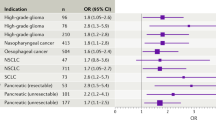
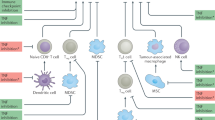
Abstract
Recognizing that the transformative effects of immunotherapy are currently limited to a minority of patients with cancer, research efforts are increasingly focused on expanding and enhancing clinical responses by combining immunotherapies; the repurposing of existing drugs is an attractive approach, given their well-characterized safety and pharmacokinetic profiles. Receptor activator of nuclear factor-κB (RANK) and the RANK ligand (RANKL) were initially described in the context of T cell–dendritic cell interactions; however, the discovery of an obligate role of RANK signalling in osteoclastogenesis led to the development of the anti-RANKL antibody denosumab for antiresorptive indications, including bone metastases. Randomized clinical trials and post-marketing surveillance studies have established the acceptable safety profile of denosumab. More recently, several case reports involving patients with advanced-stage melanoma have described remarkable responses following concurrent treatment with denosumab and immune-checkpoint inhibitors. Randomized trials assessing similar combinations in patients with melanoma or renal cell carcinoma are now underway. Herein, we discuss the hallmark clinical trials of denosumab in light of possible immunological effects of this agent. We highlight the role of immune cells as sources of RANK and RANKL in the tumour microenvironment and review data on RANKL inhibition in mouse models of cancer. Finally, we describe hypothetical immune-related mechanisms of action, which could be assessed in clinical trials of immune-checkpoint inhibitors and denosumab in patients with cancer.
Key points
-
Receptor activator of nuclear factor-κB ligand (RANKL) and its cognate receptor, RANK, are expressed by distinct immune cells in the tumour microenvironment (TME) and might also be expressed in tumour cells, the stroma, and non-malignant tissues.
-
Accumulating observational and preclinical evidence suggests that RANKL–RANK interactions between cells in the TME have immunosuppressive effects.
-
Endogenous inhibitors of RANKL include soluble osteoprotegerin (OPG) and leucine-rich repeat-containing G protein-coupled receptor 4; pharmacological inhibitors include denosumab (a monoclonal antibody) and an OPG–Fc fusion protein (discontinued from clinical development after phase I trials).
-
Denosumab is FDA approved for indications including the prevention of skeletal-related events arising from bone metastases in cancer; however, several trials are now testing the immune anticancer activities of denosumab, including as a partner to immune-checkpoint inhibitors.
-
Among immune cells infiltrating human or mouse cancers, RANK can be expressed on immature dendritic cells, immunosuppressive M2-type macrophages, myeloid-derived suppressor cells, and natural killer cells, whereas CD8+ and CD4+ T cells (including regulatory T cells) can express RANKL.
-
Possible mechanisms whereby RANKL inhibition could improve the effects of immune-checkpoint inhibition in cancer include interruption of an immunosuppressive myeloid–lymphocyte axis, cross-modulation of the TME, and interruption of central tolerance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
O’Donnell, J. S., Long, G. V., Scolyer, R. A., Teng, M. W. & Smyth, M. J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 52, 71–81 (2017).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Zappasodi, R., Merghoub, T. & Wolchok, J. D. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell 33, 581–598 (2018).
De Henau, O. et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature 539, 443–447 (2016).
Glodde, N. et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity 47, 789–802.e9 (2017).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Mariathasan, S. et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Ribas, A. et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119.e10 (2017).
Strauss, J. et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin. Cancer Res. 15, 1287–1295 (2018).
Smyth, M. J., Ngiow, S. F., Ribas, A. & Teng, M. W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016).
Hay, M., Thomas, D. W., Craighead, J. L., Economides, C. & Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 32, 40–51 (2014).
Scannell, J. W., Blanckley, A., Boldon, H. & Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 11, 191–200 (2012).
Lacey, D. L. et al. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 11, 401–419 (2012).
Kotsopoulos, J., Singer, C. & Narod, S. A. Can we prevent BRCA1-associated breast cancer by RANKL inhibition? Breast Cancer Res. Treat. 161, 11–16 (2017).
Nolan, E. et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat. Med. 22, 933–939 (2016).
Anderson, D. M. et al. A homologue of the TNF receptor and its ligand enhance T cell growth and dendritic-cell function. Nature 390, 175–179 (1997).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Wong, B. R. et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 272, 25190–25194 (1997).
Yasuda, H. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. USA 95, 3597–3602 (1998).
Luo, J. et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 22, 539–546 (2016).
Boyce, B. F. & Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 473, 139–146 (2008).
Dougall, W. C. & Chaisson, M. The RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer Metastasis Rev. 25, 541–549 (2006).
Dougall, W. C. et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 13, 2412–2424 (1999).
Smyth, M. J., Yagita, H. & McArthur, G. A. Combination anti-CTLA-4 and anti-RANKL in metastatic melanoma. J. Clin. Oncol. 34, e104–e106 (2016).
Bostwick, A. D., Salama, A. K. & Hanks, B. A. Rapid complete response of metastatic melanoma in a patient undergoing ipilimumab immunotherapy in the setting of active ulcerative colitis. J. Immunother. Cancer 3, 19 (2015).
Dougall, W. C. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin. Cancer Res. 18, 326–335 (2012).
Rodan, G. A. & Fleisch, H. A. Bisphosphonates: mechanisms of action. J. Clin. Invest. 97, 2692–2696 (1996).
Jobke, B., Milovanovic, P., Amling, M. & Busse, B. Bisphosphonate-osteoclasts: changes in osteoclast morphology and function induced by antiresorptive nitrogen-containing bisphosphonate treatment in osteoporosis patients. Bone 59, 37–43 (2014).
Stopeck, A. T. et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 28, 5132–5139 (2010).
Fizazi, K. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377, 813–822 (2011).
Henry, D. H. et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 29, 1125–1132 (2011).
Raje, N. S. et al. Impact of denosumab (DMB) compared with zoledronic acid (ZA) on renal function in the treatment of myeloma bone disease. J. Clin. Oncol. 35, (15 suppl.), Abstr. 8005 (2017).
Doshi, S. et al. Denosumab dose selection for patients with bone metastases from solid tumors. Clin. Cancer Res. 18, 2648–2657 (2012).
Dougall, W. C., Holen, I. & Gonzalez Suarez, E. Targeting RANKL in metastasis. Bonekey Rep. 3, 519 (2014).
de Groot, A. F., Appelman-Dijkstra, N. M., van der Burg, S. H. & Kroep, J. R. The anti-tumor effect of RANKL inhibition in malignant solid tumors - a systematic review. Cancer Treat. Rev. 62, 18–28 (2018).
Scagliotti, G. V. et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J. Thorac. Oncol. 7, 1823–1829 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02129699 (2018).
Gnant, M. et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 386, 433–443 (2015).
Gnant, M. et al. The impact of adjuvant denosumab on disease-free survival: results from 3,425 postmenopausal patients of the ABCSG-18 trial. Cancer Res. 76, Abstr. S2-02 (2016).
Gnant, M. et al. Adjuvant denosumab in early breast cancer: disease-free survival analysis of 3,425 postmenopausal patients in the ABCSG-18 trial. J. Clin. Oncol. 36, Abstr. 500 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01077154 (2018).
Smith, M. R. et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379, 39–46 (2012).
Palmerini, E. et al. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur. J. Cancer 76, 118–124 (2017).
Rutkowski, P. et al. Neoadjuvant denosumab treatment of locally advanced giant cell tumor of bone (GCTB). J. Clin. Oncol. 35, 11026–11026 (2017).
Chawla, S. et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 14, 901–908 (2013).
Roux, S. et al. RANK (receptor activator of nuclear factor kappa B) and RANK ligand are expressed in giant cell tumors of bone. Am. J. Clin. Pathol. 117, 210–216 (2002).
Thomas, D. M. & Skubitz, K. M. Giant cell tumour of bone. Curr. Opin. Oncol. 21, 338–344 (2009).
Atkins, G. J. et al. RANK Expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. J. Bone. Miner. Res. 21, 1339–1349 (2006).
Schramek, D. et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468, 98–102 (2010).
Gonzalez-Suarez, E. et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468, 103–107 (2010).
Sigl, V. et al. RANKL/RANK control Brca1 mutation-driven mammary tumors. Cell Res. 26, 761–774 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03382574 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01864798 (2018).
Nguyen, B. et al. D-BEYOND: a window of opportunity trial evaluating denosumab, a RANK-ligand (RANKL) inhibitor and its biological effects in young pre-menopausal women diagnosed with early breast cancer. Cancer Res. 78, Abstr. CT101 (2018).
Rao, S. et al. RANK rewires energy homeostasis in lung cancer cells and drives primary lung cancer. Genes Dev. 31, 2099–2112 (2017).
Branstetter, D. RANK and RANK ligand (RANKL) expression in primary lung cancer [abstract]. World Conference on Lung Cancer https://library.iaslc.org/search-speaker?search_speaker=14360 (2013).
Faget, J., Contat, C., Zangger, N., Peters, S. & Meylan, E. RANKL signaling sustains primary tumor growth in genetically engineered mouse models of lung adenocarcinoma. J. Thorac. Oncol. 13, 387–398 (2017).
Branstetter, D. G. et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin. Cancer Res. 18, 4415–4424 (2012).
Liede, A. et al. An observational study of concomitant immunotherapies and denosumab in patients with advanced melanoma or lung cancer. Oncoimmunology https://doi.org/10.1080/2162402X.2018.1480301 (2018).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Postow, M. A. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017 (2015).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Gettinger, S. et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J. Clin. Oncol. 34, 2980–2987 (2016).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Ribas, A. et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315, 1600–1609 (2016).
Carbone, D. P. et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 376, 2415–2426 (2017).
Khozin, S. et al. Characteristics of real-world metastatic non-small cell lung cancer patients treated with nivolumab and pembrolizumab during the year following approval. Oncologist 23, 328–336 (2018).
Cowey, C. L. et al. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 13, 1699–1710 (2017).
Angela, Y., Gutzmer, R., Satzger, I., Oberndörfer, F. & Tolk, H. Kombination von denosumab und checkpoin inhibitoren - eine retrospektive analyse von 10 patienten mit metastasierenden melanom und knochenmetastasen [poster]. Arbeitsgemeinschaft Dermatol. Onkol. (ADO) 15, P13 (2017).
McClung, M. R. et al. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 354, 821–831 (2006).
Cummings, S. R. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361, 756–765 (2009).
Bone, H. G. et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 5, 513–523 (2017).
Watts, N. B. et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos. Int. 23, 327–337 (2012).
Golden, W. et al. Findings from denosumab (prolia®) postmarketing safety surveillance for serious infections [abstract 918]. Ann. Rheum. Dis. 74, 1204 (2015).
Fizazi, K. et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 27, 1564–1571 (2009).
Lipton, A. et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J. Clin. Oncol. 25, 4431–4437 (2007).
Curtis, J. R. et al. Risk of hospitalized infection among rheumatoid arthritis patients concurrently treated with a biologic agent and denosumab. Arthritis Rheumatol. 67, 1456–1464 (2015).
von Keyserlingk, C. et al. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Semin. Arthritis Rheum. 41, 178–186 (2011).
Lipton, A. et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer 48, 3082–3092 (2012).
Bekker, P. J. et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J. Bone Miner. Res. 19, 1059–1066 (2004).
Rossini, M. et al. Effects of denosumab on peripheral lymphocyte subpopulations. Endocrine 53, 857–859 (2016).
Ominsky, M. S. et al. Denosumab, a fully human RANKL antibody, reduced bone turnover markers and increased trabecular and cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys. Bone 49, 162–173 (2011).
Josien, R. et al. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J. Exp. Med. 191, 495–502 (2000).
Wong, B. R. et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell–specific survival factor. J. Exp. Med. 186, 2075–2080 (1997).
Totsuka, T. et al. RANK-RANKL signaling pathway is critically involved in the function of CD4+CD25+ regulatory T cells in chronic colitis. J. Immunol. 182, 6079–6087 (2009).
Guerrini, M. M. & Takayanagi, H. The immune system, bone and RANKL. Arch. Biochem. Biophys. 561, 118–123 (2014).
Danks, L. & Takayanagi, H. Immunology and bone. J. Biochem. 154, 29–39 (2013).
Williamson, E., Bilsborough, J. M. & Viney, J. L. Regulation of mucosal dendritic cell function by receptor activator of NF-kappa B (RANK)/RANK ligand interactions: impact on tolerance induction. J. Immunol. 169, 3606–3612 (2002).
Loser, K. et al. Epidermal RANKL controls regulatory T cell numbers via activation of dendritic cells. Nat. Med. 12, 1372–1379 (2006).
Meng, Y. H. et al. RANKL-mediated harmonious dialogue between fetus and mother guarantees smooth gestation by inducing decidual M2 macrophage polarization. Cell Death Dis. 8, e3105 (2017).
Barbaroux, J. B., Beleut, M., Brisken, C., Mueller, C. G. & Groves, R. W. Epidermal receptor activator of NF-kappaB ligand controls Langerhans cells numbers and proliferation. J. Immunol. 181, 1103–1108 (2008).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Demoulin, S. A. et al. Cervical (pre)neoplastic microenvironment promotes the emergence of tolerogenic dendritic cells via RANKL secretion. Oncoimmunology 4, e1008334 (2015).
Bachmann, M. F. et al. TRANCE, a tumor necrosis factor family member critical for CD40 ligand–independent T helper cell activation. J. Exp. Med. 189, 1025–1031 (1999).
Wiethe, C., Dittmar, K., Doan, T., Lindenmaier, W. & Tindle, R. Enhanced effector and memory CTL responses generated by incorporation of receptor activator of NF-κB (RANK)/RANK ligand costimulatory molecules into dendritic cell immunogens expressing a human tumor-specific antigen. J. Immunol. 171, 4121–4130 (2003).
Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 (1992).
Tran Janco, J. M., Lamichhane, P., Karyampudi, L. & Knutson, K. L. Tumor-infiltrating dendritic cells in cancer pathogenesis. J. Immunol. 194, 2985–2991 (2015).
Kambayashi, Y. et al. The possible interaction between receptor activator of nuclear factor kappa-B ligand expressed by extramammary paget cells and its ligand on dermal macrophages. J. Invest. Dermatol. 135, 2547–2550 (2015).
Fujimura, T. et al. Receptor activator of NF-kappaB ligand promotes the production of CCL17 from RANK+ M2 macrophages. J. Invest. Dermatol. 135, 2884–2887 (2015).
Sawant, A. et al. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 73, 672–682 (2013).
Danilin, S. et al. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology 1, 1484–1494 (2012).
Zhuang, J. et al. Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+ myeloid-derived suppressor cells. PLOS One 7, e48871 (2012).
Li, H. et al. Human osteoclasts are inducible immunosuppressive cells in response to T cell–Derived IFN-γ and CD40 ligand in vitro. J. Bone Miner. Res. 29, 2666–2675 (2014).
Li, H. et al. Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells. Blood 116, 210–217 (2010).
Kiesel, J. R., Buchwald, Z. S. & Aurora, R. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J. Immunol. 182, 5477–5487 (2009).
An, G. et al. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication. Blood 128, 1590–1603 (2016).
Pearse, R. N. et al. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc. Natl Acad. Sci. USA 98, 11581–11586 (2001).
Giuliani, N., Bataille, R., Mancini, C., Lazzaretti, M. & Barillé, S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood 98, 3527–3533 (2001).
Roux, S. et al. RANK (receptor activator of nuclear factor-kappaB) and RANKL expression in multiple myeloma. Br. J. Haematol. 117, 86–92 (2002).
Schmiedel, B. J. et al. RANKL expression, function, and therapeutic targeting in multiple myeloma and chronic lymphocytic leukemia. Cancer Res. 73, 683–694 (2013).
Kaplan, R. N., Rafii, S. & Lyden, D. Preparing the “soil”: the premetastatic niche. Cancer Res. 66, 11089–11093 (2006).
Abe, M. et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 104, 2484–2491 (2004).
Schmiedel, B. J. et al. Receptor activator for NF-kappaB ligand in acute myeloid leukemia: expression, function, and modulation of NK cell immunosurveillance. J. Immunol. 190, 821–831 (2013).
Taylor, C. R. et al. Distribution of RANK and RANK ligand in normal human tissues as determined by an optimized immunohistochemical method. Appl. Immunohistochem. Mol. Morphol. 25, 299–307 (2017).
Irshad, S. et al. RORγt+ innate lymphoid cells promote lymph node metastasis of breast cancers. Cancer Res. 77, 1083–1096 (2017).
Renema, N., Navet, B., Heymann, M.-F., Lezot, F. & Heymann, D. RANK–RANKL signalling in cancer. Biosci. Rep. 36, e00366 (2016).
Zhau, H. E. et al. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin. Exp. Metastasis 25, 601–610 (2008).
Zhang, J. et al. In vivo real-time imaging of TGF-beta-induced transcriptional activation of the RANK ligand gene promoter in intraosseous prostate cancer. Prostate 59, 360–369 (2004).
Mikami, S. et al. Increased RANKL expression is related to tumour migration and metastasis of renal cell carcinomas. J. Pathol. 218, 530–539 (2009).
Sasaki, A. et al. Receptor activator of nuclear factor-kappaB ligand (RANKL) expression in hepatocellular carcinoma with bone metastasis. Ann. Surg. Oncol. 14, 1191–1199 (2007).
Cao, Y., Zhu, J., Jia, P. & Zhao, Z. scRNASeqDB: a database for RNA-seq based gene expression profiles in human single cells. Genes 8, E368 (2017).
Blake, S. J. & Teng, M. W. Role of IL-17 and IL-22 in autoimmunity and cancer. Actas Dermosifiliogr. 105 (Suppl. 1), 41–50 (2014).
Glatzer, T. et al. RORgammat(+) innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity 38, 1223–1235 (2013).
Kuang, D. M. et al. B7-H1-expressing antigen-presenting cells mediate polarization of protumorigenic Th22 subsets. J. Clin. Invest. 124, 4657–4667 (2014).
Kirchberger, S. et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 210, 917–931 (2013).
Carrega, P. et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 6, 8280 (2015).
Bando, J. K. et al. The tumor necrosis factor superfamily member RANKL suppresses effector cytokine production in group 3 innate lymphoid cells. Immunity 48, 1208–1219.e4 (2018).
Chevalier, M. F. et al. ILC2-modulated T cell–to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Invest. 127, 2916–2929 (2017).
Trabanelli, S. et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 8, 593 (2017).
Palafox, M. et al. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 72, 2879–2888 (2012).
Wang, R. et al. Regulation of activation-induced receptor activator of NF-κB ligand (RANKL) expression in T cells. Eur. J. Immunol. 32, 1090–1098 (2002).
Hochweller, K. & Anderton, S. M. Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur. J. Immunol. 35, 1086–1096 (2005).
Branstetter, D. RANK and RANK Ligand (RANKL) expression in primary human lung cancer [abstract]. International Association for the Study of Lung Cancer https://library.iaslc.org/search-speaker?search_speaker=14360 (2013).
Yoldi, G. et al. RANK signaling blockade reduces breast cancer recurrence by inducing tumor cell differentiation. Cancer Res. 76, 5857–5869 (2016).
Ahern, E. et al. Co-administration of RANKL and CTLA4 antibodies enhances lymphocyte-mediated anti-tumor immunity in mice. Clin. Cancer Res. 23, 5789–5801 (2017).
Ahern, E. et al. RANKL blockade improves efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade in mouse models of cancer. Oncoimmunology 7, e1431088 (2018).
Tan, W. et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470, 548–553 (2011).
Coleman, R. et al. Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat. Rev. 34, 629–639 (2008).
Rogers, A. & Eastell, R. Circulating osteoprotegerin and receptor activator for nuclear factor kappaB ligand: clinical utility in metabolic bone disease assessment. J. Clin. Endocrinol. Metab. 90, 6323–6331 (2005).
Blair, J. M., Zhou, H., Seibel, M. J. & Dunstan, C. R. Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nat. Clin. Pract. Clin. Oncol. 3, 41–49 (2006).
Mountzios, G. et al. Abnormal bone remodeling process is due to an imbalance in the receptor activator of nuclear factor-kappaB ligand (RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors metastatic to the skeleton. Acta Oncol. 46, 221–229 (2007).
Jung, K. et al. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int. J. Cancer 111, 783–791 (2004).
Leeming, D. J. et al. The relative use of eight collagenous and noncollagenous markers for diagnosis of skeletal metastases in breast, prostate, or lung cancer patients. Cancer Epidemiol. Biomarkers Prev. 15, 32–38 (2006).
Jung, K. et al. Serum osteoprotegerin and receptor activator of nuclear factor-kappa B ligand as indicators of disturbed osteoclastogenesis in patients with prostate cancer. J. Urol. 170, 2302–2305 (2003).
Terpos, E. et al. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102, 1064–1069 (2003).
Vitovski, S., Phillips, J. S., Sayers, J. & Croucher, P. I. Investigating the interaction between osteoprotegerin and receptor activator of NF-kappaB or tumor necrosis factor-related apoptosis-inducing ligand: evidence for a pivotal role for osteoprotegerin in regulating two distinct pathways. J. Biol. Chem. 282, 31601–31609 (2007).
Trofimov, S., Pantsulaia, I., Kobyliansky, E. & Livshits, G. Circulating levels of receptor activator of nuclear factor-kappaB ligand/osteoprotegerin/macrophage-colony stimulating factor in a presumably healthy human population. Eur. J. Endocrinol. 150, 305–311 (2004).
Schett, G. et al. Soluble rankl and risk of nontraumatic fracture. JAMA 291, 1108–1113 (2004).
Hofbauer, L. C., Schoppet, M., Schuller, P., Viereck, V. & Christ, M. Effects of oral contraceptives on circulating osteoprotegerin and soluble RANK ligand serum levels in healthy young women. Clin. Endocrinol. 60, 214–219 (2004).
Tanos, T. et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci. Transl. Med. 5, 182ra155 (2013).
Asselin-Labat, M.-L. et al. Control of mammary stem cell function by steroid hormone signalling. Nature 465, 798 (2010).
Streicher, C. et al. Estrogen regulates bone turnover by targeting RANKL expression in bone lining cells. Sci. Rep. 7, 6460 (2017).
Martin, A. et al. Estrogens antagonize RUNX2-mediated osteoblast-driven osteoclastogenesis through regulating RANKL membrane association. Bone 75, 96–104 (2015).
Martin, A. et al. Estrogens and androgens inhibit association of RANKL with the pre-osteoblast membrane through post-translational mechanisms. J. Cell. Physiol. 232, 3798–3807 (2017).
Eghbali-Fatourechi, G. et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Invest. 111, 1221–1230 (2003).
Bakhru, P. et al. Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity. JCI Insight 2, e93265 (2017).
Salmon, H. et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016).
Khan, I. S. et al. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J. Exp. Med. 211, 761–768 (2014).
Lopes, N., Vachon, H., Marie, J. & Irla, M. Administration of RANKL boosts thymic regeneration upon bone marrow transplantation. EMBO Mol. Med. 9, 835–851 (2017).
Sun, M. & Fink, P. J. A. New class of reverse signaling costimulators belongs to the TNF family. J. Immunol. 179, 4307–4312 (2007).
Secchiero, P. et al. Role of the RANKL/RANK system in the induction of interleukin-8 (IL-8) in B chronic lymphocytic leukemia (B-CLL) cells. J. Cell. Physiol. 207, 158–164 (2006).
Chen, N. J., Huang, M. W. & Hsieh, S. L. Enhanced secretion of IFN-gamma by activated Th1 cells occurs via reverse signaling through TNF-related activation-induced cytokine. J. Immunol. 166, 270–276 (2001).
Zhang, S. et al. Osteoclast regulation of osteoblasts via RANKRANKL reverse signal transduction in vitro. Mol. Med. Rep. 16, 3994–4000 (2017).
Kamijo, S. et al. Amelioration of bone loss in collagen-induced arthritis by neutralizing anti-RANKL monoclonal antibody. Biochem. Biophys. Res. Commun. 347, 124–132 (2006).
Smyth, M. J. et al. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J. Immunol. 176, 1582–1587 (2006).
Santini, D. et al. Expression pattern of receptor activator of NFkappaB (RANK) in a series of primary solid tumors and related bone metastases. J. Cell. Physiol. 226, 780–784 (2011).
Santini, D. et al. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLOS One 6, e19234 (2011).
Cross, S. S. et al. Expression of receptor activator of nuclear factor kappabeta ligand (RANKL) and tumour necrosis factor related, apoptosis inducing ligand (TRAIL) in breast cancer, and their relations with osteoprotegerin, oestrogen receptor, and clinicopathological variables. J. Clin. Pathol. 59, 716–720 (2006).
Van Poznak, C. et al. Expression of osteoprotegerin (OPG), TNF related apoptosis inducing ligand (TRAIL), and receptor activator of nuclear factor kappaB ligand (RANKL) in human breast tumours. J. Clin. Pathol. 59, 56–63 (2006).
Farrugia, A. N. et al. Receptor activator of nuclear factor-kappaB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res. 63, 5438–5445 (2003).
Trager, U. et al. The immune response to melanoma is limited by thymic selection of self-antigens. PLOS One 7, e35005 (2012).
Malchow, S. et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339, 1219–1224 (2013).
Metzger, T. C. & Anderson, M. S. Control of central and peripheral tolerance by Aire. Immunol. Rev. 241, 89–103 (2011).
Rossi, S. W. et al. RANK signals from CD4(+)3(-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 204, 1267–1272 (2007).
Roberts, N. A. et al. Rank signaling links the development of invariant γδ T cell progenitors and aire+ medullary epithelium. Immunity 36, 427–437 (2012).
Hikosaka, Y. et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29, 438–450 (2008).
Akiyama, T. et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29, 423–437 (2008).
Gray, D., Abramson, J., Benoist, C. & Mathis, D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J. Exp. Med. 204, 2521–2528 (2007).
Gray, D. H. D. et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108, 3777–3785 (2006).
Fletcher, A. L., Acton, S. E. & Knoblich, K. Lymph node fibroblastic reticular cells in health and disease. Nat. Rev. Immunol. 15, 350–361 (2015).
Brendolan, A. & Caamano, J. Mesenchymal cell differentiation during lymph node organogenesis. Front. Immunol. 3, https://doi.org/10.3389/fimmu.2012.00381 (2012).
Sugiyama, M. et al. Expression pattern changes and function of RANKL during mouse lymph node microarchitecture development. Int. Immunol. 24, 369–378 (2012).
Kong, Y. Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999).
Kim, D. et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member trance. J. Exp. Med. 192, 1467–1478 (2000).
Randall, T. D., Carragher, D. M. & Rangel-Moreno, J. Development of secondary lymphoid organs. Annu. Rev. Immunol. 26, 627–650 (2008).
Yoshida, H. et al. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer’s patches. Immunity 17, 823–833 (2002).
Hess, E. et al. RANKL induces organized lymph node growth by stromal cell proliferation. J. Immunol. 188, 1245–1254 (2012).
Katakai, T. et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J. Immunol. 181, 6189–6200 (2008).
Luan, X. et al. Crystal structure of human RANKL complexed with its decoy receptor osteoprotegerin. J. Immunol. 189, 245–252 (2012).
Lam, J., Nelson, C. A., Ross, F. P., Teitelbaum, S. L. & Fremont, D. H. Crystal structure of the TRANCE/RANKL cytokine reveals determinants of receptor-ligand specificity. J. Clin. Invest. 108, 971–979 (2001).
Wada, T., Nakashima, T., Hiroshi, N. & Penninger, J. M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 12, 17–25 (2006).
Wong, B. R., Josien, R. & Choi, Y. TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. J. Leukoc. Biol. 65, 715–724 (1999).
Nelson, C. A., Warren, J. T., Wang, M. W. H., Teitelbaum, S. L. & Fremont, D. H. RANKL employs distinct binding modes to engage RANK and the OPG decoy receptor. Structure 20, 1971–1982 (2012).
Locksley, R. M., Killeen, N. & Lenardo, M. J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03280667 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03161756 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02900469 (2018).
Australian and New Zealand Clinical Trials Registry. Pre-Operative programmed death-1 (PD1) checkpoint blockade and receptor activator of NFkB ligand (RANKL) inhibition in non-small cell lung cancer (NSCLC) (POPCORN) - a trial for patients with resectable NSCLC. ANZCTR https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375442&isReview=true (2018).
Borstkanker Onderzoek Group. Studieoverzicht - 2017-02 PERIDENO. Borstkanker Onderzoek Group https://www.boogstudycenter.nl/studie/287/perideno.html (2017).
Acknowledgements
The work of E.A. has been supported by a University of Queensland Australian Postgraduate Award. The work of M.J.S. has been supported by a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (grant 1078671), an NHMRC Program Grant (grant 1132519), and The Cancer Council of Queensland (grant 1102242). The work of M.W.L.T. has been supported by a New Concept Grant funded by It’s a Bloke Thing through the Prostate Cancer Foundation of Australia’s Research Program.
Reviewer information
Nature Reviews Clinical Oncology thanks B. Boyle, F. Saad and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
E.A. researched data for the article, E.A., W.C.D., and M.W.L.T made substantial contributions to discussions of content and writing the manuscript, and all authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.J.S. has received research funding from Aduro Biotech, Bristol-Myers Squibb, and Tizona Therapeutics. W.C.D. has received speaker’s honoraria from Amgen. M.W.L.T. has received speaker’s honoraria from Arcus Biosciences, Boehringer Ingelheim, and MSD. E.A. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahern, E., Smyth, M.J., Dougall, W.C. et al. Roles of the RANKL–RANK axis in antitumour immunity — implications for therapy. Nat Rev Clin Oncol 15, 676–693 (2018). https://doi.org/10.1038/s41571-018-0095-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-018-0095-y
This article is cited by
-
Downregulation of osteoprotegerin in colorectal cancer cells promotes liver metastasis via activating tumor-associated macrophage
Scientific Reports (2023)
-
TRPS1 regulates the opposite effect of progesterone via RANKL in endometrial carcinoma and breast carcinoma
Cell Death Discovery (2023)
-
OncoTherad® is an immunomodulator of biological response that downregulate RANK/RANKL signaling pathway and PD-1/PD-L1 immune checkpoint in non-muscle invasive bladder cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Immune microenvironment: novel perspectives on bone regeneration disorder in osteoradionecrosis of the jaws
Cell and Tissue Research (2023)
-
Bone metastasis of hepatocellular carcinoma: facts and hopes from clinical and translational perspectives
Frontiers of Medicine (2022)