Sci. 102, 1257–1263 (2011).
Shiraishi, K. et al. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1α. Clin. Cancer Res. 14, 1159–1166 (2008).
Akutsu, Y. et al. Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by GRP94/gp96 against squamous cell carcinoma in mice. Int. J. Oncol. 31, 509–515 (2007).
Grass, G. D., Krishna, N. & Kim, S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer. 40, 10–24 (2016).
Bernstein, M. B., Krishnan, S., Hodge, J. W. & Chang, J. Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat. Rev. Clin. Oncol. 13, 516–524 (2016). This comprehensive summary of how radiation can potentiate the immune system and best be coupled with ICI argued that, from a preclinical perspective, applying local-site radiotherapy with ICI might bolster ICI systemic effects.
Gaipl, U. S. et al. Kill and spread the word: stimulation of antitumor immune responses in the context of radiotherapy. Immunotherapy 6, 597–610 (2014).
Schaue, D. & McBride, W. H. Links between innate immunity and normal tissue radiobiology. Radiat. Res. 173, 406–417 (2010).
Zhang, X. & Niedermann, G. Abscopal effects with hypofractionated schedules extending into the effector phase of the tumor-specific T cell response. Int. J. Radiat. Oncol. Biol. Phys. 101, 63–73 (2018).
Frey, B. et al. Hypofractionated irradiation has immune stimulatory potential and induces a timely restricted infiltration of immune cells in colon cancer tumors. Front. Immunol. 8, 231 (2017).
Marciscano, A. E. et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin. Cancer Res. 24, 5058–5071 (2018).
Hellevik, T. & Martinez-Zubiaurre, I. Radiotherapy and the tumor stroma: the importance of dose and fractionation. Front. Oncol. 4, 1 (2014).
Ganss, R., Ryschich, E., Klar, E., Arnold, B. & Hämmerling, G. J. Combination of T cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 62, 1462–1470 (2002).
Matsumura, S. et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 181, 3099–3107 (2008).
Liang, H. et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell–mediated killing. J. Immunol. 190, 5874–5881 (2013).
Persa, E. et al. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Lett. 368, 252–261 (2015).
Vatner, R. E. & Formenti, S. C. Myeloid-derived cells in tumors: effects of radiation. Semin. Radiat. Oncol. 25, 18–27 (2015).
Xu, J. et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 73, 2782–2794 (2013).
Liu, Y. et al. Variations of circulating endothelial progenitor cells and transforming growth factor-beta-1 (TGF-β1) during thoracic radiotherapy are predictive for radiation pneumonitis. Radiat. Oncol. 8, 189 (2013).
Moeller, B. J., Cao, Y., Li, C. Y. & Dewhirst, M. W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5, 429–441 (2004).
Liang, H. et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 8, 1736 (2017).
Vanpouille-Box, C. et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618 (2017).
Chakraborty, M. et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T cell killing. Cancer Res. 64, 4328–4337 (2004).
Chang, J. Y. et al. Phase II randomized clinical trial comparing immunotherapy plus stereotactic ablative radiotherapy (I-SABR) versus SABR alone for stage I, selected stage IIa or isolated lung parenchymal recurrent non-small cell lung cancer: I-SABR. J. Clin. Oncol. 36 (Suppl.), TPS8580 (2018).
Tang, C. et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin. Cancer Res. 23, 1388–1396 (2017). Analysis of data from a subset of patients revealing that the location of the irradiated tumour (lung versus liver) is important for eliciting an immune response, raising the issue of tumour-specific and site-specific heterogeneity dictating the likelihood of eliciting antitumour immune priming and responses. Specifically, successful activation of multiple tumour–microenvironment interfaces would require the irradiation of multiple sites, and the optimal response would come from all-site irradiation, because every site would be a target of radiotherapy.
Garnett, C. T. et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 64, 7985–7994 (2004).
Chakraborty, M. et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J. Immunol. 170, 6338–6347 (2003).
Schaue, D., Ratikan, J. A., Iwamoto, K. S. & McBride, W. H. Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys. 83, 1306–1310 (2012).
Lee, Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009).
Lugade, A. A. et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174, 7516–7523 (2005).
Heppner, G. H. & Shekhar, M. Tumor heterogeneity is fundamental to the tumor ecosystem. Oncology 28, 780–781 (2014).
Heppner, G. H. Tumor heterogeneity. Cancer Res. 44, 2259–2265 (1984).
Marusyk, A. & Polyak, K. Tumor heterogeneity: causes and consequences. Biochim. Biophys. Acta Rev. Cancer. 1805, 105–117 (2010).
Easwaran, H., Tsai, H. C. & Baylin, S. B. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol. Cell. 54, 716–727 (2014).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
D’Urso, C. M. et al. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in B2m gene expression. J. Clin. Invest. 87, 284–292 (1991).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Wong, A. C. et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy. Cancer 122, 2242–2250 (2016).
Salama, J. K. et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clin. Cancer Res. 14, 5255–5259 (2008).
Inoue, T. et al. Clinical outcomes of stereotactic brain and/or body radiotherapy for patients with oligometastatic lesions. Jpn J. Clin. Oncol. 40, 788–794 (2010).
Pfannschmidt, J. & Dienemann, H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer 69, 251–258 (2010).
Khan, A. J. et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma. Radiother. Oncol. 81, 163–167 (2006).
Sheu, T. et al. Propensity score–matched analysis of comprehensive local therapy for oligometastatic non-small cell lung cancer that did not progress after front-line chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 90, 850–857 (2014).
Iyengar, P. et al. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 4, e173501–e173501 (2018).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 9, 239 (2009).
Takayama, T. et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 356, 802–807 (2000).
Antonia, S. J. et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 377, 1919–1929 (2017). This report from the PACIFIC trial provides further support for the success of radiotherapy and immunotherapy when radiation is used to treat existing gross disease. This study draws on the concept that using radiotherapy to treat all gross disease might be required to fully bolster ICI. It also lends clinical credence to the concept that micrometastases might be easier for the immune system to clear when all gross disease is irradiated.
Beer, T. M. et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 35, 40–47 (2017).
Alley, E. W. et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 18, 623–630 (2017).
Hansen, A. R. et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann. Oncol. 29, 1807–1813 (2018).
Frenel, J.-S. et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1–positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J. Clin. Oncol. 35, 4035–4041 (2017).
Mehnert, J. M. et al. Pembrolizumab for patients with PD-L1–positive advanced carcinoid or pancreatic neuroendocrine tumors: results from the KEYNOTE-028 study [abstract 427O]. Ann. Oncol. 28, v142–v157 (2017).
Ott, P. A. et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J. Clin. Oncol. 35, 3823–3829 (2017).
Bang, Y. J. et al. Safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: interim results of KEYNOTE-028 [abstract 525]. Eur. J. Cancer 51, S112 (2015).
Cohen, R. B. et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study. Am. J. Clin. Oncol. https://doi.org/10.1097/COC.0000000000000429 (2018).
Matulonis, U. A. et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: interim results from the phase 2 KEYNOTE-100 study. J. Clin. Oncol. 36, S5511 (2018).
Doi, T. et al. Safety and antitumor activity of the anti–programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J. Clin. Oncol. 36, 61–67 (2018).
Ott, P. A. et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1–positive endometrial cancer: results from the KEYNOTE-028 study. J. Clin. Oncol. 35, 2535–2541 (2017).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377, 2500–2501 (2017).
Theelen, W. et al. Randomized phase II study of pembrolizumab after SBRT versus pembrolizumab alone in patients with advanced NSCLC, preliminary results [PUB013]. J. Thorac. Oncol. 12, S1453 (2017).
McBride, S. M. et al. A phase II randomized trial of nivolumab with stereotactic body radiotherapy (SBRT) versus nivolumab alone in metastatic (M1) head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 36, S6009 (2018).
Moreno, V. et al. Cemiplimab, a human monoclonal anti-PD-1, alone or in combination with radiotherapy: phase 1 NSCLC expansion cohorts [abstract MA04.01]. Presented at the 19th IASLC World Conference on Lung Cancer in Toronto, Canada (23–26 Sep 2018).
US Department of Health & Human Services. Common terminology criteria for adverse events (CTCAE) v4.03. NIH.gov https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf (2010).
Hwang, W. L., Pike, L. R., Royce, T. J., Mahal, B. A. & Loeffler, J. S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 15, 477–494 (2018).
Qin, R. et al. Safety and efficacy of radiation therapy in advanced melanoma patients treated with ipilimumab. Int. J. Radiat. Oncol. Biol. Phys. 96, 72–77 (2016).
Aboudaram, A. et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: a safe and effective combination. Melanoma Res. 27, 485–491 (2017).
Barker, C. A. et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol. Res. 1, 92–98 (2013).
Diao, K. et al. Combination ipilimumab and radiosurgery for brain metastases: tumor, edema, and adverse radiation effects. J. Neurosurg. https://doi.org/10.3171/2017.7.JNS171286 (2018).
Patel, K. R. et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am. J. Clin. Oncol. 40, 444–450 (2017).
Williams, N. L. et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 99, 22–30 (2017).
Martin, A. M. et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 4, 1123–1124 (2018).
Colaco, R. J., Martin, P., Kluger, H. M., Yu, J. B. & Chiang, V. L. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J. Neurosurg. 125, 17–23 (2016).
Welsh, J. W. et al. Phase 2 5-arm trial of ipilimumab plus lung or liver stereotactic radiation for patients with advanced malignancies. Int. J. Radiat. Oncol. Biol. Phys. 99, 1315 (2017).
Fan, Q., Nanduri, A., Mazin, S. & Zhu, L. Emission guided radiation therapy for lung and prostate cancers: a feasibility study on a digital patient. Med. Phys. 39, 7140–7152 (2012).
Fan, Q. et al. Toward a planning scheme for emission guided radiation therapy (EGRT): FDG based tumor tracking in a metastatic breast cancer patient. Med. Phys. 40, 081708 (2013).
Yang, J., Yamamoto, T., Mazin, S. R., Graves, E. E. & Keall, P. J. The potential of positron emission tomography for intratreatment dynamic lung tumor tracking: a phantom study. Med. Phys. 41, 021718 (2014).
Wang, H. et al. Validation of an accelerated ‘demons’ algorithm for deformable image registration in radiation therapy. Phys. Med. Biol. 50, 2887 (2005).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Postow, M. A., Callahan, M. K. & Wolchok, J. D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 33, 1974–1982 (2015).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Darby, S. C. et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 368, 987–998 (2013).
Chang, J. Y. et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a “no fly zone”. Int. J. Radiat. Oncol. Biol. Phys. 88, 1120–1128 (2014).
Hobbs, B. P. & Landin, R. Bayesian basket trial design with exchangeability monitoring. Stat. Med. 37, 3557–3572 (2018).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Acknowledgements
The work of the authors is supported in part by Cancer Center Support (Core) Grant CA016672 from the National Institutes of Health to The University of Texas MD Anderson Cancer Center, and by the Joan and Herb Kelleher Charitable Foundation. The authors gratefully acknowledge the editorial contributions of C. F. Wogan, MS, ELS, of the MD Anderson Division of Radiation Oncology in developing this report.
Author information
Authors and Affiliations
Contributions
Both authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
J.Y.C. reports receiving financial support from Varian for travel to meetings in the past 3 years, grants from Bristol-Myers Squibb, being a shareholder of Global Oncology One (in unrelated work), and participating in advisory scientific discussions supported by AstraZeneca. E.D.B. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brooks, E.D., Chang, J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol 16, 123–135 (2019). https://doi.org/10.1038/s41571-018-0119-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-018-0119-7
This article is cited by
-
Progress of immune checkpoint inhibitors therapy for non-small cell lung cancer with liver metastases
British Journal of Cancer (2024)
-
Safety and efficacy of radiotherapy combined with chemotherapy for recurrent metastatic renal pelvic and ureteral carcinoma
World Journal of Urology (2024)
-
Initial analysis of the synergy of programmed cell death-1 (PD-1) inhibitor and concurrent chemoradiotherapy treatment for recurrent/metastatic head and neck squamous cell carcinoma patients
Radiation Oncology (2023)
-
Role of consolidative thoracic radiation in extensive-stage small-cell lung cancer with first-line chemoimmunotherapy: a retrospective study from a single cancer center
Discover Oncology (2023)
-
Keine abskopalen Effekte bei Immuntherapie plus stereotaktischer Radiotherapie
InFo Hämatologie + Onkologie (2023)