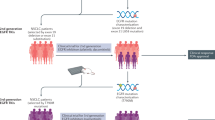
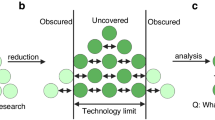
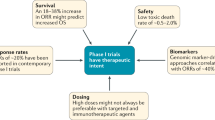
Abstract
The traditional approach to drug development in oncology, with discrete phases of clinical testing, is becoming untenable owing to expansion of the precision medicine paradigm, whereby patients are stratified into multiple subgroups according to the underlying cancer biology. Seamless approaches to drug development in oncology hold great promise of accelerating the accessibility of novel therapeutic agents to the public but are also accompanied by important trade-offs, including the limited availability of information on the clinical benefit and safety of novel agents at the time of market entry. In this Perspectives article, we describe several opportunities, in the form of novel trial designs or modelling strategies, to improve the efficiency of drug development in oncology, as well as new mechanisms to obtain information about anticancer therapies throughout their life cycle, such as innovative functional imaging techniques or the use of real-world clinical data.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gan, H. K., You, B., Pond, G. R. & Chen, E. X. Assumptions of expected benefits in randomized phase III trials evaluating systemic treatments for cancer. J. Natl Cancer Inst. 104, 590–598 (2012).
Hwang, T. J. et al. Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern. Med. 176, 1826–1833 (2016).
American Association for Cancer Research. AACR cancer progress report 2011. Roswell Park https://www.roswellpark.org/sites/default/files/node-files/asset/nid91575-2011-aacr-cpr-text-web.pdf (2011).
Wong, C. H., Siah, K. W. & Lo, A. W. Estimation of clinical trial success rates and related parameters. Biostatistics https://doi.org/10.1093/biostatistics/kxx069 (2018).
Dagher, R. et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin. Cancer Res. 8, 3034 (2002).
Kazandjian, D. et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist 19, e5–e11 (2014).
Minasian, L. et al. Optimizing dosing of oncology drugs. Clin. Pharmacol. Ther. 96, 572–579 (2014).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
National Academies of Sciences, Engineering, and Medicine. Policy Issues in the Clinical Development and Use of Immunotherapy for Cancer Treatment: Proceedings of a Workshop. (The National Academies Press, Washington, DC, 2016).
Institute of Medicine. Biomarker Tests for Molecularly Targeted Therapies: Key to Unlocking Precision Medicine. (The National Academies Press, Washington, DC, 2016).
National Academies of Sciences, Engineering, and Medicine. The Drug Development Paradigm in Oncology: Proceedings of a Workshop. (The National Academies Press, Washington, DC, 2018).
Kemp, R. & Prasad, V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 15, 134 (2017).
Lonial, S. & Anderson, K. C. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia 28, 258–268 (2014).
Anderson, K. C. et al. The role of minimal residual disease testing in myeloma treatment selection and drug development: current value and future applications. Clin. Cancer Res. 23, 3980–3993 (2017).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Vilar, E. & Gruber, S. B. Microsatellite instability in colorectal cancer — the stable evidence. Nat. Rev. Clin. Oncol. 7, 153–162 (2010).
US Food and Drug Administration. Guidance for industry: pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. FDA www.fda.gov/downloads/drugs/guidances/ucm305501.pdf (2014).
Quantum Leap Healthcare Collaborative. The I-SPY trials. I-SPY Trials https://www.ispytrials.org/ (2018).
Wahl, R. L., Jacene, H., Kasamon, Y. & Lodge, M. A. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 50, 122S–150S (2009).
Clegg, N. J. et al. ARN-509: a novel anti-androgen for prostate cancer treatment. Cancer Res. 72, 494–1503 (2012).
Rathkopf, D. E. et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 31, 3525–3530 (2013).
Fox, J. J. et al. Positron emission tomography/computed tomography–based assessments of androgen receptor expression and glycolytic activity as a prognostic biomarker for metastatic castration-resistant prostate cancer. JAMA Oncol. 4, 217–224 (2018).
Wang, Y. et al. 18F-fluoroestradiol PET/CT measurement of estrogen receptor suppression during a phase I trial of the novel estrogen receptor-targeted therapeutic GDC-0810: using an imaging biomarker to guide drug dosage in subsequent trials. Clin. Cancer Res. 23, 3053–3060 (2017).
Michel, L. S. et al. PET of poly (ADP-ribose) polymerase activity in cancer: preclinical assessment and first in-human studies. Radiology 282, 453–463 (2016).
Kim, J. et al. Use of PRO measures to inform tolerability in oncology trials: implications for clinical review, IND safety reporting and clinical site inspections. Clin. Cancer Res. 24, 1780–1784 (2018).
Fontes Jardim, D. L. et al. Impact of a biomarker-based strategy on oncology drug development: a meta-analysis of clinical trials leading to FDA approval. J. Natl Cancer Inst. 107, djv253 (2015).
Schwaederle, M. et al. Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: a meta-analysis. JAMA Oncol. 2, 1452–1459 (2016).
Mandrekar, S. J. & Sargent, D. J. Clinical trial designs for predictive biomarker validation: one size does not fit all. J. Biopharm. Stat. 19, 530–542 (2009).
Finn, R. S. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 16, 25–35 (2015).
National Cancer Institute. NCI-Molecular Analysis for Therapy Choice (NCI-MATCH) Trial. Cancer.gov https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match (2017).
Shepherd, F. A. et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 353, 123–132 (2005).
Herbst, R. S. et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 23, 5892–5899 (2005).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Pao, W. et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA 101, 13306–13311 (2004).
Fukuoka, M. et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 29, 2866–2874 (2011).
Jänne, P. A. et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J. Clin. Oncol. 30, 2063–2069 (2012).
Kwak, E. L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
O’Connor, J. P. B. et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 14, 169–186 (2017).
Tighiouart, M., Piantadosi, S. & Rogatko, A. Dose finding with drug combinations in cancer phase I clinical trials using conditional escalation with overdose control (EWOC). Stat. Med. 33, 3815–3829 (2014).
Jänne, P. A. et al. Dose finding of small-molecule oncology drugs: optimization throughout the development life cycle. Clin. Cancer Res. 22, 2613–2617 (2016).
Cook, N., Hansen, A. R., Siu, L. L. & Abdul Razak, A. R. Early phase clinical trials to identify optimal dosing and safety. Mol. Oncol. 9, 997–1007 (2015).
Goldstein, D. A. et al. A phamacoeconomic analysis of personalized dosing versus fixed dosing of pembrolizumab in first-line PD-L1 positive non-small cell lung cancer. J. Natl Cancer Inst. 109, djx063 (2017).
Tighiouart, M., Li, Q. & Rogatko, A. A. Bayesian adaptive design for estimating the maximum tolerated dose curve using drug combinations in cancer phase I clinical trials. Stat. Med. 36, 280–290 (2017).
Myers, R. H., Montgomery, D. C. & Anderson-Cook, C. M. Response Surface Methodology: Process and Product Optimization using Designed Experiments. (John Wiley & Sons, Inc., Hoboken, NJ, 2016).
Emens, L. A. et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor–secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J. Clin. Oncol. 27, 5911–5918 (2009).
Wages, N. A., Portell, C. A., Williams, M. E., Conaway, M. R. & Petroni, G. R. Implementation of a model-based design in a phase Ib study of combined targeted agents. Clin. Cancer Res. 23, 7158–7164 (2017).
Raju, G., Gurumurthi, K. & Domike, R. Benefit-risk analysis for decision-making: an approach. Clin. Pharmacol. Ther. 100, 654–671 (2016).
Raju, G. et al. A benefit–risk analysis approach to capture regulatory decision-making: non-small cell lung cancer. Clin. Pharmacol. Ther. 100, 672–684 (2016).
Piantadosi, S. Clinical Trials: A Methodologic Perspective. 3rd edn 286–292 (John Wiley & Sons, Inc., Hoboken, NJ, 2017).
Prowell, T. M., Theoret, M. R. & Pazdur, R. Seamless oncology-drug development. N. Engl. J. Med. 374, 2001–2003 (2016).
Cohen, M. H. et al. Approval summary for Imatinib Mesylate capsules in the treatment of chronic myelogenous leukemia. Clin. Cancer Res. 8, 935 (2002).
Theoret, M. R. et al. Expansion cohorts in first-in-human solid tumor oncology trials. Clin. Cancer Res. 21, 4545–4551 (2015).
Ou, S. H., Bartlett, C. H., Mino-Kenudson, M., Cui, J. & Iafrate, A. J. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 17, 1351–1375 (2012).
Khoja, L., Butler, M. O., Kang, S. P., Ebbinghaus, S. & Joshua, A. M. Pembrolizumab. J. Immunother. Cancer 3, 36 (2015).
Patnaik, A. et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin. Cancer Res. 21, 4286–4293 (2015).
Kang, S. P. et al. Pembrolizumab KEYNOTE-001: an adaptive study leading to accelerated approval for two indications and a companion diagnostic. Ann. Oncol. 28, 1388–1398 (2017).
Bates, S. E. et al. Advancing clinical trials to streamline drug development. Clin. Cancer Res. 21, 4527–4535 (2015).
Simon, R. Critical review of umbrella, basket, and platform designs for oncology clinical trials. Clin. Pharmacol. Ther. 102, 934–941 (2017).
Woodcock, J. & LaVange, L. M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 377, 62–70 (2017).
Berry, D. A. Emerging innovations in clinical trial design. Clin. Pharmacol. Ther. 99, 82–91 (2016).
Baghdadi, T. A. et al. Palbociclib (P) in patients (Pts) with pancreatic cancer (PC) and gallbladder or bile duct cancer (GBC) with CDKN2A alterations: results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study [abstract]. J. Clin. Oncol. 36 (Suppl), 2532 (2018).
National Cancer Institute. NCI-MATCH precision medicine clinical trial releases new findings, strengthens path forward for targeted cancer therapies. Cancer.gov https://www.cancer.gov/news-events/press-releases/2018/nci-match-first-results (2018).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
Neelapu, S. S. et al. Axicabtagene Ciloleucel CAR T cell therapy in refractory large B cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Mangat, P. K. et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry Study. JCO Precis. Oncol. https://doi.org/10.1200/PO.18.00122 (2018).
Clinical Trials Transformation Initiative. PROJECT: Data Monitoring Committees (DMCs). CTTI https://www.ctti-clinicaltrials.org/projects/data-monitoring-committees-dmcs (2018).
Badenas, J. M. Globalization of clinical trials. Slideshare https://www.slideshare.net/josepmariabadenas/globalization-of-clinical-trials-2010-josep-m-badenas (2010).
Sheiner, L. B. Learning versus confirming in clinical drug development. Clin. Pharmacol. Ther. 61, 275–291 (1997).
US Food and Drug Administration. Real-time oncology review pilot program. FDA https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/OCE/ucm612927.htm (2018).
Beaver, J. A. et al. 25-year experience of US Food and Drug Administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 4, 849–856 (2018).
US Food and Drug Administration. Drugs@FDA: FDA approved drug products. FDA http://www.accessdata.fda.gov/scripts/cder/daf (2018).
US National Library of Medicine. Homepage. ClinicalTrials.gov https://clinicaltrials.gov/ct2/home (2018).
Miksad, R. A. & Abernethy, A. P. Harnessing the power of real-world evidence (RWE): a checklist to ensure regulatory-grade data quality. Clin. Pharmacol. Ther. 103, 202–205 (2018).
Mudad, R., Patel, M. B., Margunato-Debay, S., Garofalo, D. & Lal, L. S. Comparative effectiveness and safety of nab-paclitaxel plus carboplatin versus gemcitabine plus carboplatin in first-line treatment of advanced squamous cell non-small cell lung cancer in a US community oncology setting. Lung Cancer 8, 179–190 (2017).
Khozin, S., Blumenthal, G. M. & Pazdur, R. Real-world data for clinical evidence generation in oncology. J. Natl Cancer Inst. 109, djx187 (2017).
Sutter, S. Pink sheet — real-world evidence may find a home on breakthrough pathway. FOCR https://www.focr.org/news/pink-sheet-real-world-evidence-may-find-home-breakthrough-pathway (2016).
Agarwala, V. et al. Real-world evidence in support of precision medicine: clinico-genomic cancer data as a case study. Health Aff. 37, 765–772 (2018).
Basch, E. Toward patient-centered drug development in oncology. N. Engl. J. Med. 369, 397–400 (2013).
American Cancer Society Cancer Action Network. Barriers to patient enrollment in therapeutic clinical trials for cancer. April, 2017. ACSCAN https://www.acscan.org/policy-resources/clinical-trial-barriers (2017).
Kim, E. S. et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J. Clin. Oncol. 35, 3737–3744 (2017).
Institute of Medicine. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program (The National Academies Press, Washington, DC, 2010).
Visvanathan, K. et al. Untapped potential of observational research to inform clinical decision making: American Society of Clinical Oncology research statement. J. Clin. Oncol. 35, 1845–1854 (2017).
Schilsky, R. L. Finding the evidence in real-world evidence: moving from data to information to knowledge. J. Am. Coll. Surg. 224, 1–7 (2017).
Roland, M. & Torgerson, D. J. What are pragmatic trials? Br. Med. J. 316, 285 (1998).
US Food and Drug Administration. Use of electronic health record data in clinical investigations: guide for industry. FDA https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm501068.pdf?utm_campaign=FDA%20Issues%20Final%20Guidance%20for%20Industry%3A%20Use%20of%20EHR%20Data%20in%20Clinical%20Investigations&utm_medium=email&utm_source=Eloqua (2018).
Bach, P. B. Limits on Medicare’s ability to control rising spending on cancer drugs. N. Engl. J. Med. 360, 626–633 (2009).
Bach, P. Price & value of cancer drug. MSKCC https://www.mskcc.org/research-programs/health-policy-outcomes/cost-drugs (2018).
Mailankody, S. & Prasad, V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol. 1, 539–540 (2015).
President’s Cancer Panel. Promoting value, affordability, and innovation in cancer drug treatment. Cancer.gov https://prescancerpanel.cancer.gov/report/drugvalue/Part2Rec1.html (2018).
National Academies of Sciences, Engineering, and Medicine. Making Medicines Affordable: A National Imperative. (The National Academies Press, Washington, DC, 2018).
Mandelblatt, J. S., Ramsey, S. D., Lieu, T. A. & Phelps, C. E. Evaluating frameworks that provide value measures for health care interventions. Value Health 20, 185–192 (2017).
Gibson, T. B., Maclean, R. J., Chernew, M. E., Fendrick, A. M. & Baigel, C. Value-based insurance design: benefits beyond cost and utilization. Am. J. Manag. Care 21, 32–35 (2015).
Drug Pricing Lab. Value-based pricing versus outcomes-based contracting. Drug Pricing Lab https://drugpricinglab.org/our-work/value-based-pricing-vs-outcomes-based-contracting/ (2017).
Acknowledgements
The responsibility for the content of this article rests with the authors and does not necessarily represent the views of the National Academies of Sciences, Engineering and Medicine, its committees, its sponsors or its convening activities. The activities of the National Cancer Policy Forum are supported by its sponsoring members, which currently include the Centers for Disease Control and Prevention, the NIH/National Cancer Institute, the American Association for Cancer Research, the American Cancer Society, the American College of Radiology, ASCO, the American Society of Hematology, the Association of American Cancer Institutes, Bristol-Myers Squibb, the Cancer Support Community, the CEO Roundtable on Cancer, Flatiron Health, Helsinn Therapeutics (US), the LIVESTRONG Foundation, Merck, the National Comprehensive Cancer Network, Novartis Oncology, the Oncology Nursing Society and Pfizer. The authors thank the speakers and participants for their contributions to the workshop.
Reviewer information
Nature Reviews Clinical Oncology thanks G. M. Blumenthal, F. Pignatti, E. D. Saad and H. J. West for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to all aspects of manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
S.J.N. receives partial salary support from the sponsors of the National Cancer Policy Forum. M.L.R. is an employee and stockholder of Pfizer. H.H. is a member of the board of directors of Ion Beam Applications. A.A. is an employee of Flatiron Health. K.A. is an advisory board member for Bristol-Myers Squibb (BMS), Celgene and Millennium Takeda, a scientific advisory board member of Gilead, and the National Cancer Institute and is a founder of C4 Therapeutics and Oncopep. A.W.G. receives salary support from the sponsors of the National Academies of Sciences, Engineering and Medicine (NASEM) Forum on Drug Discovery, Development and Translation. R.D.H. receives research funding that supports his salary from Abbvie, Amgen, Arqule, AstraZeneca, BMS, Calithera, Celgene, Corvus, Eli Lilly, Five Prime Therapeutics, Genmab, Halozyme, Ignyta, Incyte, Merck, Nektar, Pfizer, Regeneron, Rgenix, Sanofi, Syndax, Takeda and Vertex. R.L.S. is a principal investigator in the Targeted Agent and Profiling Utilization Registry (TAPUR) study, which receives grant support from AstraZeneca, Bayer, BMS, Genentech, Lilly, Merck and Pfizer. R.P., S.P., M.M.B. and D.S. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Clinicaltrials.gov: https://clinicaltrials.gov/
FDA Hematology/Oncology approvals and safety notifications: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm
National Cancer Policy Forum: nationalacademies.org/NCPF
Glossary
- Bayesian learning process
-
The interpretation of information from accumulating data using Bayes’ theorem to modify a prior belief.
- Data and Safety Monitoring Boards
-
(DSMBs). Committees of impartial experts who can assess the risks and benefits for study participants on an ongoing basis and recommend whether the trial should continue or terminate early; the use of DSMBs is a universally employed method to monitor an ongoing clinical trial.
- Envelope simulation
-
A method of generating simulated data from a rectangular region (‘envelope’) of the dose–response plane to support a model that aids dose-finding studies.
- Surface design methods
-
Experimental designs in which two or more factors are varied to test their effects on a response. The response can then be plotted as a surface to look for peaks and valleys (high and low responses).
- Traditional type I and II error properties
-
Properties of many clinical trials in which the probability of type I errors (false-positive result) is set to 0.05 and that of type II errors (false-negative result) is set to 0.1–0.2. These choices are very common but not always appropriate for some questions.
Rights and permissions
About this article
Cite this article
Nass, S.J., Rothenberg, M.L., Pentz, R. et al. Accelerating anticancer drug development — opportunities and trade-offs. Nat Rev Clin Oncol 15, 777–786 (2018). https://doi.org/10.1038/s41571-018-0102-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-018-0102-3
This article is cited by
-
A review on reported phytochemicals as druggable leads with antimalarial potential
Medicinal Chemistry Research (2023)
-
Association between control group therapy and magnitude of clinical benefit of cancer drugs
Scientific Reports (2022)
-
Modeling neoplastic disease with spheroids and organoids
Journal of Hematology & Oncology (2020)