Abstract
NTRK gene fusions involving either NTRK1, NTRK2 or NTRK3 (encoding the neurotrophin receptors TRKA, TRKB and TRKC, respectively) are oncogenic drivers of various adult and paediatric tumour types. These fusions can be detected in the clinic using a variety of methods, including tumour DNA and RNA sequencing and plasma cell-free DNA profiling. The treatment of patients with NTRK fusion-positive cancers with a first-generation TRK inhibitor, such as larotrectinib or entrectinib, is associated with high response rates (>75%), regardless of tumour histology. First-generation TRK inhibitors are well tolerated by most patients, with toxicity profiles characterized by occasional off-tumour, on-target adverse events (attributable to TRK inhibition in non-malignant tissues). Despite durable disease control in many patients, advanced-stage NTRK fusion-positive cancers eventually become refractory to TRK inhibition; resistance can be mediated by the acquisition of NTRK kinase domain mutations. Fortunately, certain resistance mutations can be overcome by second-generation TRK inhibitors, including LOXO-195 and TPX-0005 that are being explored in clinical trials. In this Review, we discuss the biology of NTRK fusions, strategies to target these drivers in the treatment-naive and acquired-resistance disease settings, and the unique safety profile of TRK inhibitors.
Key points
-
NTRK fusions, encoding TRK fusion proteins, are oncogenic drivers of a wide variety of adult and paediatric tumours, supporting a basket trial approach to drug development.
-
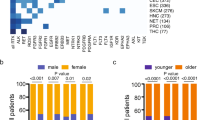
These alterations are found at high frequencies (up to or greater than 90%) in rare cancer types (secretory breast carcinoma, mammary analogue secretory carcinoma, cellular or mixed congenital mesoblastic nephroma and infantile fibrosarcoma) and at lower frequencies (commonly <1%) in a range of other tumour types.
-
NTRK fusions are clinically actionable: first-generation TRK tyrosine kinase inhibitors (larotrectinib or entrectinib) result in histology-agnostic responses in both adult and paediatric patients with NTRK fusion-positive cancers.
-
Resistance to TRK inhibition can be mediated by the acquisition of NTRK kinase domain mutations, including solvent-front and gatekeeper mutations; second-generation TRK inhibitors have been developed to overcome these mechanisms of resistance.
-
First-generation TRK inhibitors are generally well-tolerated and, with consideration of the biological roles of TRK receptors in normal development and adulthood, the occasional on-target adverse effects are predictable.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Robert, C. et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 372, 30–39 (2015).
Geyer, C. E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355, 2733–2743 (2006).
Planchard, D. et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet. Oncol. 17, 984–993 (2016).
Shaw, A. T. et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 371, 1963–1971 (2014).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Solomon, B. J. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371, 2167–2177 (2014).
Hyman, D. M. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 373, 726–736 (2015).
Hyman, D. M. et al. AKT inhibition in solid tumors with AKT1 mutations. J. Clin. Oncol. 35, 2251–2259 (2017).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
Drilon, A. et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase i trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 7, 400–409 (2017).
Pulciani, S. et al. Oncogenes in solid human tumours. Nature 300, 539–542 (1982).
Martin-Zanca, D., Hughes, S. H. & Barbacid, M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 319, 743–748 (1986).
Martin-Zanca, D., Oskam, R., Mitra, G., Copeland, T. & Barbacid, M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol. Cell. Biol. 9, 24–33 (1989).
Klein, R., Jing, S. Q., Nanduri, V., O’Rourke, E. & Barbacid, M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell 65, 189–197 (1991).
Kaplan, D. R., Hempstead, B. L., Martin-Zanca, D., Chao, M. V. & Parada, L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science 252, 554–558 (1991).
Klein, R., Parada, L. F., Coulier, F. & Barbacid, M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 8, 3701–3709 (1989).
Lamballe, F., Klein, R. & Barbacid, M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell 66, 967–979 (1991).
Davies, A. M. et al. Neurotrophin-4/5 is a mammalian-specific survival factor for distinct populations of sensory neurons. J. Neurosci. 13, 4961–4967 (1993).
Soppet, D. et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 65, 895–903 (1991).
Deinhardt, K. & Chao, M. V. Trk receptors. Handb. Exp. Pharmacol. 220, 103–119 (2014).
Snider, W. D. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77, 627–638 (1994).
Lemmon, M. A. & Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010).
Arevalo, J. C. et al. A novel mutation within the extracellular domain of TrkA causes constitutive receptor activation. Oncogene 20, 1229–1234 (2001).
Zaccaro, M. C., Ivanisevic, L., Perez, P., Meakin, S. O. & Saragovi, H. U. p75 Co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains. J. Biol. Chem. 276, 31023–31029 (2001).
Barbacid, M. The Trk family of neurotrophin receptors. J. Neurobiol. 25, 1386–1403 (1994).
Huang, E. J. & Reichardt, L. F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 (2003).
Clary, D. O. & Reichardt, L. F. An alternatively spliced form of the nerve growth factor receptor TrkA confers an enhanced response to neurotrophin 3. Proc. Natl Acad. Sci. USA 91, 11133–11137 (1994).
Brodeur, G. M. et al. Trk receptor expression and inhibition in neuroblastomas. Clin. Cancer Res. 15, 3244–3250 (2009).
Tacconelli, A. et al. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell 6, 347–360 (2004).
Tacconelli, A., Farina, A. R., Cappabianca, L., Gulino, A. & Mackay, A. R. Alternative TrkAIII splicing: a potential regulated tumor-promoting switch and therapeutic target in neuroblastoma. Future Oncol. 1, 689–698 (2005).
Strohmaier, C., Carter, B. D., Urfer, R., Barde, Y. A. & Dechant, G. A splice variant of the neurotrophin receptor trkB with increased specificity for brain-derived neurotrophic factor. EMBO J. 15, 3332–3337 (1996).
Boeshore, K. L., Luckey, C. N., Zigmond, R. E. & Large, T. H. TrkB isoforms with distinct neurotrophin specificities are expressed in predominantly nonoverlapping populations of avian dorsal root ganglion neurons. J. Neurosci. 19, 4739–4747 (1999).
Luberg, K., Wong, J., Weickert, C. S. & Timmusk, T. Human TrkB gene: novel alternative transcripts, protein isoforms and expression pattern in the prefrontal cerebral cortex during postnatal development. J. Neurochem. 113, 952–964 (2010).
Valenzuela, D. M. et al. Alternative forms of rat TrkC with different functional capabilities. Neuron 10, 963–974 (1993).
Stoilov, P., Castren, E. & Stamm, S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem. Biophys. Res. Commun. 290, 1054–1065 (2002).
Tsoulfas, P., Stephens, R. M., Kaplan, D. R. & Parada, L. F. TrkC isoforms with inserts in the kinase domain show impaired signaling responses. J. Biol. Chem. 271, 5691–5697 (1996).
Esteban, P. F. et al. A kinase-deficient TrkC receptor isoform activates Arf6-Rac1 signaling through the scaffold protein tamalin. J. Cell Biol. 173, 291–299 (2006).
Ohira, K. et al. A truncated tropomyosin-related kinase B receptor, T1, regulates glial cell morphology via Rho GDP dissociation inhibitor 1. J. Neurosci. 25, 1343–1353 (2005).
Cunningham, M. E. & Greene, L. A. A function-structure model for NGF-activated TRK. EMBO J. 17, 7282–7293 (1998).
Reichardt, L. F. Neurotrophin-regulated signalling pathways. Phil. Trans. R. Soc. Series B Biol. Sci. 361, 1545–1564 (2006).
Minichiello, L. et al. Point mutation in trkB causes loss of NT4-dependent neurons without major effects on diverse BDNF responses. Neuron 21, 335–345 (1998).
Postigo, A. et al. Distinct requirements for TrkB and TrkC signaling in target innervation by sensory neurons. Genes Dev. 16, 633–645 (2002).
Wai, D. H. et al. The ETV6-NTRK3 gene fusion encodes a chimeric protein tyrosine kinase that transforms NIH3T3 cells. Oncogene 19, 906–915 (2000).
Barbacid, M., Lamballe, F., Pulido, D. & Klein, R. The trk family of tyrosine protein kinase receptors. Biochim. Biophys. Acta 1072, 115–127 (1991).
Levi-Montalcini, R. The nerve growth factor: thirty-five years later. Biosci. Rep. 7, 681–699 (1987).
Cohen, S., Levi-Montalcini, R. & Hamburger, V. A. Nerve growth-stimulating factor isolated from sarcom as 37 and 180. Proc. Natl Acad. Sci. USA 40, 1014–1018 (1954).
Frade, J. M. & Barde, Y. A. Nerve growth factor: two receptors, multiple functions. Bioessays 20, 137–145 (1998).
Patel, T. D., Jackman, A., Rice, F. L., Kucera, J. & Snider, W. D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 25, 345–357 (2000).
Teng, H. K. et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 25, 5455–5463 (2005).
Mahadeo, D., Kaplan, L., Chao, M. V. & Hempstead, B. L. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J. Biol. Chem. 269, 6884–6891 (1994).
Hempstead, B. L., Martin-Zanca, D., Kaplan, D. R., Parada, L. F. & Chao, M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature 350, 678–683 (1991).
Bibel, M., Hoppe, E. & Barde, Y. A. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 18, 616–622 (1999).
Makkerh, J. P. et al. p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 6, 936–941 (2005).
Eggert, A., Sieverts, H., Ikegaki, N. & Brodeur, G. M. p75 mediated apoptosis in neuroblastoma cells is inhibited by expression of TrkA. Med. Pediatr. Oncol. 35, 573–576 (2000).
Rabizadeh, S. et al. Induction of apoptosis by the low-affinity NGF receptor. Science 261, 345–348 (1993).
Geiger, T. R., Song, J. Y., Rosado, A. & Peeper, D. S. Functional characterization of human cancer-derived TRKB mutations. PLOS ONE 6, e16871 (2011).
Harada, T. et al. Role and relevance of TrkB mutations and expression in non-small cell lung cancer. Clin. Cancer Res. 17, 2638–2645 (2011).
Marchetti, A. et al. Frequent mutations in the neurotrophic tyrosine receptor kinase gene family in large cell neuroendocrine carcinoma of the lung. Hum. Mut. 29, 609–616 (2008).
Miranda, C., Mazzoni, M., Sensi, M., Pierotti, M. A. & Greco, A. Functional characterization of NTRK1 mutations identified in melanoma. Genes Chromosomes Cancer 53, 875–880 (2014).
Tomasson, M. H. et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood 111, 4797–4808 (2008).
Reuther, G. W., Lambert, Q. T., Caligiuri, M. A. & Der, C. J. Identification and characterization of an activating TrkA deletion mutation in acute myeloid leukemia. Mol. Cell. Biol. 20, 8655–8666 (2000).
Eggert, A. et al. Expression of the neurotrophin receptor TrkA down-regulates expression and function of angiogenic stimulators in SH-SY5Y neuroblastoma cells. Cancer Res. 62, 1802–1808 (2002).
Lagadec, C. et al. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene 28, 1960–1970 (2009).
Lange, A. M. & Lo, H. W. Inhibiting TRK proteins in clinical cancer therapy. Cancers 10, 105 (2018).
Rajan, N. et al. Dysregulated TRK signalling is a therapeutic target in CYLD defective tumours. Oncogene 30, 4243–4260 (2011).
Nakagawara, A., Azar, C. G., Scavarda, N. J. & Brodeur, G. M. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol. Cell. Biol. 14, 759–767 (1994).
Evans, A. E. et al. Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clin. Cancer Res. 5, 3594–3602 (1999).
Evans, A. E. et al. Effect of CEP-751 (KT-6587) on neuroblastoma xenografts expressing TrkB. Med. Pediatr. Oncol. 36, 181–184 (2001).
Minturn, J. E. et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother. Pharmacol. 68, 1057–1065 (2011).
Vaishnavi, A., Le, A. T. & Doebele, R. C. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 5, 25–34 (2015).
Schram, A. M., Chang, M. T., Jonsson, P. & Drilon, A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 14, 735–748 (2017).
Coulier, F., Martin-Zanca, D., Ernst, M. & Barbacid, M. Mechanism of activation of the human trk oncogene. Mol. Cell. Biol. 9, 15–23 (1989).
Knezevich, S. R., McFadden, D. E., Tao, W., Lim, J. F. & Sorensen, P. H. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat. Genet. 18, 184–187 (1998).
Hechtman, J. F. et al. Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am. J. Surg. Pathol. 41, 1547–1551 (2017).
Singh, D. et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 337, 1231–1235 (2012).
Borrello, M. G. et al. The oncogenic versions of the Ret and Trk tyrosine kinases bind Shc and Grb2 adaptor proteins. Oncogene 9, 1661–1668 (1994).
Miranda, C., Greco, A., Miele, C., Pierotti, M. A. & Van Obberghen, E. IRS-1 and IRS-2 are recruited by TrkA receptor and oncogenic TRK-T1. J. Cell. Physiol. 186, 35–46 (2001).
Ranzi, V. et al. The signaling adapters fibroblast growth factor receptor substrate 2 and 3 are activated by the thyroid TRK oncoproteins. Endocrinology 144, 922–928 (2003).
Jin, W. et al. Cellular transformation and activation of the phosphoinositide-3-kinase-Akt cascade by the ETV6-NTRK3 chimeric tyrosine kinase requires c-Src. Cancer Res. 67, 3192–3200 (2007).
Tognon, C. E. et al. A tripartite complex composed of ETV6-NTRK3, IRS1 and IGF1R is required for ETV6-NTRK3-mediated membrane localization and transformation. Oncogene 31, 1334–1340 (2012).
Drilon, A. et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann. Oncol. 27, 920–926 (2016).
Vaishnavi, A. et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat. Med. 19, 1469–1472 (2013).
Vaishnavi, A. et al. EGFR mediates responses to small-molecule drugs targeting oncogenic fusion kinases. Cancer Res. 77, 3551–3563 (2017).
Chen, S. et al. A new ETV6-NTRK3 cell line model reveals MALAT1 as a novel therapeutic target - a short report. Cell. Oncol. 41, 93–101 (2018).
Cook, P. J. et al. Somatic chromosomal engineering identifies BCAN-NTRK1 as a potent glioma driver and therapeutic target. Nat. Commun. 8, 15987 (2017).
Roberts, K. G. et al. ETV6-NTRK3 induces aggressive acute lymphoblastic leukemia highly sensitive to selective TRK inhibition. Blood 132, 861–865 (2018).
Witte, K. et al. TFG-1 function in protein secretion and oncogenesis. Nat. Cell Biol. 13, 550–558 (2011).
Amatu, A., Sartore-Bianchi, A. & Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 1, e000023 (2016).
Halalsheh, H. et al. Dramatic bone remodeling following larotrectinib administration for bone metastasis in a patient with TRK fusion congenital mesoblastic nephroma. Pediat. Blood Cancer 65, e27271 (2018).
Davis, J. L. et al. Infantile NTRK-associated mesenchymal tumors. Pediatr. Dev. Pathol. 21, 68–78 (2018).
Laetsch, T. W. et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet. Oncol. 19, 705–714 (2018).
Tognon, C. et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2, 367–376 (2002).
Taylor, J. et al. Oncogenic TRK fusions are amenable to inhibition in hematologic malignancies. J. Clin. Invest. 31, 3819–3825 (2018).
Benayed, R. et al. Comprehensive detection of targetable fusions in lung adenocarcinomas by complementary targeted DNAseq and RNAseq assays. J. Clin. Oncol. 36, 12076–12076 (2018).
Drilon, A. et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 7, 963–972 (2017).
Russo, M. et al. Acquired resistance to the TRK inhibitor entrectinib in colorectal cancer. Cancer Discov. 6, 36–44 (2016).
Rudzinski, E. R. et al. Pan-Trk immunohistochemistry identifies NTRK rearrangements in pediatric mesenchymal tumors. Am. J. Surg. Pathol. 42, 927–935 (2018).
Menichincheri, M. et al. Discovery of entrectinib: a new 3-aminoindazole as a potent anaplastic lymphoma kinase (ALK), c-ros oncogene 1 kinase (ROS1), and pan-tropomyosin receptor kinases (pan-TRKs) inhibitor. J. Med. Chem. 59, 3392–3408 (2016).
Doebele, R. C. et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov. 5, 1049–1057 (2015).
Kazandjian, D. et al. Benefit-risk summary of crizotinib for the treatment of patients with ROS1 alteration-positive, metastatic non-small cell lung cancer. Oncol. 21, 974–980 (2016).
Malik, S. M. et al. U. S. Food and Drug Administration approval: crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin. Cancer Res. 20, 2029–2034 (2014).
Singh, H. et al. U. S. Food and Drug Administration approval: cabozantinib for the treatment of advanced renal cell carcinoma. Clin. Cancer Res. 23, 330–335 (2017).
Shamroe, C. L. & Comeau, J. M. Ponatinib: a new tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann. Pharmacother. 47, 1540–1546 (2013).
Karimi-Shah, B. A. & Chowdhury, B. A. Forced vital capacity in idiopathic pulmonary fibrosis — FDA review of pirfenidone and nintedanib. N. Engl. J. Med. 372, 1189–1191 (2015).
Zou, H. Y. et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 67, 4408–4417 (2007).
Christensen, J. G. et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 6, 3314–3322 (2007).
Cui, J. J. et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J. Med. Chem. 54, 6342–6363 (2011).
Bergethon, K. et al. ROS1 rearrangements define a unique molecular class of lung cancers. J. Clin. Oncol. 30, 863–870 (2012).
Bowles, D. W., Kessler, E. R. & Jimeno, A. Multi-targeted tyrosine kinase inhibitors in clinical development: focus on XL-184 (cabozantinib). Drugs Today (Barc) 47, 857–868 (2011).
O’Hare, T. et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 16, 401–412 (2009).
Fuse, M. J. et al. Mechanisms of resistance to NTRK inhibitors and therapeutic strategies in NTRK1-rearranged cancers. Mol. Cancer Ther. 16, 2130–2143 (2017).
Hilberg, F. et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 68, 4774–4782 (2008).
Mok, S., Duffy, C., Du, R. & Allison, J. P. Increase antitumor activity of immunotherapy by blocking colony stimulating factor 1 receptor and tropomyosin receptor kinase [abstract]. Cancer Immunol. Res. 4 (Suppl), A146 (2016).
Patwardhan, P. P., Ivy, K. S., Musi, E., de Stanchina, E. & Schwartz, G. K. Significant blockade of multiple receptor tyrosine kinases by MGCD516 (Sitravatinib), a novel small molecule inhibitor, shows potent anti-tumor activity in preclinical models of sarcoma. Oncotarget 7, 4093–4109 (2016).
Smith, K. M. et al. Anti-tumor activity of entrectinib, a pan-TRK, ROS1 and ALK inhibitor, in ETV6-NTRK3-positive acute myeloid leukemia. Mol. Cancer Ther. 17, 455–463 (2017).
Kiga, M. et al. Preclinical characterization and antitumor efficacy of DS-6051b, a novel, orally available small molecule tyrosine kinase inhibitor of ROS1 and NTRKs. Eur. J. Cancer 69, S35–S36 (2016).
Smith, B. D. et al. Altiratinib inhibits tumor growth, invasion, angiogenesis, and microenvironment-mediated drug resistance via balanced inhibition of MET, TIE2, and VEGFR2. Mol. Cancer Ther. 14, 2023–2034 (2015).
Smith, B. D. et al. Altiratinib is a potent inhibitor of TRK kinases and is efficacious in TRK-fusion driven cancer models [abstract]. Cancer Res. 75, 790 (2015).
Kawada, I. et al. Dramatic antitumor effects of the dual MET/RON small-molecule inhibitor LY2801653 in non-small cell lung cancer. Cancer Res. 74, 884–895 (2014).
Yan, S. B. et al. MET-targeting antibody (emibetuzumab) and kinase inhibitor (merestinib) as single agent or in combination in a cancer model bearing MET exon 14 skipping. Invest. New Drugs 36, 536–544 (2017).
Konicek, B. W. et al. Merestinib (LY2801653) inhibits neurotrophic receptor kinase (NTRK) and suppresses growth of NTRK fusion bearing tumors. Oncotarget 9, 13796–13806 (2018).
Yan, S. B. et al. LY2801653 is an orally bioavailable multi-kinase inhibitor with potent activity against MET, MST1R, and other oncoproteins, and displays anti-tumor activities in mouse xenograft models. Invest. New Drugs 31, 833–844 (2013).
Katayama, R. et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci.Transl Med. 4, 120ra117 (2012).
Awad, M. M., Engelman, J. A. & Shaw, A. T. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N. Engl. J. Med. 369, 1173 (2013).
Kim, S. et al. Heterogeneity of genetic changes associated with acquired crizotinib resistance in ALK-rearranged lung cancer. J. Thorac. Oncol. 8, 415–422 (2013).
Zhai, D., Deng, W., Huang, J., Rogers, E. & Cui, J. J. TPX-0005, an ALK/ROS1/TRK inhibitor, overcomes multiple resistance mechanisms by targeting SRC/FAK signaling [abstract]. Cancer Res. 77, 3161 (2017).
Kozaki, R., Yoshizawa, T., Tsukamoto, K., Kato, H. & Kawabata, K. A potent and selective TRK inhibitor ONO-5390556, shows potent antitumor activity against both TRK-rearranged cancers and the resistant mutants [abstract]. Cancer Res. 76, 2954A (2016).
Drilon, A. E. et al. A phase 1 study of the next-generation ALK/ROS1/TRK inhibitor ropotrectinib (TPX-0005) in patients with advanced ALK/ROS1/NTRK + cancers (TRIDENT-1). J. Clin. Oncol. 36, 2513 (2018).
Smeyne, R. J. et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368, 246–249 (1994).
Klein, R. et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 75, 113–122 (1993).
Klein, R. et al. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature 368, 249–251 (1994).
Wagner, N. et al. Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms’ tumor transcription factor Wt1. Genes Dev. 19, 2631–2642 (2005).
Tessarollo, L. et al. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc. Natl Acad. Sci. USA 94, 14776–14781 (1997).
Kermani, P. & Hempstead, B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc. Med. 17, 140–143 (2007).
Dissen, G. A. et al. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology 137, 198–209 (1996).
Kermani, P. et al. Neurotrophins promote revascularization by local recruitment of TrkB + endothelial cells and systemic mobilization of hematopoietic progenitors. J. Clin. Invest. 115, 653–663 (2005).
Kawamura, K., Kawamura, N., Mulders, S. M., Sollewijn Gelpke, M. D. & Hsueh, A. J. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc. Natl Acad. Sci. USA 102, 9206–9211 (2005).
Greco, A., Villa, R., Fusetti, L., Orlandi, R. & Pierotti, M. A. The Gly571Arg mutation, associated with the autonomic and sensory disorder congenital insensitivity to pain with anhidrosis, causes the inactivation of the NTRK1/nerve growth factor receptor. J. Cell. Physiol. 182, 127–133 (2000).
Indo, Y. et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat. Genet. 13, 485–488 (1996).
Yeo, G. S. et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 7, 1187–1189 (2004).
Xu, B. et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6, 736–742 (2003).
Richardson, C. A. & Leitch, B. Phenotype of cerebellar glutamatergic neurons is altered in stargazer mutant mice lacking brain-derived neurotrophic factor mRNA expression. J. Comparative Neurol. 481, 145–159 (2005).
Ashraf, S., Bouhana, K. S., Pheneger, J., Andrews, S. W. & Walsh, D. A. Selective inhibition of tropomyosin-receptor-kinase A (TrkA) reduces pain and joint damage in two rat models of inflammatory arthritis. Arthritis Res. Ther. 18, 97 (2016).
Hirose, M., Kuroda, Y. & Murata, E. NGF/TrkA signaling as a therapeutic target for pain. Pain Pract. 16, 175–182 (2016).
Acknowledgements
The authors thank A. Drilon, S. Misale and C. Verma for assisting with the creation and design of the figures in this article. The work of the authors is supported by Cycle for Survival and the National Institutes of Health awards P30 CA008748 and R01CA226864. E.C. is a recipient of a MSK Society Scholar Prize. Given the space limitations of this review, the authors apologize for their inability to cite everyone who has contributed to this field of inquiry.
Reviewer information
Nature Reviews Clinical Oncology thanks T. Laetsch and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
E.C., M.S. and A.D. made substantial contributions to each stage of the preparation of this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
A.D. has received honoraria (as an advisory board member) from Bayer, Ignyta, Loxo Oncology, Pfizer, Roche/Genentech and TP Therapeutics, and research funding from Loxo Oncology. M.S. has received research funding from Daiichi Sankyo and Puma Biotechnology. E.C. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cocco, E., Scaltriti, M. & Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 15, 731–747 (2018). https://doi.org/10.1038/s41571-018-0113-0
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-018-0113-0
This article is cited by
-
A benchmarking framework for the accurate and cost-effective detection of clinically-relevant structural variants for cancer target identification and diagnosis
Journal of Translational Medicine (2024)
-
Synthesis, anticancer evaluation, molecular docking and ADME study of novel pyrido[4ʹ,3ʹ:3,4]pyrazolo[1,5-a]pyrimidines as potential tropomyosin receptor kinase A (TrKA) inhibitors
BMC Chemistry (2024)
-
Oncogenic alterations in advanced NSCLC: a molecular super-highway
Biomarker Research (2024)
-
DeKinomics pulse-chases kinase functions in living cells
Nature Chemical Biology (2024)
-
LTK mutations responsible for resistance to lorlatinib in non-small cell lung cancer harboring CLIP1-LTK fusion
Communications Biology (2024)